Abstract
Aims
Busulfan and treosulfan are cytotoxic agents used in the conditioning regime prior to paediatric haematopoietic stem cell transplantation (HSCT). These agents cause suppression of myeloid cells leaving patients severely immunocompromised in the early post‐HSCT period. The main objectives were: (i) to establish a mechanistic pharmacokinetic–pharmacodynamic (PKPD) model for the treatment and engraftment effects on neutrophil counts comparing busulfan and treosulfan‐based conditioning, and (ii) to explore current dosing schedules with respect to time to HSCT.
Methods
Data on 126 patients, 72 receiving busulfan (7 months–18 years, 5.1–47.0 kg) and 54 treosulfan (4 months–17 years, 3.8–35.8 kg), were collected. In total, 8935 neutrophil count observations were recorded during the study period in addition to drug concentrations to develop a mechanistic PKPD model. Absolute neutrophil count profiles were modelled semimechanistically, accounting for transplant effects and differing set points pre‐ and post‐transplant.
Results
PK were best described by 2‐compartment models for both drugs. The Friberg semimechanistic neutropenia model was applied with a linear model for busulfan and a maximum efficacy model for treosulfan describing drug effects at various stages of neutrophil maturation. System parameters were consistent across both drugs. The HSCT was represented by an amount of progenitor cells enhancing the neutrophils' proliferation and maturation compartments. Alemtuzumab was found to enhance the proliferative rate under which the absolute neutrophil count begin to grow after HSCT.
Conclusion
A semimechanistic PKPD model linking exposure to either busulfan or treosulfan to the neutrophil reconstitution dynamics was successfully built. Alemtuzumab coadministration enhanced the neutrophil proliferative rate after HSCT. Treosulfan administration was suggested to be delayed with respect to time to HSCT, leaving less time between the end of the administration and stem cell infusion.
Keywords: busulfan, conditioning, hematopoietic stem cell transplant, population modelling, treosulfan
What is already known about this subject
Busulfan pharmacokinetics (PK) have been widely studied in relation to clinical and transplant outcomes. Even though much less is known about the treosulfan therapeutic range, there have been some PK analyses as well.
What this study adds
We report busulfan and treosulfan PK and neutrophil pharmacodynamics, accounting for the PK and pharmacodynamic interindividual variability for the short‐term immune recovery after paediatric haematopoietic stem cell transplantation.
1. INTRODUCTION
The bifunctional DNA alkylating agent busulfan is the most common chemotherapy agent used in HSCT conditioning regimens, alone or in combination with other drugs. The busulfan analogue treosulfan is a prodrug of 2 or more bifunctional alkylating agents produced in vivo by nonenzymatic reactions.1 Treosulfan exhibits strong immunosuppressive characteristics with low proinflammatory cytokine release. This facilitates stem cell engraftment and associates with a lower risk of graft vs host disease. Busulfan and treosulfan act on all cells of the granulopoietic series at any stage of their cycle2 through DNA alkylation, interstrand DNA crosslinking and/or chromosomal aberration to induce apoptosis.3, 4, 5
Considerable interpatient variability exists in the effectiveness and toxicity of busulfan‐containing conditioning regimens. Rejection, relapse and toxicity in HSCT are associated with busulfan plasma exposure measured as area under the plasma concentration–time curve (AUC) or average steady state concentration (Css), although recent guideline harmonisation suggests AUC in mg h/L should be used.6 Therefore, personalizing busulfan doses to a target AUC is thought to improve the clinical outcomes.6, 7 Treosulfan is considered to have a wider therapeutic index, which has made it an attractive candidate for the use in conditioning regimens as an alternative to busulfan,8 but recent evidence suggests that treosulfan dose individualisation may also be required.9, 10 A relatively high interindividual variability (IIV), but low interoccasion variability has been reported for treosulfan pharmacokinetics (PK) so individualisation based on therapeutic drug monitoring (TDM) is feasible.9, 10, 11, 12 This fact is also applied to busulfan, for which TDM is currently done in the clinical setting.6 In addition, the overall benefit risk and cost inform busulfan and treosulfan TDM feasibility.
Despite the fact that the PK characteristics of busulfan and treosulfan have been widely studied,6, 9, 13, 14, 15 there are not, to our knowledge, any publications comparing innate immune reconstitution in the early post HSCT period. Sepsis and conditioning toxicity are major causes of early HSCT mortality and morbidity,16 and the dynamics of short‐term innate immune reconstitution have recently been shown to predict long‐term CD4+ T cell recovery.17
Neutrophils are involved in the first line of defence against pathogens as part of the innate immune system, which develops and matures during foetal life, with possible differences in newborns compared to older children or adults.18 Subsequently, age‐related changes might be important in neutrophil dynamics after busulfan or treosulfan‐based myeloablative treatment.18, 19 The therapeutic challenge in improving outcomes for transplanted children relies on the enhancement of immune reconstitution.16, 20 Identifying patient‐ and/or drug‐related characteristics associated with the dynamics of neutrophil reconstitution and predicting individual trajectories may prove to be useful in understanding post‐HSCT recovery.
Therefore, the objectives of the study were to: (i) develop a mechanistic paediatric PK–pharmacodynamic (PD) model of neutrophil counts over time and look whether any parameters scale with age; (ii) identify patient or treatment characteristics associated with the interindividual variability in neutrophil dynamics; (iii) compare the myeloablative effects of busulfan and treosulfan in the shape of the pharmacodynamic effect curve; and (iv) perform model‐based simulations to evaluate the dosing schedules of busulfan and treosulfan.
2. MATERIAL AND METHODS
All patients provided informed consent consistent with the International Conference on Harmonization of technical Requirements for Registration of Pharmaceuticals for Human Use—Good Clinical Practice and local legislation. The study was performed in accordance with the declaration of Helsinki and the use of de‐identified data received ethical approval (17/LO/0008).
2.1. Drug administration and sample collection
Busulfan (1–2 mg/kg) was administered intravenously in a 2‐ or 3‐hour infusion for 4 days prior to HSCT, either every day, twice daily or every 6 hours. Typically, the administration was on day −6 to −3 pretransplant. Treosulfan was administered daily over 3 days, usually on days −7 to −5 prior HSCT, as a 2‐hour intravenous infusion, at the following dose levels: 10 g/m2 (<3 months), 12 g/m2 (3–12 months) or 14 g/m2 (>12 months).21
Blood samples to determine busulfan concentrations in plasma were obtained in all patients prior to the first administration, and 5, 10 and 30 minutes, 1, 2 and 4 hours after the end of the infusion. Treosulfan samples were obtained prior to infusion, and immediately and 1, 2 and 4 hours postinfusion. Busulfan blood samples were analysed using gas chromatography–mass spectrometry, being the limit of detection 0.04 μmol/L. Analytical determination of treosulfan in plasma was described by Chiesa et al.10 and the limit of quantification of treosulfan was 10 μg/mL.
