Abstract
Aims
Chinese children are more susceptible to the development of thiopurine‐induced leukopenia compared with Caucasian populations. The aim of our study was to establish a 6‐mercaptopurine (6‐MP) dose–concentration–response relationship through exploration of pharmacogenetic factors involved in the thiopurine‐induced toxicities in Chinese paediatric patients afflicted by acute lymphoblastic leukaemia (ALL).
Methods
Blood samples were obtained from ALL children treated with 6‐MP. We determined the metabolite steady‐state concentrations of 6‐MP in red blood cells (RBCs) by using high‐performance liquid chromatography. Pharmacogenetic analysis was carried out on patients' genomic DNA using the MassArray genotyping platform.
Results
Sixty children afflicted by ALL who received 6‐MP treatment were enrolled in this study. The median concentration of 6‐thioguanine in patients afflicted by leukopenia was 235.83 pmol/8 × 108 RBCs, which was significantly higher than for patients unafflicted by leukopenia (178.90 pmol/8 × 108 RBCs; P = 0.029). We determined the population special target 6‐thioguanine threshold to have equalled 197.50 pmol/8 × 108 RBCs to predict leukopenia risk in Chinese paediatric patients afflicted by ALL. Among 36 candidate single nucleotide polymorphisms, our results indicated that NUDT15 (rs116855232) and IMPDH1 (rs2278293) were correlated with a 5.50‐fold and 5.80‐fold higher risk of leukopenia, respectively. MTHFR rs1801133 variants were found to have had a 4.46‐fold significantly higher risk of hepatotoxicity vs wild‐type genotype.
Conclusion
Our findings support the idea that predetermination of genotypes and monitoring of thiopurine metabolism for Chinese paediatric patients afflicted by ALL is necessary to effectively predict the efficacy of treatments and to minimize the adverse effects of 6‐MP maintenance therapy.
Keywords: acute lymphoblastic leukaemia, paediatric, pharmacogenetics, thiopurine metabolite levels
What is already known about this subject
6‐Mercaptopurine has been used to treat children afflicted by with acute lymphoblastic leukaemia.
Chinese children are more likely to have thiopurine‐induced leukopenia compared with Caucasian populations.
TPMT genotyping before the initiation of thiopurine therapy is recommended, but the Chinese population has been demonstrated to have very low frequencies of TPMT variants.
What this study adds
A population specific threshold of 6‐thioguanine = 197.50 pmol/8 × 108 red blood cells was demonstrated to have efficacy in predicting the risk of leukopenia in Chinese children afflicted by acute lymphoblastic leukaemia.
Predetermination of genotypes of NUDT15, IMPDH1 and MTHFR was recommended in order to most efficiently and effectively minimize resultant side effects from maintenance therapy treatments with 6‐mercaptopurine therapy.
1. INTRODUCTION
Over the past 30 years, important advancements in the application of chemotherapeutic regimens for the treatment of acute lymphatic leukaemia (ALL) have been made. Such advancements have resulted in an increased the 5‐year survival rate from <10% in past decades to as high as 90% in recent times.1 Progress in the treatment of ALL has mostly been related to advancements in diagnostics, optimization of chemotherapy, and because of better control of and outcomes from drug‐related adverse events.
Prolonged daily exposure to thiopurines is an important component of the contemporary daily treatment for patients afflicted by ALL. However, up to 40% of patients have courses of thiopurine treatment that are interrupted because of considerable drug‐related adverse effects. Adverse effects are especially important in the contexts of leukopenia and hepatotoxicity, which are the most common and are life‐threatening types of toxicities that afflict children.2 Interestingly, Chinese populations are more susceptible to the development of thiopurine‐induced haematotoxicity compared with Caucasian populations. For example, in an examination of people of an East Asian origin afflicted by ALL and treated with thiopurines, a higher risk (21.9–44.0%)3, 4, 5, 6, 7, 8 of haematotoxicity was observed than when compared to similarly treated Caucasian patients (1.8–5.0%)9, 10, 11 (Table S1).
Pharmacogenetic testing is crucial and should be applied in daily practice such as to optimize the efficacy of thiopurine treatments and so as to prevent adverse events. Thiopurine S‐methyltransferase (TPMT) is an important enzyme that induces metabolises thiopurines by methylation in thiopurine catabolic pathways. Research has confirmed that single nucleotide polymorphisms (SNPs) associated with and as part of TPMT gene result in the loss of the enzyme's function and increase the risk of a leukopenia affliction. The guidelines from the Clinical Pharmacogenetics Implementation Consortium recommend the examination of TPMT genotypes before initiation of thiopurine therapy.12 Higher prevalence of adverse events in Chinese compared to Caucasian populations is not explainable by different frequencies of genetic variations as frequencies of TPMT variants in Chinese populations have been documented to equal only 0.9%.13, 14
Recent genome‐wide correlation studies have reported that the nucleoside diphosphate‐linked moiety X‐type motif 15 (NUDT15) is strongly associated with thiopurine‐induced leukopenia in patients of Asian descent15, 16 and indicated that the Chinese population had a frequency of occurrence of approximately 22.5% of NUDT15 variants.17 However, although NUDT15 acts as a strong predictor for thiopurine‐induced leukopenia, it was not found to have been involved in inducing elevation of the levels of 6‐thioguanine nucleotides (6‐TGN), which suggested that there is a thiopurine metabolism‐independent mechanism at play. The dose–concentration–response (DCR) relationship between thiopurine metabolites and adverse events has thus far however not been evaluated for Chinese children.
