Abstract
Background
Neurofibromatosis of types 1 and 2 (NF1, NF2) and schwannomatosis are the diseases that make up the neurofibromatosis spectrum. With respective incidences of 1 in 3000, 1 in 33 000, and 1 in 60 000 births, they form part of the group of rare tumor-suppressor syndromes. They give rise to a greater tumor burden for the nervous system than any other type of neoplastic disease. New approaches to symptomatic treatment are emerging.
Method
This review is based on articles retrieved by a selective literature search on the pathogenesis, diagnosis, and treatment of the neurofibromatoses.
Results
NF1 and NF2 are monogenic diseases, while the genetics of schwannomatosis is complex. The three entities are clinically and pathophysiologically distinct. An important aspect of their tumor biology is the alternation of growth phases and growth pauses. Correlations between genotypes and phenotypes are variable, while new mutations and genetic mosaics are common. Ninety-nine percent of patients with NF1 have six or more café-au-lait spots by the age of 12 months; 90–95% of patients with NF2 develop bilateral vestibular schwannomas. In schwannomatosis, pain is the most prominent symptom; two-thirds of those affected develop spinal schwannomas. The severity and prognosis of these disorders are not closely correlated with the radiological findings; rather, neurologic deficits, malignant transformation, and psychosocial stress are of greater clinical importance. Advances in knowledge of pathophysiology have led to the development of targeted treatment approaches. Examples include the off-label treatment of vestibular schwannomas with bevacizumab and of plexiform neurofibromas with MEK inhibitors.
Conclusion
Patients with neurofibromatoses need individualized care. They should be treated in centers of expertise where interdisciplinary consultation is available and new types of pharmacotherapy can be provided.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. The CME questions on this article can be found at http://daebl.de/RY95. Answers must be submitted by 14 May 2021.
Participation is possible only over the Internet: cme.aerzteblatt.de
Neurofibromatosis types 1 and 2 (NF1, NF2) and schwannomatosis (SWN) constitute the neurofibromatosis disease spectrum. The neurofibromatoses are rare, but they give rise to a greater tumor burden for the nervous system than any other neoplastic disease (1). The three entities differ fundamentally from one another (e1– e3): NF1 and NF2 are inherited in an autosomal dominant pattern, with an approximately 50% rate of de novo mutations in each. The genetic mechanism of SWN is more complex; the rate of new mutations probably lies between 50% and 80% (2, e4).
In this review, we present the definition and pathophysiology of the neurofibromatoses and discuss the current diagnostic and therapeutic strategies in the light of the relevant international literature.
Type 1 neurofibromatosis (NF1)
NF1 (von Recklinghausen’s disease) is a genetic tumor-disposition syndrome associated with malformations and tumor growth in the nervous system and other organ systems (1– 7). Its frequency is approximately 1 per 3000 neonates, regardless of sex or ethnic background (8). The diagnosis is made on clinical grounds with the aid of the criteria defined by the National Institutes of Health (NIH) (table). The life expectancy of the affected persons is approximately 15 years less than normal, mainly because of malignancies, but also due to heart attack and stroke (3, e5).
Table. Diagnostic criteria for the neurofibromatoses (1, e64, e65).
| Type 1 neurofibromatosis (NF1) [3,4] | Type 2 neurofibromatosis (NF2) [e66] | Schwannomatosis [e67] |
| Two or more of the following features | ||
| – ≥ 6 café-au-lait spots (> 5 mm before puberty, > 15 mm after puberty) – ≥ 2 neurofibromas or ≥ 1 plexiform neurofibroma – axillary or inguinal freckling – ≥ 2 Lisch nodules (iris hamartomas) – optic nerve glioma – typical bony changes (dysplasia of sphenoid bone, thinning of cortex in long bones [with or without pseudarthrosis]) – first-degree relative with NF1 |
Definite: bilateral vestibular nerve schwannomas (VS) or – NF2 in a first-degree relative and – unilateral VS and age < 30 or – two of the following: meningioma, glioma, schwannoma, juvenile posterior lens opacification/juvenile cortical cataract Possible or probable: – unilateral VS and age < 30 and at least one of the following: meningioma, glioma, schwannoma, juvenile posterior lens opacification/juvenile cortical cataract – > 2 meningiomas and VS and age < 30 or one of the following: glioma, schwannoma, juvenile posterior lens opacification/juvenile cortical cataract |
Definite: age > 30 and ≥ 2 non-intradermal schwannomas, of which at least one has been histologically confirmed, and no evidence of VS on high-resolution MRI and no constitutional NF2 mutation or 1 histologically confirmed non-vestibular schwannoma and a first-degree relative who meets the above criteria Possible: age < 30 and 2 non-dermal schwannomas, of which at least one has been histologically confirmed, and no evidence of vs on high-resolution mri and no constitutional nf2 mutation or age > 45 and ≥ 2 non-dermal schwannomas, of which at least one has been histologically confirmed, and no manifestations of eighth cranial nerve dysfunction and no constitutional NF2 mutation or radiological evidence of a non-vestibular schwannoma and a first-degree relative who meets the criteria for definite schwannomatosis |
| Genetic tests [1]: blood analysis for constitutional mutations and tumor analysis (if tumor material is available, fixed or frozen) for somatic mutations (mosaics) | ||
| NF1 gene analysis | NF2 gene analysis | NF2 gene analysis—SMARCB1/INI1, LZTR1 |
Diagnostic criteria
Some 70% of persons with NF1 (95% confidence interval [55; 82]), fulfill two of the criteria listed in the Table before their first birthday, thereby establishing the diagnosis. Two criteria are met by the eighth birthday in 97%; all patients have marked, characteristic features of NF1 by the time they are 20 years old (4).