Busulfan AUC was calculated by noncompartmental analysis, following the first dose. Based on this the final dose was adjusted such that the cumulative AUC for all doses was within the target range specified by each treatment protocol. In our data, 14% of patients required dose increases whereas 7% patients underwent dose reductions.
In addition to busulfan and treosulfan, other conditioning drugs were given prior transplant. Busulfan was given either alone or in combination with cyclophosphamide, melphalan, alemtuzumab, fludarabine or thiotepa. By contrast, treosulfan was given in combination only with fludarabine, thiotepa or alemtuzumab. The administration of these drugs were not taken into account due to the lack of information regarding drug administration and exposure.
2.2. Data analysis
Population models were firstly developed for the time course of busulfan plasma concentrations and then for the blood absolute neutrophil count (ANC) data. The population PK characteristics of treosulfan in the studied populations have been reported elsewhere,10 having PK sampling for 20 patients out of the 54 that had neutrophil count data. The model‐predicted individual patient PK parameters of busulfan and treosulfan were incorporated into the dataset comprising the dosing history, ANC values and covariates to generate the individual PK profiles and perform the PKPD modelling. For those patients without PK samples, individual predictions based on dose and covariates were used. Data were logarithmically transformed.
2.2.1. Model selection criteria
Selection between models was based on the minimum value of the objective function provided by NONMEM, differences of 3.84, 7.88 and 10.83 in this value are considered an improvement at a significance level of P < .05, <.005 and <.001, respectively, with 1 degree of freedom. In addition, selection between models was also done based on other information, such as graphic diagnostics (goodness of fit plots, visual predictive checks, residual plots).
2.2.2. Model development
A 3‐step approach was followed during the PK and PKPD analyses.
First, the base models were built, selecting the structural (see below) and the stochastic parts of the population models, the latter including IIV in model parameters and their covariance, and residual error. IIV was modelled exponentially, and residual error was initially described with an additive model in the logarithmic domain. In the busulfan PK analysis, interoccasion variability effect was also explored, where the first occasion involved data after the first dose, and the second occasion values of concentrations after dose adjustment.
Then, covariates were selected using the stepwise covariate model approach22 implemented in the Pearl Speaks NONMEM software.23 Table 1 lists the covariates tested for significance. The stepwise covariate model procedure is based on forward inclusion followed by backward elimination of covariates. During these, the levels of significance used to incorporate the covariate and to keep it in the model were set to 0.05 and 0.001, respectively.
Table 1.
Summary of patient demographic, treatment and disease characteristics. Values are represented as n (%)
| n = 126 | Busulfan (72) | Treosulfan (54) | |
|---|---|---|---|
| Sex | Male | 42 (58.33) | 33 (61.11) |
| Female | 30 (41.67) | 21 (38.89) | |
| Age (mo) a | 51.25 (7.39–227.14) | 33.28 (4.5–209.97) | |
| Weight (kg)a | 14 (5.17–47) | 10.1 (3.76–35.8) | |
| Conditioning | Alone | 5 (6.94) | |
| + Cyclo | 5 (6.94) | ||
| + Cyclo/Melph | 10 (13.89) | ||
| + Cyclo/Melph/Alem | 5 (6.94) | ||
| + Cyclo/Alem or ATG | 5 (6.94) | ||
| + Flu | 9 (12.50) | 9 (16.67) | |
| + Flu/Thio | 7 (12.96) | ||
| + Flu/Thio/ATG | 8 (14.81) | ||
| + Flu/Alem or ATG | 27 (37.50) | 19 (35.19) | |
| + Melph | 6 (8.33) | ||
| + Flu/Alem/Thio | 11 (20.37) | ||
| Diagnosis | Immunodeficiency | 30 (41.67) | 26 (48.15) |
| Cancer | 30 (41.67) | 18 (33.33) | |
| Others | 12 (16.67) | 10 (18.52) | |
| Transplant type | Bone marrow | 62 (86.11) | 48 (88.89) |
| Gene therapy | 7 (9.72) | 4 (7.41) | |
| Peripheral blood | 3 (4.16) | ‐ | |
| Umbilical cord | ‐ | 2 (3.70) | |
| Donor type | Autologous | 12 (16.67) | 1 (1.85) |
| Haploidentical | ‐ | 4 (7.41) | |
| Mismatched family donor | 2 (2.78) | 4 (7.41) | |
| Mismatched unrelated donor | 19 (26.39) | 20 (37.04) | |
| Matched sibling donor | 21 (29.17) | 3 (5.56) | |
| Matched unrelated donor | 18 (25.00) | 17 (31.48) | |
| Unknown | ‐ | 5 (9.26) | |
| Cytomegalovirus | No | 38 (52.78) | 29 (53.70) |
| Yes | 27 (37.50) | 19 (35.19) | |
| Unknown | 7 (9.72) | 6 (11.11) | |
| Epstein–Barr virus | No | 33 (45.83) | 27 (50.00) |
| Yes | 32 (44.44) | 22 (40.74) | |
| Unknown | 7 (9.72) | 5 (9.26) | |
| Granulocyte‐Colony stimulating factors | No | 25 (34.72) | 22 (40.74) |
| Yes | 46 (63.89) | 31 (57.41) | |
| Unknown | 1 (1.39) | 1 (1.85) | |
| Deceased | 10 (13.89) | 9 (16.67) | |
Values are represented as mean (range). Cyclo, Cyclophosphamide; Flu, Fludarabine; Melph, Melphalan; Alem, Alemtuzumab; Thio, Thiotepa; ATG, Anti‐thymocyte globulin.
Finally, the selected models were evaluated using prediction‐corrected visual predictive check (pcVPC)24 and numerical predictive checks, 2 simulation‐based diagnostics, where 500 datasets with the same study characteristics as the original were simulated. Briefly, a pcVPC allows to compare graphically the 2.5th, 50th and 97.5th observation percentiles with the prediction percentiles to detect if there is a systematic match of the prediction percentile with the observation percentile. For numerical predictive checks, comparison of the distributions of the time to nadir, nadir and recovery ANC (defined as 3 consecutive days with normal neutrophil counts) conditions obtained from raw data and simulations were performed.
In addition, parameter precision was further evaluated performing 200 nonparametric bootstrap analysis, and listing the 5th, 50th and 95th percentiles of each parameter distribution.
2.2.3. Software used
Population PK and PKPD analyses were performed with NONMEM v7.3.0.25 Compilations were achieved using gfortran v4.8.5 (Free Software Foundation, Inc., Boston, MA, USA). Graphical and all other statistical analyses, including evaluation of NONMEM outputs, were performed with Pearl Speaks NONMEM (PsN v4.6.0),21 R v3.2.526 and packages Xpose v4.5.3,22 and ggplot2 v.2.2.0.27
2.2.4. PK modelling of busulfan and treosulfan
Busulfan disposition was characterised using compartmental models parameterized in apparent volumes of distribution for each of the compartments, distribution clearances between compartments, and total elimination clearance. Treosulfan disposition was characterised in the same way as busulfan, however this was part of previous work developed in the same institution, and since for only 20 patients out of the 54 PK information was available, the previously developed model10 was used to characterise treosulfan exposure.