Given the knowledge gap in the understanding of the dynamics and mechanisms underlying higher prevalence of thiopurine‐induced adverse events in Chinese children, we undertook the present study in order to establish a 6‐mercaptopurine DCR relationship. Further, we sought to examine which pharmacogenetic factors were involved in thiopurine‐induced toxicities in Chinese paediatric patients afflicted by ALL.
2. METHODS
2.1. Patient recruitment and treatment
Paediatric patients who were undergoing chemotherapy or continuous follow‐up after completion of chemotherapy at the Children's Hospital of Hebei Province and Shandong Provincial Qianfoshan Hospital and who were being treated according to the Chinese Children Cancer Group (CCCG) protocol‐ALL 2015 during the period from 2016 to 2018. These patients were enrolled into our study.18 ALL patients received maintenance therapy that included oral administration of 6‐mercaptopurine (6‐MP; for >4 weeks) and completion of ≥6 months according to the CCCG protocol‐ALL 2015. During maintenance therapy, patients received: intravenous vincristine, a monthly pulse of dexamethasone, weekly oral administration of methotrexate at 25 mg/m2, daily oral administration of 6‐MP at 50 mg/m2, and intrathecal methotrexate once every 2 months. The initial dose of 6‐MP was given based on the results from TPMT genotyping. The initial dose was preadjusted based upon finding related to occurrences of the NUDT15 genotype and blood counts (Figure 1).
Figure 1.
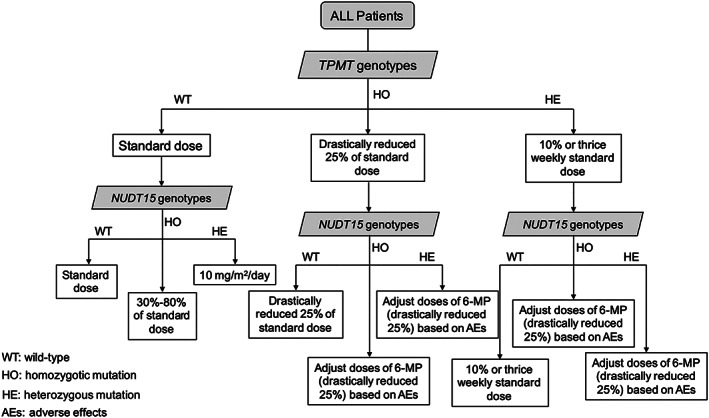
Recommended starting doses and adjustment schema for thiopurines based treatments for the TPMT and NUDT15 phenotypes
We evaluated measures of DCR 3 weeks after the initial treatment (steady‐state). Patients who had changed the amounts of doses before the 3 weeks interval were excluded from further analysis. Doses of 6‐MP were adjusted to maintain a target count of white blood cell count between 2.0–3.0 × 109/L. We graded the degree and severity of leukopenia by using the common toxicity criteria and as follows: Grade 3, 1.0–2.0 × 109/L; and Grade 4, <1.0 × 109/L. We defined hepatotoxicity as when the levels of aspartate aminotransferase or alanine transaminase were at least 2‐fold above the upper limit without cytolysis according to and following guidelines in the Common Terminology Criteria for Adverse Events version 4.0. Our definition for patients that experienced relapse according to CCCG protocol included any observations of: ≥25% lymphoblasts in bone marrow (bone marrow relapse); lymphoblasts on smears of cerebrospinal fluid; cytocentrifuged preparations with a cerebrospinal fluid mononuclear cell count >5/μL; tumour infiltration in the central nervous system (central nervous system relapse); and or an observation of testicle infiltrates (testicle relapse). Measures of leukopenia were determined after dose reduction or discontinuation of 6‐MP, both in the absence of other apparent causes for the leukopenia or as a result of its confirmed disappearance. All aspects of the design and undertaking of our study were reviewed and approved by the Institutional Ethics Board of the Children's Hospital of Hebei Province, China. We obtained informed consent from the parents or guardians of all the enrolled patients.
2.2. Sample collection and analysis of red blood cells 6‐MP metabolite concentrations
We obtain peripheral blood samples were obtained at steady‐state plasma concentrations in order to separate plasma and erythrocytes using standardized procedures. RBC counts from samples were subsequently determined by using a cell counting device (Pentra 120 counter; Horiba) in order to normalize thiopurine metabolite levels to standardized units of measurement (pmol/8 × 108 red blood cells, RBCs). We measured concentrations of RBCs 6‐TGN and 6‐methylmercaptopurine nucleotides (6‐MMPN) by using a Shimadzu 2030C high‐performance liquid chromatography system (Shimadzu, Tokyo, Japan). The calibration curves for 6‐TGN and 6‐MMPN were linear, and each had correlation coefficients = 0.999. Intraday and interday coefficients of variation were <10% over the entire concentration range for all of the metabolites we analysed. Limits of quantification were = 15 and 60 pmol/8 × 108 RBCs for intraday and interday coefficients of variation, respectively.