Genetics and molecular pathophysiology
The NF1 gene (17q11.2) encodes the tumor suppressor protein neurofibromin (e6, e7), which stabilizes the proto-oncogene Ras in its inactive form (e8) and thereby inhibits cellular proliferation. The disorder is inherited in autosomal dominant fashion, with complete penetrance and variable expression. Genotype–phenotype correlations have been established for a small number of mutations: for example, a deletion of the entire gene (17q11.2 microdeletion) is associated with a severe disease course (5), and splice-site mutations are associated with spinal tumors in particular. Genetic mosaics generally lead to a mild form of the disease (e6, e7). Prenatal diagnosis of NF1 is possible in principle, as the disease causes severe manifestations before the patient reaches the age of 18 in more than just isolated cases. In Germany, preimplantation diagnosis (PID) must be approved by the local ethics committee. Prenatal diagnosis and PID are used only exceptionally, not routinely.
Clinical manifestations
Neurofibroma
Most patients with NF1 have cutaneous neurofibromas (CNF) (Figure 1a). These usually first appear in adolescence and become more numerous in adulthood (3). In addition, 30–50% of NF1 patients have subcutaneous and plexiform neurofibromas (PNF) (Figures 1a, Figures 1b). PNF are already present in the embryo; they are benign at first and grow in reticular fashion, displacing normal tissue. In around 8–13% of NF1 patients, PNF degenerate into malignant peripheral nerve sheath tumors (MPNST); these tend to metastasize early and are the main cause of the lower life expectancy of persons with NF1 (9).
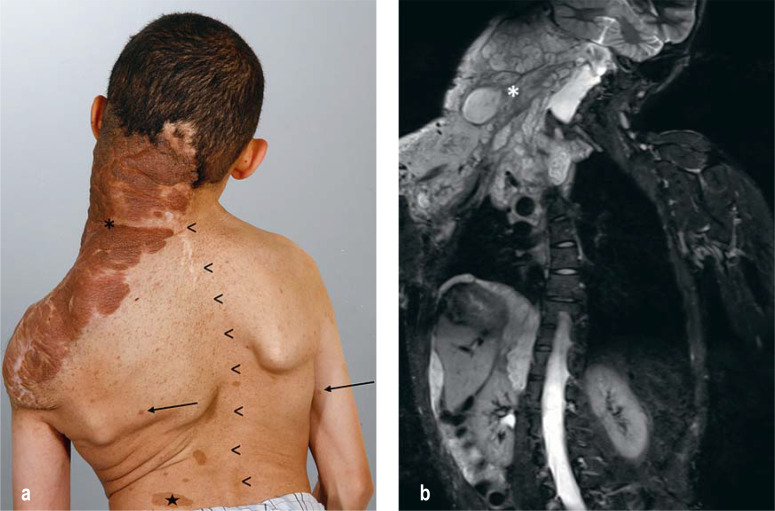
Figure 1:
Features of type 1 neurofibromatosis (NF1)
a) There are small, nodular cutaneous neurofibromas (arrows) and changes of skin pigmentation. A large plexiform neurofibroma (*) extends across the right side of the neck to the back and arm. Scoliosis (arrowheads) and café-au-lait spots (star) are also seen, but are not specific and occur in other genetic syndromes as well (Silver–Russell syndrome, multiple endocrine neoplasia type IIb [MEN IIb], Legius syndrome, McCune–Albright syndrome, etc.).
b) MRI reveals the nodular-infiltrative growth pattern of the plexiform neurofibroma (PNF). Further tumor manifestations are seen around the thoracic and abdominal organs.