2.2.5. PKPD modelling of ANC
ANC dynamics were initially characterised by the semimechanistic neutropenia PKPD model developed by Friberg et al.28 Briefly, the following granulopoietic processes were considered: (i) proliferation represented by 1 compartment; (ii) maturation and differentiation, being accounted for by 3 transit compartments; and (iii) appearance in the systemic circulation and degradation. Cell proliferation, maturation/differentiation and degradation were described by the first order rate constants: proliferation rate constant (Kprol), transit rate constant (Ktr) and elimination from circulation rate constant (Kcirc), respectively. In compartments different from circulating compartments, it was assumed that all compartments in the model share the same initial (baseline) condition, the level of ANC at the start of the study period, which implies that Kprol, Ktr and Kcirc have equal values. These values were derived from the mean transit time (MTT), being Ktr equal to the number of transit compartments plus 1 divided by MTT, in this case: ktr = 4/MTT. The model incorporated a feedback mechanism modulating Kprol, triggered by the ratio between circulation ANC at any time (Circ) and the ANC at equilibrium (Circeq), and governed by the parameter γ. Busulfan and treosulfan reduce Kprol and consequently induce neutropenia as a function of their predicted concentrations in plasma. Extension of this model to allow the drugs to deplete cells in each of the transit compartments was tested.
3. RESULTS
3.1. Study population
A retrospective analysis of paediatric patients undergoing HSCT at a tertiary paediatric hospital (Great Ormond Street Hospital, London, UK) was carried out. Between 2010 and 2016, 126 patients aged 4.5 months to 18.9 years underwent HSCT and received either busulfan or treosulfan. Of those 126 patients, 113 children (90%) received allogeneic HSCT and 13 (10%), autologous HSCT. Table 1 provides a summary of patients' characteristics including demographics, treatments, host and donor types, and disease among others. In addition, a more detailed description of the patients' diagnoses is included in Table S1.
3.2. Population PK model of busulfan and treosulfan
A total of 534 plasma concentrations of busulfan taken during routine therapeutic drug monitoring (TDM) from 72 children were used in the PK analysis. The plasma concentration vs time profiles of busulfan were best described by a 2‐compartment model. Weight affecting clearance (CL) and volume, and post‐menstrual age affecting CL through a maturation function, were included as covariates in the model. In Supplementary material 2, the results corresponding to the PK model are shown, along with the list of the model parameters corresponding to the selected PK model (Table S2). The number of samples below the limit of quantification (BLQ) represented only 5% in the PK analysis, and were therefore ignored. All parameters were obtained with good precision. The pcVPC of busulfan PK model, showing that the median tendency and the dispersion of the busulfan plasma concentration data are well described, is shown in Figure S1.
A total of 20 patients underwent PK sampling for treosulfan as part of a clinical trial reported elsewhere.10 The PK of treosulfan studied population was also best described by a 2‐compartment model. For patients not undergoing PK sampling population predictions (based on dose history and covariates) were used. Supplementary material 2 lists the PK parameters of treosulfan.
3.3. Structural PD model of neutrophil dynamics
For the ANC dynamics characterization, 8935 samples were collected from 1 month prior to 3 months post‐HSCT. The limit of quantification for the ANC was 0.06 × 109 cells/L, and the number of BLQ samples represented 6% in the ANC characterization, but as they were gathered around the nadir time, representing almost 20% of the data at nadir, they were taken into account for the analysis, modelled on the basis of the M3 method,28 using the F_Flag functionality and the PHI function as described by Ahn et al.29 Figure 1 shows the raw data corresponding to the neutrophil count dynamics.
Figure 1.
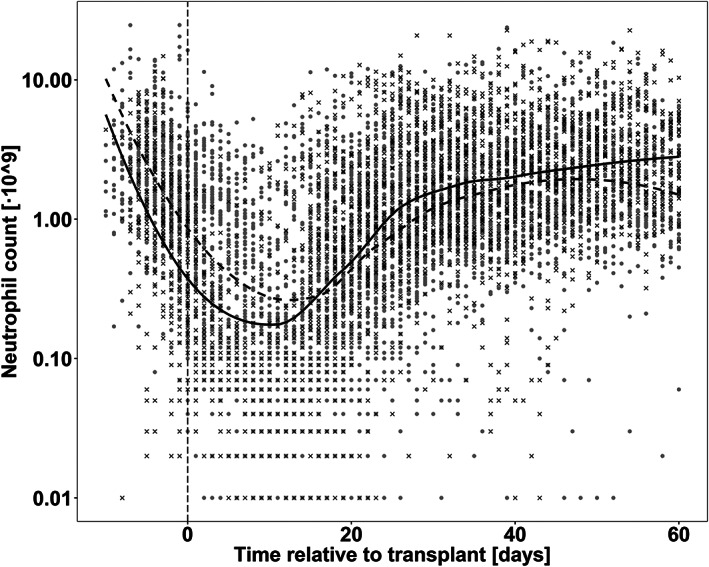
Observed neutrophil counts of all the studied patients. Grey dots represent patients receiving busulfan, and black crosses observations from treosulfan patients. Black lines represent the mean tendency for busulfan and treosulfan in dashed and solid lines, respectively. Vertical dashed line represents the time at which the HSCT is infused
Starting from the Friberg semimechanistic model30 several modifications resulted in a significantly (P < .001) better description of the data (Figure 2 gives a schematic overview of the model). Firstly, the pretransplant baseline was not the same as the post‐transplant set point. This was achieved by taking the initial ANC levels and imputing them from the individual data taking into account the residual error magnitude.31 A steady‐state, different from the baseline condition, was estimated with a typical value of 0.788 × 109 cells/L and an IIV of 75.9%, representing a 76.9 and a 68.4% decrease with respect to the median of the initial conditions in the patients receiving busulfan‐based or treosulfan‐based conditioning, respectively. This is an interesting finding, because it highlights the fact that it is not the same population of cells that is going to grow after the HSCT, after eradicating the original neutrophil population. The amount of progenitor cells delivered by HSCT was represented in the model by an amount of progenitor cells arbitrarily set to a typical value of 1 but allowed to differ among patients, with an IIV estimate of 114%, reflecting considerable differences in the load of transplanted cells. HSCT represents an exogenous cell population, changing cell proliferation and maturation resulting in a 2.03‐fold increase in Kprol and Ktr with respect to the pre‐HSCT system. This change in the system had an activation (latency) period of 9.1 days associated with a 99.5% estimate in IIV. Additionally, Kprol and γ changed in a significant manner (P < .001) after HSCT, increasing a 5% with respect to the cell population pre‐HSCT.
Figure 2.