2.3. SNP selection and genetic analyses
We reviewed 36 thiopurine‐related genetic polymorphisms downloaded from the PharmGKB databases. Genes with minor allele frequency <0.01 results in the PubMed database were excluded for our assessment of individual in Chinese populations. We implemented the genotyping step during maintenance therapy or after completion of maintenance therapy. We extracted total genomic DNA extraction from whole blood samples using the Blood Clot DNA Kit (DP335–02, Tiangen Ltd, Beijing, China) following all the manufacturer's protocols. We quantified measures of genomic DNA using a DNA Nanodrop 2000 spectrophotometer (Thermo‐Fisher Scientific, Waltham, MA, USA). We analysed genotypes by Sequenom's MassARRAY system genotyping platform system.
2.4. Statistical analysis
We conducted statistical analyses using SPSS 22.0 statistics software (SPSS Inc, Chicago, IL, USA; Version 22.0) and by using Prism version 6.0 (Graph Pad software, La Jolla, CA, USA; Version 6.0). We compared concentrations of 6‐TGN and 6‐MMPN using the Mann–Whitney U‐test. We compared frequencies of occurrences of leukopenia and hepatotoxicity for genetic polymorphisms by using a univariate χ2 test. We used multivariate logistic regression analyses were performed to identify the associations of leukopenia and hepatotoxicity with candidate SNPs. In addition, we used receiver operating characteristic curve analyses to predict the development of leukopenia and hepatotoxicity. A 2‐sided P value ≤0.05 was considered the level of statistically significance at which the null of no difference among treatment groups would be rejected.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,19 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.20
3. RESULTS
3.1. Demographic characteristics of patients
There were 74 afflicted children enrolled in our study. Of these, 14 were excluded because changes in the dose before DCR and pharmacogenetics analysis, which indicated that the 6‐MP metabolite concentrations did not achieve the steady‐state at 3 weeks of treatment. Thus, 60 children, aged 2.1–12.5 years were included in the analyses. Patients received a standard dose of 50 mg/m2/day of 6‐MP during maintenance therapy. Treatment duration for patients ranged from 3.7 to 20.7 months (mean 9.3 months). The frequencies of 36 thiopurine‐related genetic polymorphisms in 60 patients are shown in Table S2. Of the 60 patients assessed, no genetic polymorphisms in TPMT*2, TPMT*3A and TPMT*3B were observed. Only 5 patients were found to have harboured the TPMT*1/*3C variant with a frequency of 4.17%. The frequencies of these genotypes did not deviate from Hardy–Weinberg equilibrium (P > 0.05). Demographic data for patients are summarized in Table 1.
Table 1.
Demographic data of patients with acute lymphoblastic leukaemia
| Characteristic | Subject (n = 60) |
|---|---|
| Sex | |
| Male, n (%) | 26 (43.30) |
| Female, n (%) | 34 (56.70) |
| Age at diagnosis (range), y | 5.6 (2.1–12.5) |
| Body weight (range), kg | 22.9 (10.1–58.2) |
| Body surface area (BSA, range), m2 | 0.86 (0.45–1.61) |
| 6‐MP maintenance dose (range), mg/day | 42.3 (25.0–87.5) |
| Duration of 6‐MP therapy (range), mo | 9.3 (3.7–20.7) |
| Median 6‐TGN level (range), pmol/8 × 108 RBCs | 217.13 (89.38–674.77) |
| Median 6‐MMPN level (range), pmol/8 × 108 RBCs, | 3055.65 (459.96–9175.84) |
| Median 6‐MMPN/6‐TGN ratio (range) | 13.12 (1.54–54.55) |
| Median 6‐TGN/6‐MP dosage ratio (range) | 4.98 (1.49–26.38) |
| Immunologic subtype | |
| B cell, n (%) | 55 (91.67) |
| T cell, n (%) | 5 (8.33) |
| Risk group | |
| Standard‐risk, n (%) | 8 (13.33) |
| Median‐risk, n (%) | 52 (86.67) |
| Toxicity | |
| Leukopenia (%) | 36 (60.0) |
| Grade 4, n (%) | 4 (11.11) |
| Grade 3, n (%) | 32 (88.89) |
| Hepatotoxicity (%) | 23 (38.3) |
RBCs, red blood cells; 6‐MMPN, 6‐methyl mercaptopurine nucleotides; 6‐MP, 6‐mercaptopurine; 6‐TGN, 6‐thioguanine nucleotides.
3.2. DCR relationship between thiopurine metabolites and adverse events
During maintenance therapy, the measured median concentrations of 6‐TGN and 6‐MMPN with the standard 6‐MP dose = 217.13 pmol/8 × 108 RBCs (range: 89.38 to 674.77 pmol/8 × 108 RBCs) and 3055.65 pmol/8 × 108 RBCs (range: 459.96 to 9175.84 pmol/8 × 108 RBCs), respectively. Upon follow‐ups with patients who had a median value of 1.8 years from diagnosis (range from 1.2 to 3.1), we found that there were 3 patients who experienced relapse (involving bone‐marrow) and 1 patient died from associated complications. Two of these patients died from bone marrow depression induced by corresponding infection. Concentrations of 6‐TGN for patients with relapse who did not die = 262.94 ± 39.17 pmol/8 × 108 RBCs, and for patients who died = 279.93 and for patients without relapse = 242.32 pmol/8 × 108 RBCs. Analyses indicated that these concentrations did not significantly differ for patients with relapse or without.