Other tumors
Children with NF1 have an approximately sevenfold elevation of the lifetime risk of hematopoietic malignancies, particularly myeloid leukemia. NF-associated and sporadic tumors carry the same prognosis and are treated identically. In women under age 50 in particular, the risk of breast cancer is significantly elevated (approximately fivefold) (3). Benign pheochromocytoma of the adrenal glands is much more common in NF1 patients than in the general population, although still rare (incidence 5% vs. < 1%) (3). Children with NF1 are also at elevated risk of rhabdomyosarcoma, predominantly in the bladder and the prostate gland (3, e9). Gastrointestinal stroma tumors (GIST) have been found at elevated frequency in some study populations (6); endocrine tumors have also been described occasionally (6).
Central nervous system
Patients with NF1 have an elevated risk of developing both low-grade and high-grade gliomas. The most common type is pilocytic astrocytoma of the optic nerve, seen in 15% of NF1 patients of whom approximately half are asymptomatic (3). The brainstem is the second most common site. The most important element of the care of patients with low-grade glioma is monitoring the progression of the disease. Optic nerve gliomas can grow markedly in early childhood but often remain stable from age 7 years onward (7). The lifetime risk of high-grade glioma is elevated at least fivefold. Focal areas of T2-signal intensity (FASI) are seen in the cerebral MRI scans of at least 60% of children with NF1, but their clinical significance remains unclear (e10– e14). The affected children are of mildly lower intelligence on average, and 60–81% have specific partial performance deficits (dyslexia, dyscalculia, etc.) or an attention deficit disorder (AD[H]D) (10, e15– e18). Approximately 11% have a disorder from the autism spectrum, a markedly higher prevalence than in the general population (1 %); these frequency figures cannot, however, be considered representative of the totality of children with NF1, because of a preselection effect (e19).
Pigmentation anomalies
Pigmentation anomalies are not diseases in and of themselves, but they are diagnostically useful. Café-au-lait spots are often the initial clinical manifestation of NF1. Ninety-nine percent of NF1 patients have six or more such spots measuring at least 5 mm in diameter by the end of their first year of life (table) (4, e20, e21). Axillary and inguinal freckling is a further sign seen in only 40% of toddlers with NF1, but in 90% of patients by age 7 (4). Small, melanocytic iris hamartomas (Lisch nodules) are seen in 93% of adults with NF1 (e22).
Musculoskeletal manifestations
The typical skeletal abnormalities are scoliosis, sphenoid wing dysplasia, and congenital tibial dysplasia and osteopenia. Children with NF1 have a 3.4-fold elevation of the fracture risk, and adults over age 41 have a 5.2-fold elevation (33.3% vs. 6.3% fracture rate, p < 0.001); this is correlated, among other things, with a significantly lower vitamin D3 level (11, e23).
Treatment
No causally directed treatment of NF1 is yet available. The most important elements of management are early diagnosis and symptom-oriented treatment. Genetic counseling is especially important. Cognitive deficits and developmental delays should be recognized early and addressed with suitable special-needs education. AD(H)D can be specifically treated with stimulants (methylphenidate) in both childhood and adulthood; randomized, placebo-controlled trials have shown that this improves attentiveness (a 3.9 ± 1.1 point reduction on the simplified Conners scale, p = 0.0003) (e24). Moreover, long-term effects on the development of intelligence and on the quality of life have been demonstrated (e25, e26). Cutaneous neurofibromas can be surgically resected for either esthetic or medical reasons (pain, inflammation).
The surgical treatment of PNF is an interdisciplinary challenge. MRI and PET are indispensable for risk stratification. Regressive changes in the tumor, growth acceleration, and changes in glucose utilization can constitute evidence of malignant transformation. For malignant peripheral nerve sheath tumors (MPNST), complete surgical resection is the single treatment approach offering a chance of cure; the 5-year survival rate with chemotherapy is less than 20%. Annual ophthalmological examinations are more sensitive than imaging studies for the detection of symptomatic optic nerve glioma. For patients with impaired visual acuity, chemotherapy is indicated (carboplatin and vincristine; SIOP-LGG). Radiotherapy is controversial because of the elevated risk of second malignancies and higher-grade gliomas. Vitamin D3 deficiency, if present, should be substituted. For inoperable PNF, a currently available option is off-label treatment with MEK inhibitors. These drugs work by inhibiting the molecule MEK, which plays a role in the Ras downstream signaling pathway and thus affects the cell proliferation in NF1-associated tumors. In an initial trial, tumors were reduced in size in 17 of the 24 children so treated (12).