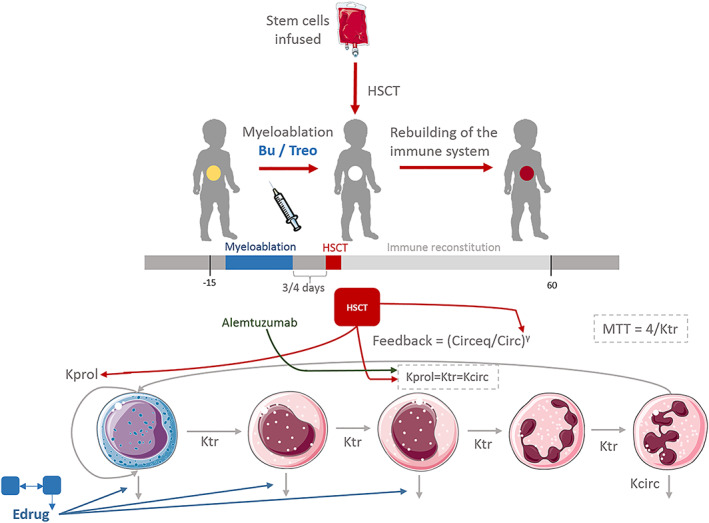
Model framework established in which the pharmacokinetics of busulfan (Bu) and treosulfan (Treo) are linked to the neutrophil dynamics in a short term after transplant, and then is related to graft rejection in a long term. HSCT, haematopoietic stem cell transplantation; MTT, mean transit time; Kprol, proliferation rate constant; Ktr, transit rate constant; Kcirc, elimination from circulation rate constant
Drug effects were included in the model as an elimination of cells from the proliferative compartment, and, in addition, as an extended effect with elimination from the first and second transit compartments, reflecting the pharmacological effect of the drugs, which act on cells on different maturation stages.2 Busulfan effects were included as a linear model with a killing constant equal to 0.7 L/μmol. Treosulfan effects were best described with a maximum efficacy (Emax) model. The estimated parameters were (i) Emax, the maximum fractional decrease in Kprol, of 1.2, and (ii) C50, the predicted plasma concentration eliciting half of Emax of 1.4 × 10−4 mg/L. Even though the pharmacodynamics of both drugs differ, the net effect affecting the depletion of the neutrophils of busulfan and treosulfan is similar and comparable, as shown in Figure S2.
3.4. Covariate model of neutrophil dynamics
Only 1 significant covariate effect was identified. Children receiving alemtuzumab as part of the conditioning regimen, had an increased effect on the outcome of the transplant by improving its neutrophil response, which was translated in a 2.98‐fold increase in Kprol and Ktr, instead of the 2.03‐fold increase found in patients without alemtuzumab on its myeloablative conditioning.
The selected PKPD model is represented schematically in Figure 2 and mathematically in Supplementary material 4. Table 2 also lists the model parameter estimates, indicating that they were obtained with good precision.
Table 2.
Population parameter estimates of the immune reconstitution model
| Parameter | Estimate (RSE%) | Confidence intervals [5th – 95th] | |
|---|---|---|---|
| Pharmacodynamic parameters | CIRCEQ (109/L) | 0.79 (18.91) | [0.52–0.81] |
| IIV CIRCEQ (109/L) (CV%) | 75.90 (26.17) | [73.48–90.5] | |
| MTT (days) | 8.02 (12.04) | [6.91–10.63] | |
| IIV MTT (CV%) | 35.41 (36.95) | [35.35–45.82] | |
| GAMMA | 0.10 (13.93) | [0.07–0.11] | |
| IIV GAMMA (CV%) | 77.10 (11.23) | [72.80–80.62] | |
| KKILL BUSULFAN (L/μmol) | 0.70 (13.45) | [0.45–0.71] | |
| IIV KKILL (CV%) | 54.91 (46.72) | [44.71–55.71] | |
| EMAX TREOSULFAN | 1.20 (10.71) | [1.13–1.52] | |
| C50 TREOSULFAN (mg/L) | 1.40·10−4 (12.23) | [1.1·10−4 – 1.5·10−4] | |
| IIV C50 (CV%) | 44.73 (39.81) | [44.68–47.83] | |
| Transplant effect: Kprol and gamma | 1.05 (2.77) | [1.01–1.08] | |
| Transplant effect: Transit | 1.03 (11.41) | [0.88–1.15] | |
| Transplant effect: Transit + Alem | 1.98 (12.46) | [1.63–2.32] | |
| Latency time transplant (days) | 9.07 (25.13) | [8.90–17.20] | |
| IIV latency time transplant (CV%) | 99.5 (13.43) | [78.68–99.70] | |
| IIV bioavailability transplant (CV%) | 114 (26.01) | [113.13–134.31] | |
| Residual error[log (109/L)] | 0.60 (5.78) | [0.59–0.71] |
CIRCEQ; absolute neutrophil count at steady state; IIV; interindividual variability; MTT; mean transit time; KKILL; killing constant; Kprol; proliferation constant; Alem; coadministration of alemtuzumab; CV; coefficient of variation.
pcVPCs of the immune reconstitution model stratified by conditioning group are shown in Figure 3. In addition, pcVPCs stratified by main conditioning drug are shown in Figure S3. The immune reconstitution model performed adequately and was able to describe the central tendency of the data as well as the spread. Figure 4 shows that the model adequately describes the distribution of 3 relevant clinical metrics as are the value of the nadir (split in grades), the time to nadir, and time to recovery to baseline ANC.
Figure 3.
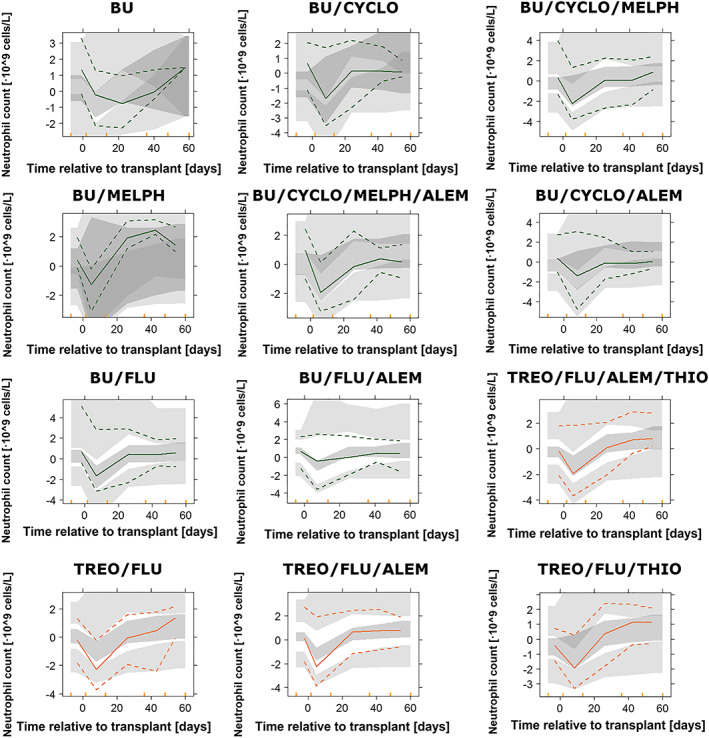
Results of the prediction‐corrected visual predictive checks from 500 simulated profiles for the pharmacokinetic–pharmacodynamic model, stratified by conditioning group. Coloured points represent raw data, and coloured lines correspond to the 2.5th, 50th and 97.5th percentiles of the raw data. Coloured shaded areas represent the 95% prediction intervals of the 2.5th, 50th and 97.5th percentiles of 500 simulated datasets. Cyclo, Cyclophosphamide; Flu, Fludarabine; Melph, Melphalan; Alem, Alemtuzumab; Thio, Thiotepa; ATG, Anti‐thymocyte globulin
Figure 4.