Thiopurine‐induced leukopenia and hepatotoxicity were observed in 36 (60.0%) and 23 (38.3%) patients, respectively, during maintenance therapy. The impact of 6‐MP metabolite concentrations on leukopenia and hepatotoxicity was evaluated. Median 6‐TGN concentration in patients with leukopenia was 235.83 pmol/8 × 108 RBCs (range: 89.38 to 674.77 pmol/8 × 108 RBCs), which was a level found to have been significantly higher than in patients without leukopenia (178.90 pmol/8 × 108 RBCs; range: 93.00 to 494.24 pmol/8 × 108 RBCs; P = 0.029; Figure 2). We did not identify a significant correlation of hepatotoxicity and 6‐MMPN concentrations. In addition, we found that the concentrations of 6‐TGN and 6‐MMPN in patients without hepatotoxicity were similar to levels observed for patients with hepatotoxicity.
Figure 2.
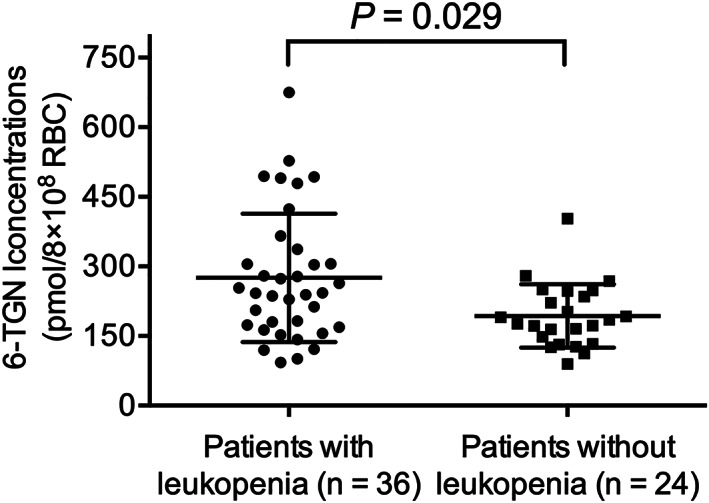
Association of 6‐MP metabolite 6‐TGN concentrations with thiopurine‐induced leukopenia. Median concentrations of 6‐TGN in patients with leukopenia (235.83 pmol/8 × 108 RBCs; range: 89.38–674.77 pmol/8 × 108 RBCs) were increase in patients without leukopenia (178.90 pmol/8 × 108 RBCs; range: 93.00–494.24 pmol/8 × 108 RBCs; P = 0.029)
We determined the optimal value of a 6‐TGN concentration value that could be used to predict the risk of leukopenia by using receiver operating characteristic curve analyses. Optimum levels for sensitivity (66.67%) and specificity (72.73%) were determined for a 6‐TGN concentration threshold of 197.50 pmol/8 × 108 RBCs with an area under the curve = 0.68 (Figure 3). Furthermore, 6 of the 24 patients without leukopenia had 6‐TGN concentrations >197.50 pmol/8 × 108 RBCs while 8 of 36 patients afflicted by leukopenia had 6‐TGN concentrations <197.50 pmol/8 × 108 RBCs. Therefore, levels for the accuracy of predictions of the positive and negative rates reached as high as 82.35% and 69.23%, respectively, based upon the thresholds of 6‐TGN concentrations.
Figure 3.
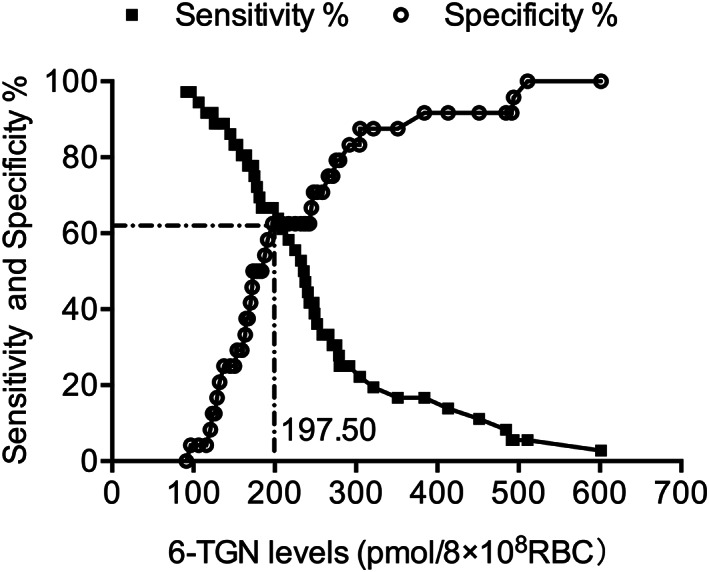
Specificity and sensitivity curves from receiver operating characteristic analysis for red blood cells (RBCs) 6‐thioguanine (6‐TGN) concentrations and leukopenia. The optimum measures of sensitivity (66.67%) and specificity (72.73%) were defined for a 6‐TGN concentration threshold of 197.50 pmol/8 × 108 RBCs with an area under the curve = 0.68
Results from univariate analyses indicated that concentration of 6‐TGN variant inosine triphosphate pyrophosphatase (ITPA) rs1127354 were significantly higher than concentrations for the wild‐type genotype (P = 0.035, Table 2). Among the 6‐MMPN concentrations analysed, variant methylenetetrahydrofolate reductase (MTHFR) rs1801133 was significantly higher compared with concentrations for wild‐type genotype based upon univariate analysis (P = 0.025, Table 3). Sex, age and other genotypes had no associations with concentrations of RBCs 6‐TGN, 6‐MMPN or 6‐TGN/6‐MP dosage ratios based upon results from both univariate and multivariate analyses.