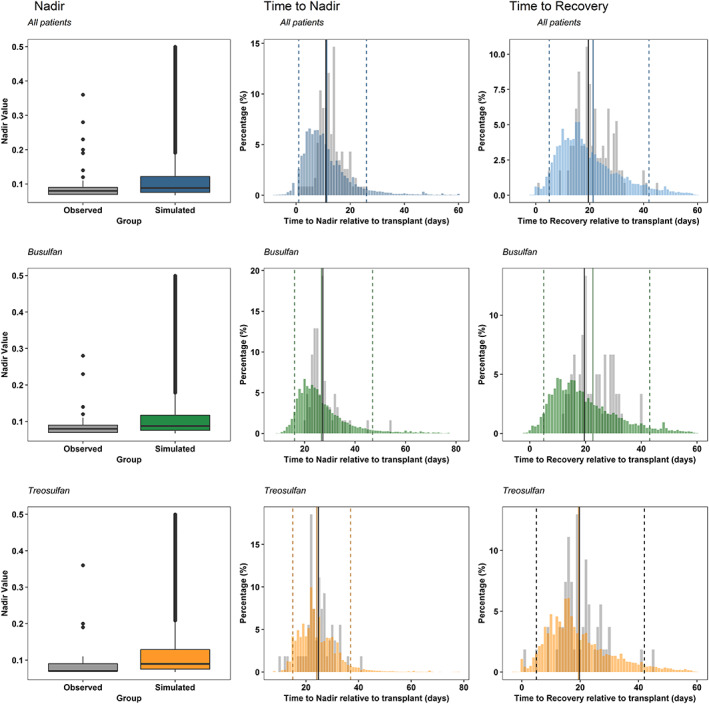
Results of the numerical predictive checks evaluating nadir grade, time to nadir and time to recovery for all of the patients after 500 simulations of the dataset (blue), and stratified by main conditioning drug; busulfan (green), and treosulfan (orange). Grey bars represent the distribution of the raw data. Black vertical line is the median of the raw distribution. Coloured bars correspond to the simulated data. Coloured solid line is the median of the simulated data, and dashed lines indicate the 95% confidence interval of the simulations
3.5. Model evaluation
Figure 5A shows typical neutrophil profiles over time of different covariate groups. It can be observed how treosulfan‐containing regimens reach a deeper nadir compared to busulfan‐containing regimens. While the presence of alemtuzumab in the myeloablative regimen does not elicit a significant effect on the magnitude or the time to nadir, people receiving alemtuzumab recover ANC faster.
Figure 5.
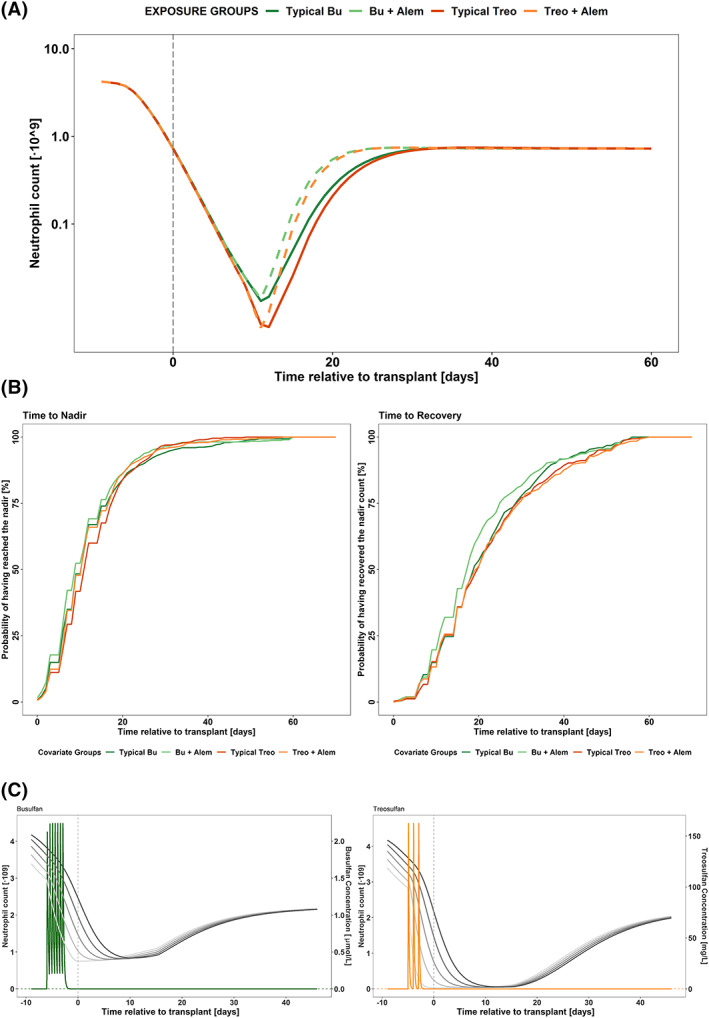
Results from the model exploration and clinical impact exercises. (A) Typical neutrophil count dynamics vs time relative to transplant for individuals belonging to different covariate groups. Vertical dashed line represents the day of transplant. (B) Kaplan–Meier curves after 500 simulations of patients receiving either busulfan or treosulfan as main conditioning drug belonging to different covariate groups. Bu, busulfan; Treo, treosulfan; Alem, alemtuzumab. (C) Typical concentration of drug and neutrophil dynamics vs time relative to transplant in days after deterministic simulations of the most common dosing schedule, for busulfan (green, left panel, twice daily) and treosulfan (orange, right panel, once daily). Left axis indicates concentration of drug, represented by a coloured line, and right axis the neutrophil count, represented by grey lines. Neutrophil count is represented in all the compartments, from proliferation (lighter line) to circulating compartments (darker lines). Dashed vertical line represents the time of transplant, dashed horizontal line reflects the value of the C50
Time to nadir and time to recovery are further explored in Figure 5B, where 500 simulations of every covariate group are analysed. Unlike what was inferred from the deterministic simulations, the coadministration of alemtuzumab does not affect the time to recovery of normal neutrophil counts.