Table 2.
Univariate analysis for variables with thiopurine‐induced leukopenia
| Leukopenia | ||
|---|---|---|
| Variable | OR (95% CI) | P values |
| Sex | 0.64 (0.22–1.81) | 0.40 |
| Age | 0.95 (0.78–1.16) | 0.61 |
| BSA | 0.61 (0.081–4.61) | 0.63 |
| 6‐TGN | 3.33 (1.13–9.80) | 0.03 |
| 6‐MMPN | 1.00 (1.00–1.001) | 0.22 |
| TPMT rs1142345 | 1.00 (0.15–6.48) | > 0.999 |
| NUDT15 rs116855232 | 5.50 (1.11–27.37) | 0.037 |
| ITPA rs1127354 | 8.80 (1.80–43.15) | 0.007 |
| IMPDH1 rs2278293 | 5.80 (1.82–18.46) | 0.003 |
BSA, body surface area; CI, confidence interval; IMPDH1, inosine monophosphate dehydrogenase 1; ITPA, inosine triphosphate pyrophosphatase; NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15; TPMT, thiopurine S‐methyltransferase; OR, odds ratio; 6‐MMPN, 6‐methyl mercaptopurine nucleotides; 6‐TGN, 6‐thioguanine nucleotides.
Table 3.
Univariate analysis for variables with thiopurine‐induced hepatotoxicity
| Variable | Hepatotoxicity | |
|---|---|---|
| OR (95% CI) | P values | |
| Sex | 1.19 (0.42–3.36) | 0.75 |
| Age | 1.08 (0.88–1.31) | 0.47 |
| BSA | 2.87 (0.37–22.07) | 0.31 |
| 6‐TGN | 1.01 (0.96–1.07) | 0.64 |
| 6‐MMPN | 1.00 (1.00–1.00) | 0.95 |
| TPMT rs1142345 | 0.35 (0.036–3.32) | 0.36 |
| NUDT15 rs116855232 | 0.79 (0.23–2.73) | 0.71 |
| ITPA rs1127354 | 1.80 (0.59–5.51) | 0.30 |
| IMPDH1 rs2278293 | 2.14 (0.69–6.68) | 0.19 |
| MTHFR rs1801133 | 4.46 (1.12–17.76) | 0.034 |
BSA, body surface area; CI, confidence interval; IMPDH1, inosine monophosphate dehydrogenase 1; ITPA, inosine triphosphate pyrophosphatase; NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15; MTHFR, methylenetetrahydrofolate reductase; OR, odds ratio; TPMT, thiopurine S‐methyltransferase; 6‐MMPN, 6‐methyl mercaptopurine nucleotides; 6‐TGN, 6‐thioguanine nucleotides.
3.3. Associations between patient genotypes and measures of risk of thiopurine‐induced adverse effects
Based upon results from univariate analyses, we found that ITPA (rs1127354), NUDT15 (rs116855232) and inosine monophosphate dehydrogenase 1 (IMPDH1; rs2278293) were significantly associated with an increased risk of leukopenia. Results from multivariate analyses did not indicate that there was statistical significance for assessment of genetic polymorphisms, except for NUDT15 (rs116855232) and IMPDH1 (rs2278293). In addition, results from univariate analyses indicated that MTHFR rs1801133 variants were significantly related with a 4.46‐fold higher risk of hepatotoxicity cs the level of risk resultant for wild‐type genotype (P = 0.034). Tables 2, 3, 4 show the genetic polymorphisms that were associated with leukopenia and hepatotoxicity. We found no significant association between sex, age and leukopenia based upon results from univariate analysis.
Table 4.
Multivariate analysis for variables with thiopurine‐induced leukopenia
| Variable | OR (95% CI) | P values |
|---|---|---|
| NUDT15 rs116855232 | 6.44 (1.02–40.66) | 0.048 |
| ITPA rs1127354 | 3.891 (0.68–21.32) | 0.13 |
| IMPDH1 rs2278293 | 5.02 (1.25–20.11) | 0.023 |
CI, confidence interval; IMPDH1, inosine monophosphate dehydrogenase 1; ITPA, inosine triphosphate pyrophosphatase; NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15; OR, odds ratio.
4. DISCUSSION
Predicting toxicity in individuals with an at‐risk genotype is a crucial component for efficacy of thiopurine‐based therapy because of its narrow therapeutic index. We enrolled patients and used a standard treatment of doses of 50 mg/m2 to examine the relationship 6‐MP and DCR. We observed a high prevalence of thiopurine‐induced leukopenia in Chinese paediatric patients afflicted by ALL.
In patients receiving the 6‐MP treatment, the interindividual variability in RBCs concentrations of the 6‐MP metabolite, 6‐MMPN, and of 6‐TGN, is a factor known to influence the efficacy of treatment and potential side effects. It also has been reported that 6‐MP has a narrow therapeutic index with a corresponding potential drug‐related toxicity in Caucasian populations.21 Excess accumulation of 6‐MMPN and 6‐TGN has been shown to induce leukopenia and hepatotoxicity by inhibiting nucleosides and protein synthesis; however, such data for Chinese population are sparse. For such a reason, we sought to evaluate measures of correlation between 6‐MP metabolite concentrations and thiopurine‐induced adverse effects in Chinese paediatric patients afflicted by ALL. We performed the evaluation by using a previously described high‐performance liquid chromatography‐based technique,22 which has been cross‐validated with Robert Debre Hospital in Paris, France. Our results indicated that mean concentrations of 6‐TGN in patients with leukopenia were 1.43‐fold higher than were mean concentrations in patients without leukopenia. Interestingly, we identified a target threshold of 6‐TGN concentration >450 pmol/8 × 108 RBCs in Caucasian populations.23 Our results indicated that there was a target threshold of 197.50 pmol/8 × 108 RBCs that could predict leukopenia risk in Chinese paediatric patients afflicted by ALL. Relatively large levels of variation might have been due to different pharmacogenetic properties of thiopurines, such as for TPMT and other variants. We did not observe statistically significant relationships between 6‐MMPN concentrations and thiopurine‐induced adverse effects.
Pharmacogenetic properties of thiopurine‐induced leukopenia have been studied extensively, especially for and in relation to TPMT. However, the influence of TPMT on thiopurine‐induced leukopenia was restricted to only 5 patients who were heterozygous for TPMT*3C in this study. Based on results from our assessment of data from the PharmGKB databases, we identified 36 candidate thiopurine‐associated SNPs (including TPMT) were included for genetic analyses. Results showed that 4 SNPs (ITPA rs1127354, NUDT15 rs116855232, IMPDH1 rs2278293 and MTHFR rs1801133) were identified as having an association with thiopurine‐induced adverse effects or thiopurine metabolism.
NUDT15 is known to dephosphorylate the thiopurine active metabolites TGTP and TdGTP, thereby preventing their incorporation into DNA and negatively influencing thiopurine‐induced leukopenia.24, 25, 26 We confirmed the influence of NUDT15 rs116855232 variants on thiopurine‐induced leukopenia in Chinese paediatric patients afflicted by ALL. Considering the great difference of mutant frequencies in different ethnic populations, standard guidelines for thiopurine dose adjustment based on NUDT15 should be beneficial and improve the outcomes of thiopurine therapy. In addition, we did not find any significant relationships between 6‐TGN concentrations, 6‐TGN/6‐MP dosage ratio, and NUDT15 genetic polymorphisms. Considering the mechanism of NUDT15 on thiopurine, DNA‐6‐TGN concentrations in white blood cells could be directly responsible for the excessive toxicity in patients with NUDT15 deficiency because NUDT15 alters the ratio of TGTP to TGMP which in turn increases DNA‐6‐TGN concentrations.27 It was indicated that DNA‐6‐TGN would be monitored in patients with NUDT15 deficiency in future study. Overall, pretreatment determinations of NUDT15 genotypes is necessary for the prediction of thiopurine‐induced leukopenia.
Furthermore, a deficiency of ITPase in patients is a risk factor for thiopurine‐induced leukopenia.28, 29 In this study, the 15.0% frequency of the ITPA rs1127354 variants was a level similar with previous research that had examined populations of Asians descent (18.1%) and was higher than the reported frequencies for Caucasians (6.0%).7, 30 It has also been reported that ITPA activity was reduced to only 25% in heterozygous and was reduced to a null level in homozygous patients.30 Our data indicated that an increased risk of thiopurine‐induced leukopenia was observed among ITPA rs1127354 variants, which might have been caused by decreased enzyme activity and increased concentrations of 6‐TGN. In the present study, IMPDH1 rs2278293 variants contributed to an increased risk of thiopurine‐induced leukopenia. IMPDH1 is a known key enzyme that diverts 6‐MP away from methylation by TPMT and instead towards 6‐TGN. However, we did not identify any association between IMPDH1 genotypes and 6‐TGN concentrations. These observations may be at least partly explained by lower IMPDH activity in patients with high 6‐methylthioinosine monophosphate concentrations (toxic methylated mercaptopurine ribonucleotides).31
In this study, we observed that there was a significant association between MTHFR rs1801133 variants and thiopurine‐induced hepatotoxicity was observed. Moreover, we observed that patients with variant MTHFR genotypes had 1.62‐fold higher 6‐MMPN concentrations than did patients with the wild‐type genotype. MTHFR homozygotes for this variant exerted 30% of otherwise normal wildtype genotype enzyme activity, while heterozygotes exerted 60%.32 It has also been found that a decrease in the level of activity of MTHFR activity might lead to reduced concentrations of TPMT cofactor S‐adenosylmethionine and thereby causing a decreased in TPMT stability.33 Therefore, we speculated that MTHFR variants may affect TPMT activity or thiopurine metabolism such as to exert thiopurine‐induced hepatotoxicity, but analysis of this effect needs to be undertaken through further genetic association studies.
From our results, we demonstrated that the comparative heterogeneity among East Asian and Caucasian populations in DCR reflected the complexity of ethnicity. In assessment of Caucasian populations, Stocco et al. found that 4.8% of patients were on 6‐MP dose that had been reduced by 30% or more because of concerns arising from haematological toxicity.9, 10, 11 In an assessment of East Asian population, a significantly higher rate of thiopurine‐induced haematological toxicity (17.7–44.0%) was observed than that of the Caucasian research. We observed a 60.0% incidence of haematological toxicity in Chinese patients afflicted by ALL and who received a standard 6‐MP dose. The main reason for this phenomenon was probably due to the intolerance of 6‐TGN and ethnicity‐ based differences of responses to pharmacogenetics in Chinese children compared to other ethnicities.