3.6. Clinical model applications
Regarding the exploration of the dosing schedule, concentration–time profiles were simulated using the most common scheme of each of the drugs: 4 consecutive days (day −7 to −4 prior transplant) every 12 hours for busulfan, and 3 daily treosulfan doses (from day −7 to −5 prior transplant). At the time of transplant, busulfan and treosulfan appear to be already cleared (Figure 5C). Whereas for busulfan, results supports the standard dosage regimens, in the case of treosulfan, simulations suggest that a later administration between days −5 and − 3 prior to transplant would allow for treosulfan to be mostly cleared by the day of transplant and mean patients are less neutropenic in the days immediately prior to transplant, as shown in Figure S4.
4. DISCUSSION
A mechanistic model of neutrophil reconstitution in children receiving HSCT has been developed with the objective of understanding the short‐term dynamics of the neutrophil counts. Whilst limited neutropenia dynamic modelling has been published in children,32 to our knowledge this is the first report of a model describing neutrophil dynamics following HSCT. This mechanistic approach links the PK of the main conditioning drugs (busulfan and treosulfan) with neutrophil dynamics. Our findings indicate that HSCT neutrophil dynamics can be captured using a modified Friberg model. The main mechanistic insight was that the time needed for the HSCT to begin exerting its effects was around 8 days, whereas the main clinical insight was that giving treosulfan closer to the day of transplant may be feasible. This latter point is particularly important as sepsis‐related mortality in the day or 2 preceding transplant is not uncommon, so limiting the time the patient is neutropenic prior to transplant whilst ensuring conditioning drugs are washed out (and hence do not affect the donor cells) is a key goal.
Successful HSCT requires a tight balance between wash out of the conditioning treatments and the length of the myelosuppresion, and it should be performed once the myeloablative drug has been eliminated and hence will not affect the donor cells. Model‐based simulations show that at the time of transplant and following current dosing schedules, plasma concentrations of both busulfan and treosulfan were negligible compared with the estimates of drug effect by the day of transplant, and therefore, both ablative treatments are washed out from the system. However, administration of treosulfan could potentially be shifted to later times (up to 2 days, from day −5 to day −3 prior transplant), without compromising the above criteria (Figure S4). Shifting treosulfan dosing forward would limit the time a patient is neutropenic before receiving the transplant, possibly avoiding severe sepsis‐related complications pretransplant. It is important to state that this is based on the assumption that treosulfan is already washed out from the system, and no residual toxic effect remains. In addition, these results must be taken with caution since this clinical study was not primarily designed to evaluate alternative schedules of myeloablative drugs with respect to time to transplant, and the impact of relevant design related aspects as blood sampling distribution on the PD model estimates and therefore on the schedule recommendations have not been explored in detail.
This fact has to be interpreted cautiously, since these simulations were made based on a value of Emax equal to 1.2 and a very low half maximal effective concentration (EC50) estimate of 1.40 × 10−4 mg/L. This constitutes a limitation, as this EC50 value is a reflection of all the drugs being given at the same time before the HSCT, meaning it cannot be interpreted as a pure treosulfan EC50, and model‐based simulations should be limited to scenarios administering similar drug cocktails as in the current 1.
Busulfan PK characteristics have been widely studied in the context of therapeutic drug monitoring, and its exposure has been related mainly to survival and hepatotoxicity.33, 34, 35, 36 The results of the population PK model developed in the current investigation (Supplementary Material 1) were consistent with those previously reported: The value of 2.84 hours for the terminal half‐life obtained in our case resulted remarkably similar to 2.77 hours as reported elsewhere.6 In addition, the values for CL (12.2 L/h/70kg) and steady‐state volume(47.82 L/70kg) were in accordance with those reported by McCune et al6 (11.4 L/h/70kg and 43.8 L/70kg, respectively).
Age was not found to be a significant covariate on any system model parameters. Although the number of circulating neutrophils are higher in preterm and newborn babies, the number returns to adult‐equivalent levels by 72 hours of life, while a neutrophil mass similar to that in adults is achieved at age 4 weeks, without involving scaling of the neutrophil mass to a standard body size.37, 38 Since HSCT is not performed in the neonatal period, this supports our finding of no age‐related effect. Relationships between model parameters and the type of disease were also tested, showing nonsignificant results. This may need to be further explored in a larger cohort.
The immune reconstitution model developed comprises elements describing the granulopoiesis physiology of the host, the transplant and drug effects. Typical estimates of the maturation/differentiation and γ had values of 8.02 days and 0.10, with an IIV of 35.4 and 77.1%, respectively. The former is in accordance to previous reports.23 However, γ in our study was estimated to 0.10 whereas typically it is around 0.16, which possibly indicates regulatory feedback in HSCT differs to nontransplant settings.
Regarding baseline values, there are notable differences between the 2 populations under study. While the median baseline value for the busulfan‐based conditioning patients is 3.42 × 109 cells/L, this value was 2.49 × 109 cells/L in children receiving treosulfan‐based conditioning regimens. This may reflect differences between the 2 populations before entering the study.
A significant improvement in fit was found when drug effect was set to reduce the number of neutrophils by eliminating cells from the proliferative and the first and second maturation compartments. This model feature is supported by the mechanism of action described for busulfan and treosulfan, which can act on cells at any stage of their cycle inducing apoptosis.2, 3, 4, 5
Simulation results indicate that the myeloablative effects of treosulfan are more pronounced than those elicited by busulfan since patients receiving treosulfan‐based conditioning reach a deeper nadir (Figure 5A, B).
While it is true that the model performs adequately for the purpose of the analysis, as clearly shown in the postpredictive checks (Figure 4), looking at the VPCs, the model is not able to describe accurately the data, as the variability seems to be over predicted, and this is a limitation of the model developed. In the case of treosulfan, the predicted median is higher than observed, and there is also some evidence for failure to predict the busulfan effect on neutrophils with the predicted value being somehow higher than the observed median from the nadir onwards. In contrast, the initial observed median neutrophil count is substantially higher than predicted. Another limitation worth mentioning is the fact that the individual parameter estimates of busulfan and treosulfan PK were used in the model developed, and this has the problem of having too narrow estimates of the uncertainty confidence interval because of the shrinkage. Nonetheless, the use of different methods of including the PK data in the PKPD analysis substantially increased computational time without significant changes in parameter estimates.
The lower treosulfan median nadir and, by contrast, the lower percentile being lower with busulfan suggest that the PK of the other drugs that have been given in combination in the conditioning pre‐HSCT may be playing a role. The covariate selected was the coadministration of alemtuzumab, the typical effects of which are graphically presented in Figure 5A. The mechanism of action of alemtuzumab causing neutropenia has not been fully elucidated,39, 40 and although neutrophil recovery has been reported to be further delayed in a group of patients receiving alemtuzumab,41, 42 in the present study, the cohorts receiving alemtuzumab show similar times to recovery of neutrophil counts. The interpretation of this observation is unclear; it may be due to confounding with donor type since this is what determines whether to administer alemtuzumab.