Our research was not without limitations. Our approach was founded upon a single‐centre study, thus, we lacked different populations and laboratory derived variables. Fourteen patients who were not achieving steady‐state concentrations of 6‐MP metabolites were excluded because 1 of primary objectives in this study was to identify 6‐MP DCR relationship at standard dose regimen of 50 mg/m2/day during maintenance therapy. The additional developmental pharmacokinetic‐pharmacogenetic study should be further explored in patients with very early dose adjustment during maintenance therapy. Our findings should be replicated using an increased number of enrolled patients and from a multicentre‐based study.
In conclusion, we found that concentrations of 6‐TGN and SNPs for NUDT15, IMPDH1 and MTHFR were associated with thiopurine‐induced toxicity during maintenance therapy. This study suggests that Chinese paediatric patients afflicted by ALL need monitoring of concentrations of 6‐TGN with a population specific target threshold of 197.50 pmol/8 × 108 RBCs for Chinese ethnicities. Further, our results indicated that predetermining genotypes of NUDT15, IMPDH1 and MTHFR is an essential step that will greatly help physicians to be able to predict the efficacy of as well as minimize adverse effects during maintenance and 6‐MP therapies.
ACKNOWLEDGEMENTS
This study was supported by National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2017ZX09304029‐001, 2017ZX09304029‐002), Young Taishan Scholars Program of Shandong Province, Qilu Young Scholars Program of Shandong University and the Young Research Program of the Health Commission of Hebei Province (20180623).
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
Y.Z., T.Y.W and W.Z. wrote the manuscript; E.J.A., T.Y.W and W.Z. designed the research; Y.Z., L.W., X.Y.Z., L.W., L.J.Z., F.Y., X.T.L, L.D., H.Y.S., G.X.H. and Y.Z. performed the research. Y.Z., X.Y.Z. and F.T. analysed the data.
Supporting information
Table S1 The risk of haematotoxicity in acute lymphoblastic leukaemia patients undergoing thiopurine treatment between East Asian and Caucasian populations
Table S2 Frequencies of 60 patient genotypes according to pharmacogenetics testing
Zhou Y, Wang L, Zhai X‐Y, et al. Precision therapy of 6‐mercaptopurine in Chinese children with acute lymphoblastic leukaemia. Br J Clin Pharmacol. 2020;86:1519–1527. 10.1111/bcp.14258
Tian‐You Wang and Wei Zhao contributed equally.
The authors confirm that the Principal Investigator for this paper is Wei Zhao and that he had direct clinical responsibility for patients.
Contributor Information
Tian‐You Wang, Email: wangtianyou@bch.com.cn.
Wei Zhao, Email: zhao4wei2@hotmail.com.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541‐1552. [DOI] [PubMed] [Google Scholar]
- 2. Liu Y, Meng Y, Wang L, Liu Z, Li J, Dong W. Associations between the NUDT15 R139C polymorphism and susceptibility to thiopurine‐induced leukopenia in Asians: a meta‐analysis. Onco Targets Ther. 2018;11:8309‐8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi R, Sohn I, Kim MJ, et al. Pathway genes and metabolites in thiopurine therapy in Korean children with acute lymphoblastic leukaemia. Br J Clin Pharmacol. 2019;85(7):1585‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimasaki N, Mori T, Torii C, et al. Influence of MTHFR and RFC1 polymorphisms on toxicities during maintenance chemotherapy for childhood acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol. 2008;30(5):347‐352. [DOI] [PubMed] [Google Scholar]
- 5. Ando M, Ando Y, Hasegawa Y, Sekido Y, Shimokata K, Horibe K. Genetic polymorphisms of thiopurine S‐methyltransferase and 6‐mercaptopurine toxicity in Japanese children with acute lymphoblastic leukaemia. Pharmacogenetics. 2001;11(3):269‐273. [DOI] [PubMed] [Google Scholar]
- 6. Zhu Y, Yin D, Su Y, et al. Combination of common and novel rare NUDT15 variants improves predictive sensitivity of thiopurine‐induced leukopenia in children with acute lymphoblastic leukemia. Haematologica. 2018;103(7):e293‐e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou H, Li L, Yang P, et al. Optimal predictor for 6‐mercaptopurine intolerance in Chinese children with acute lymphoblastic leukemia: NUDT15, TPMT, or ITPA genetic variants? BMC Cancer. 2018;18(1):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka Y, Manabe A, Nakadate H, et al. Methylenetetrahydrofolate reductase gene haplotypes affect toxicity during maintenance therapy for childhood acute lymphoblastic leukemia in Japanese patients. Leuk Lymphoma. 2014;55(5):1126‐1131. [DOI] [PubMed] [Google Scholar]
- 9. Stocco G, Cheok MH, Crews KR, et al. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin Pharmacol Ther. 2009;85(2):164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lennard L, Lilleyman JS, Maddocks JL. The effect of folate supplements on 6‐mercaptopurine remission maintenance therapy in childhood leukaemia. Br J Cancer. 1986;53(1):115‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stork LC, Matloub Y, Broxson E, et al. Oral 6‐mercaptopurine versus oral 6‐thioguanine and veno‐occlusive disease in children with standard‐risk acute lymphoblastic leukemia: report of the Children's oncology group CCG‐1952 clinical trial. Blood. 2010;115(14):2740‐2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Relling MV, Gardner EE, Sandborn WJ, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93(4):324‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zgheib NK, Akika R, Mahfouz R, et al. NUDT15 and TPMT genetic polymorphisms are related to 6‐mercaptopurine intolerance in children treated for acute lymphoblastic leukemia at the Children's cancer center of Lebanon. Pediatr Blood Cancer. 2017;64(1):146‐150. [DOI] [PubMed] [Google Scholar]
- 14. Fei X, Shu Q, Zhu H, et al. NUDT15 R139C variants increase the risk of azathioprine‐induced leukopenia in Chinese autoimmune patients. Front Pharmacol. 2018;9:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine‐induced leukopenia. Nat Genet. 2014;46(9):1017‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki H, Fukushima H, Suzuki R, et al. Genotyping NUDT15 can predict the dose reduction of 6‐MP for children with acute lymphoblastic leukemia especially at a preschool age. J Hum Genet. 2016;61(9):797‐801. [DOI] [PubMed] [Google Scholar]
- 17. Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S‐methyltransferase as predictor of risk for thiopurine‐induced leukopenia in Chinese patients with Crohn's disease. Aliment Pharmacol Ther. 2016;44(9):967‐975. [DOI] [PubMed] [Google Scholar]
- 18. Cai J, Yu J, Zhu X, et al. Treatment abandonment in childhood acute lymphoblastic leukaemia in China: a retrospective cohort study of the Chinese Children's cancer group. Arch Dis Child. 2019;104(6):522‐529. [DOI] [PubMed] [Google Scholar]
- 19. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol. 2017;174(Suppl 1):S272‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogungbenro K, Aarons L, CRESim & Epi‐CRESim Project Groups . Physiologically based pharmacokinetic model for 6‐mercpatopurine: exploring the role of genetic polymorphism in TPMT enzyme activity. Br J Clin Pharmacol. 2015;80(1):86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adam de Beaumais T, Fakhoury M, Medard Y, et al. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 2011;71(4):575‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dervieux T, Meyer G, Barham R, et al. Liquid chromatography‐tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6‐mercaptopurine therapy. Clin Chem. 2005;51(11):2074‐2084. [DOI] [PubMed] [Google Scholar]
- 24. Sato T, Takagawa T, Kakuta Y, et al. NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases. Intest Res. 2017;15(3):328‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yi ES, Choi YB, Choi R, et al. NUDT15 Variants Cause Hematopoietic Toxicity with Low 6‐TGN Levels in Children with Acute Lymphoblastic Leukemia. Cancer Res Treat. 2018;50:872‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koutsilieri S, Caudle KE, Alzghari SK, Monte AA, Relling MV, Patrinos GP. Optimizing thiopurine dosing based on TPMT and NUDT15 genotypes: it takes two to tango. Am J Hematol. 2019;94(7):737‐740. [DOI] [PubMed] [Google Scholar]
- 27. Moriyama T, Nishii R, Lin TN, et al. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2017;27(6):236‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marsh S, Van Booven DJ. The increasing complexity of mercaptopurine pharmacogenomics. Clin Pharmacol Ther. 2009;85(2):139‐141. [DOI] [PubMed] [Google Scholar]
- 29. Azimi F, Mortazavi Y, Alavi S, Khalili M, Ramazani A. Frequency of ITPA gene polymorphisms in Iranian patients with acute lymphoblastic leukemia and prediction of its myelosuppressive effects. Leuk Res. 2015;39(10):1048‐1054. [DOI] [PubMed] [Google Scholar]
- 30. Sumi S, Marinaki AM, Arenas M, et al. Genetic basis of inosine triphosphate pyrophosphohydrolase deficiency. Hum Genet. 2002;111(4–5):360‐367. [DOI] [PubMed] [Google Scholar]
- 31. Haglund S, Taipalensuu J, Peterson C, Almer S. IMPDH activity in thiopurine‐treated patients with inflammatory bowel disease ‐ relation to TPMT activity and metabolite concentrations. Br J Clin Pharmacol. 2008;65(1):69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karas Kuzelicki N, Milek M, Jazbec J, Mlinaric‐Rascan I. 5,10‐methylenetetrahydrofolate reductase (MTHFR) low activity genotypes reduce the risk of relapse‐related acute lymphoblastic leukemia (ALL). Leuk Res. 2009;33(10):1344‐1348. [DOI] [PubMed] [Google Scholar]
- 33. Karas‐Kuzelicki N, Jazbec J, Milek M, Mlinaric‐Rascan I. Heterozygosity at the TPMT gene locus, augmented by mutated MTHFR gene, predisposes to 6‐MP related toxicities in childhood ALL patients. Leukemia. 2009;23(5):971‐974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The risk of haematotoxicity in acute lymphoblastic leukaemia patients undergoing thiopurine treatment between East Asian and Caucasian populations
Table S2 Frequencies of 60 patient genotypes according to pharmacogenetics testing
Data Availability Statement
Research data are not shared.