From a mechanistic point of view, it would have been expected that the number of cells transplanted and/or donor or transplant type would show an impact on the estimated effects, facilitating post‐transplant treatment (administration of G‐CSF or steroids, for instance) and follow‐up stratification (high vs low risk groups). This potentially highlights a limitation in our data, in that differences in conditioning regimens are guided by differences in disease and donor type.
In conclusion, a semimechanistic model that predicted the short‐term neutrophil reconstitution after paediatric HSCT was developed. The predictions derived from the model suggest that treosulfan dosing could potentially be moved forward by 1 or 2 days. To our knowledge, this is the first time a semimechanistic model was used to analyse the neutrophil dynamics of children undergoing HSCT. As hospital records are increasingly electronic, the potential exists to use this model to provide predicted reconstitution trajectories, possibly creating a useful Bayesian tool to inform the immediate post‐transplant clinical management of these patients.
ACKNOWLEDGEMENTS
The research leading to these results has received funding from “la Caixa” Banking Foundation, and from the mobility grants by this foundation. This project was also supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London, and J.F.S. was supported by a UK Medical Research Council Fellowship (MRC grant MR/M008665/1). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
B.P.S., I.F.T. and J.F.S. wrote the manuscript with critical input from R.C., P.V. and B.D. B.P.S., R.C., I.F.T. and J.F.S. designed the research, R.C., P.V. and B.D. were part of the clinical team caring for the patients, H.P. analysed the busulfan PK samples and contributed to data collection, and B.P.S., I.F.T. and J.F.S. analysed the data.
Supporting information
TABLE S1 Population characteristics regarding diagnosis
TABLE S2 Population parameter estimates of busulfan and treosulfan PK
FIGURE S1 Results of the prediction‐corrected visual predictive checks from 500 simulated profiles of busulfan concentrations. Green points represent raw data and green lines correspond to the 2.5th, 50th and 97.5th percentiles. Green shaded areas represent the 95% prediction intervals of the 2.5th, 50th and 97.5th percentiles of the 500 simulated datasets.
FIGURE S2 Magnitude of the drug effect vs time with respect to transplant in days. Left panel: busulfan; right panel: treosulfan.
FIGURE S3 Results of the prediction‐corrected visual predictive checks from 500 simulated profiles of absolute neutrophil counts. Upper panel: points represent raw data and lines correspond to the median of the 2.5th, 50th and 97.5th percentiles. Shaded areas represent the 95% prediction intervals of the 2.5th, 50th and 97.5th percentiles of the 500 simulated datasets. Lower panel: coloured open circles represent the percentage of raw data reported as a measurement below the lower limit of quantification. The grey shaded area represents the 95% prediction intervals of percentages of the simulated observations below the lower limit of quantification from the 500 simulated datasets.
FIGURE S4 Typical concentration of treosulfan and neutrophil dynamics vs time relative to transplant in days after deterministic simulations of the most common dosing schedule (once daily). Left axis indicates concentration of drug, represented by a coloured line, and right axis the neutrophil count, represented by grey lines. Neutrophil count is represented in all the compartments, from proliferation (lighter line) to circulating compartments (darker lines). The panel in the left (A) is the simulation using the protocol administration schedule for treosulfan (days –7 to –5), and the panel in the right (B), shows the simulation results when the administration of treosulfan was shifted 2 days and given on days –5 to –3, showing that the drug is already cleared from the body when the haematopoietic stem cell transplantation is performed.
Solans BP, Chiesa R, Doncheva B, et al. Modelling of neutrophil dynamics in children receiving busulfan or treosulfan for haematopoietic stem cell transplant conditioning. Br J Clin Pharmacol. 2020;86:1537–1549. 10.1111/bcp.14260
Iñaki F. Trocóniz and Joseph F. Standing contributed equally to the paper seniorship.
The authors confirm that the PI for this paper is Robert Chiesa and that he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Danylesko I, Shimoni A, Nagler A. Treosulfan‐based conditioning before hematopoietic DCT: more than a Bu look‐alive. Bone Marrow Transplant. 2012;47(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 2. Krivoy N, Hoffer E, Lurie Y, et al. Busulfan use in hematopoietic stem cell transplantation: pharmacology, dose adjustment, safety and efficacy in adults and children. Curr Drug Saf. 2008;3(1):60‐66. [DOI] [PubMed] [Google Scholar]
- 3. Valdez BC, Andersson BS. Interstrand crosslink inducing agents in pretransplant conditioning therapy for hematologic malignancies. Environ Mol Mutagen. 2010;51(6):659‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feit PW, Rastrup‐Andersen N, Matagne R. Studies on epoxide formation from (2S,3S)‐threitol 1,4‐bismethanesulfonate. The preparation and biological activity of (2S,3S)‐1,2‐epoxy‐3,4‐butanediol 4‐methanesulfonate. J Med Chem. 1970;13(6):1173‐1175. [DOI] [PubMed] [Google Scholar]
- 5. Hartley JA, O'Hare CC, Baymgart J. DNA alkylation and interstrand cross‐linking by treosulfan. Br J Cancer. 1999;79(2):264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCune JS, Quinones CM, Ritchie J, et al. Harmonization of Busulfan plasma exposure unit (BPEU): a community‐initiated consensus statement. Biol Blood Marrow Transplant. 2019;25(9):1890‐1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCune JS, Baker KS, Blough DK, et al. Variation in prescribing patterns and therapeutic drug monitoring of intravenous busulfan in pediatric hematopoietic cell transplant recipients. J Clin Pharmacol. 2013;53(3):264‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sjöö F, Hassan Z, Abedi‐Valugerdi M, et al. Myeloablative and immunosuppressive properties of treosulfan in mice. Exp Hematol. 2006;34(1):115‐121. [DOI] [PubMed] [Google Scholar]
- 9. Mohanan E, Panetta JC, Lakshmi KM, et al. Pharmacokinetics and pharmacodynamics of treosulfan in patients with thalassemia major undergoing allogeneic hematopoietic stem cell transplantation. Clin Pharmacol Ther. 2018;104(3):575‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiesa R, Standing JF, Winter R, et al. Proposed therapeutic range of treosulfan in reduced toxicity pediatric allogeneic hematopoietic stem cell transplant conditioning: results from a prospective trial. Clin Pharmacol Ther. 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danielak D, Twardosz J, Kasprzyk A, Wachowiak J, Kałwak K, Główka F. Population pharmacokinetics of treosulfan and development of a limited sampling strategy in children prior to hematopoietic stem cell transplantation. Eur J Clin Pharmacol. 2017;74:79‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Stoep MYE, Bertaina A, Ten Brink MH, et al. High interpatient variability of treosulfan exposure is associated with early toxicity in paediatric HSCT: a prospective multicentre. Br J Haematol. 2017;179(5):772‐780. [DOI] [PubMed] [Google Scholar]
- 13. Románski M, Wachowiak J, Glowka F. Treosulfan pharmacokinetics and its variability in pediatric and adult patients undergoing conditioning prior to hematopoietic stem cell transplantation: current state of the art, in‐depth analysis and perspectives. Clin Pharmacokinet. 2018;57(19):1255‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cremers S, Schoemaker R, Bredius R, et al. Pharmacokinetics of intravenous busulfan in children prior to stem cell transplantation. Br J Clin Pharmacol. 2002;53(4):386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once‐daily IV Busulfan as part of pretransplantation preparative regimens: a comparison with an every 6‐hour dosing schedule. Biol Blood Marrow Transplant. 2007;13(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 16. Szabolcs P, Niedzwiecki D. Immune reconstitution in children after unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2008;14:66‐72. [DOI] [PubMed] [Google Scholar]
- 17. de Koning C, Langenhorst J, van Kersteren C, et al. Innate immune recovery predicts CD4+ T cell reconstitution after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(4):819‐826. [DOI] [PubMed] [Google Scholar]
- 18. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nussbaum C, Gloning A, Pruenster M, et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. J Leukoc Biol. 2013;93(2):175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoare RL, Veys P, Klein N, Callard R, Standing JF. Predicting CD4 T‐cell reconstitution following pediatric hematopoietic stem cell transplantation. Clin Pharmacol Ther. 2017;102(2):349‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slatter MA, Rao K, Juliana I, et al. Treosulfan and Fludarabine conditioning for Hematopoieic stem cell transplantation in children with primary immunodeficiency: UK experience. Biol Blood Marrow Transplant. 2018;24(3):529‐536. [DOI] [PubMed] [Google Scholar]
- 22. Jonsson E, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15:1463‐1468. [DOI] [PubMed] [Google Scholar]
- 23. Lindbom L, Pihlgren P, Jonsson N. PsN toolkit: a collection of computer intensive statistical methods for nonlinear mixed effect modelling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241‐257. [DOI] [PubMed] [Google Scholar]
- 24. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects models. AAPS j. 2011;13(2):143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beal S, Sheiner LB, Boeckmann A. NONMEM's user's guides (1989–2015). Ellicott city: Icon Development Solutions; 2015. [Google Scholar]
- 26. R Core Team . R: A language and environment for statistical computing. (R foundation for statistical Computing, Vienna, Austria. 2017). URL https://www.R-project.org/
- 27. Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer‐Verlag; 2016. [Google Scholar]
- 28. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481‐504. [DOI] [PubMed] [Google Scholar]
- 29. Ahn J, Karlsson M, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35(4):401‐421. [DOI] [PubMed] [Google Scholar]
- 30. Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy‐induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713‐4721. [DOI] [PubMed] [Google Scholar]
- 31. Dansirikul C, Silber HE, Karlsson MO. Approaches to handling pharmacodynamic baseline responses. J Pharmacokinet Parmacodyn. 2008;35:269‐283. [DOI] [PubMed] [Google Scholar]
- 32. Panetta JC, Schaiquevich P, Santana VM, Stewart CF. Using pharmacokinetic and pharmacodynamic modeling and simulation to evaluate importance of schedule in topotecan therapy for pediatric neuroblastoma. Clin Cancer Res. 2008;14(1):318‐325. [DOI] [PubMed] [Google Scholar]
- 33. Bartelink IH, Boelens JJ, Bredius RGM, et al. Body weight‐dependent pharmacokinetics of busulfan in paediatric haematopoietic stem cell transplantation patients: towards individualized dosing. Clin Pharmacokinet. 2012;51(5):331‐345. [DOI] [PubMed] [Google Scholar]
- 34. Sandström M, Karlsson MO, Ljungman P, et al. Population pharmacokinetic analysis resulting in a tool for dose individualization of busulphan in bone marrow transplantation recipients. Bone Marrow Transplant. 2001;28(7):657‐664. [DOI] [PubMed] [Google Scholar]
- 35. Trame MN, Bergstrand M, Karlsson MO, Boos J, Hempel G. Population pharmacokinetics of busulfan in children: increased evidence for body surface area and allometric body weight dosing of busulfan in children. Clin Cancer Res. 2011;17(21):6867‐6877. [DOI] [PubMed] [Google Scholar]
- 36. Rhee SJ, Lee JW, Yu KS, et al. Pediatric patients undergoing hematopoietic stem cell transplantation can greatly benefit from a novel once‐daily intravenous busulfan dosing normogram. Am J Hematol. 2017;92(7):607‐613. [DOI] [PubMed] [Google Scholar]
- 37. Lawrence SM, Corriden R. Age‐appropriate functions and dysfunctions of the neonatal neutrophil. Front Pediatr. 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110(1):18‐28. [DOI] [PubMed] [Google Scholar]
- 39. Siders WM, Shields J, Garron C, et al. Involvement of neutrophils and natural killer cells in the anti‐tumour activity of alemtuzumab in xenograft tumor models. Leuk Lymphoma. 2010;51(7):1293‐1304. [DOI] [PubMed] [Google Scholar]
- 40. Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128(2):260‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chakraverty R, Orti G, Roughton M, et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA‐identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116(16):3080‐3088. [DOI] [PubMed] [Google Scholar]
- 42. Morris EC, Rebello P, Thomson KJ, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T‐cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102(1):404‐406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Population characteristics regarding diagnosis
TABLE S2 Population parameter estimates of busulfan and treosulfan PK
FIGURE S1 Results of the prediction‐corrected visual predictive checks from 500 simulated profiles of busulfan concentrations. Green points represent raw data and green lines correspond to the 2.5th, 50th and 97.5th percentiles. Green shaded areas represent the 95% prediction intervals of the 2.5th, 50th and 97.5th percentiles of the 500 simulated datasets.
FIGURE S2 Magnitude of the drug effect vs time with respect to transplant in days. Left panel: busulfan; right panel: treosulfan.
FIGURE S3 Results of the prediction‐corrected visual predictive checks from 500 simulated profiles of absolute neutrophil counts. Upper panel: points represent raw data and lines correspond to the median of the 2.5th, 50th and 97.5th percentiles. Shaded areas represent the 95% prediction intervals of the 2.5th, 50th and 97.5th percentiles of the 500 simulated datasets. Lower panel: coloured open circles represent the percentage of raw data reported as a measurement below the lower limit of quantification. The grey shaded area represents the 95% prediction intervals of percentages of the simulated observations below the lower limit of quantification from the 500 simulated datasets.
FIGURE S4 Typical concentration of treosulfan and neutrophil dynamics vs time relative to transplant in days after deterministic simulations of the most common dosing schedule (once daily). Left axis indicates concentration of drug, represented by a coloured line, and right axis the neutrophil count, represented by grey lines. Neutrophil count is represented in all the compartments, from proliferation (lighter line) to circulating compartments (darker lines). The panel in the left (A) is the simulation using the protocol administration schedule for treosulfan (days –7 to –5), and the panel in the right (B), shows the simulation results when the administration of treosulfan was shifted 2 days and given on days –5 to –3, showing that the drug is already cleared from the body when the haematopoietic stem cell transplantation is performed.
Data Availability Statement
Research data are not shared.