Type 2 neurofibromatosis (NF2)
NF2 is a tumor disposition syndrome of autosomal dominant inheritance that is found in approximately 1 per 33,000 births (etable 1) (13, e27). Half of all cases arise through de novo mutations (14).
eTable 1. Incidence and new mutation rates of the neurofibromatoses.
| Incidence (per number of births) | Hereditary cases/new mutations | |
| NF1 | 1:3000 | 50:50 |
| NF2 | 1:33 000 | 50:50 |
| SWN | 1:60 000 | 20:80 |
NF1/2, Types 1 and 2 neurofibromatosis; SWN, schwannomatosis
The mortality of the disease has steadily declined in recent years because of early detection, treatment in multidisciplinary centers, and innovative therapies aimed at the preservation of neurological function. The mean life span of persons with NF2 in industrialized countries is now over 60 years (15).
Diagnostic criteria
The growth of schwannomas (also known as neurinomas) on both vestibulocochlear nerves, i.e., bilateral vestibular nerve schwannoma, is pathognomonic for NF2 (5). “Acoustic neuroma” is an older term for vestibular nerve schwannoma (5).
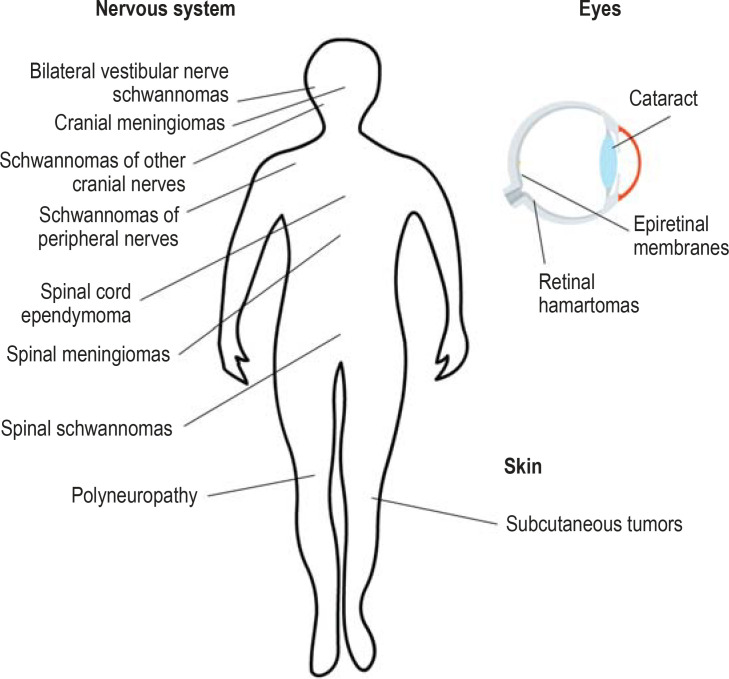
As 41% of patients initially presenting with NF2 do not yet have pronounced bilateral vestibular nerve schwannoma, a number of diagnostic standards have been developed, including the well-established Manchester criteria, which serve as the basis for the NIH criteria (table). Other types of benign tumor are common in NF2 as well (etable 2). The reported frequencies of the main clinical criteria (neurological, ocular, and cutaneous manifestations) (figure 2) are variable, sometimes markedly so (16).
eTable 2. The frequency of tumors in patients with NF2 (11, 14).
| Tumor entity | Frequency |
| Vestibular nerve schwannoma | 90–95% |
| Schwannoma of other cranial nerves | 24–51% |
| (Sub-)cutaneous schwannoma | 59–68% |
| Intracranial meningioma | 45–58% |
| Peripheral nerve schwannoma | 43–48% |
| Ependymoma | 33–53% |
| Mesothelioma | Rare (associated with asbestos) |
| Malignant schwannoma | Rare |
NF2, Type 2 neurofibromatosis
Figure 2.
Clinical features of type 2 neurofibromatosis:
bilateral vestibular nerve schwannomas are pathognomonic.
Genetics and molecular pathophysiology
The NF2 gene (22q11.2) was identified in 1993 (e28, e29). It encodes the tumor suppressor protein merlin. Persisting repair processes by Schwann cells in an altered axonal microenvironment probably contribute to schwannoma formation (17).
Loss of merlin leads to the activation of proliferative signal pathways by way of Ras modulation, which affects targets that have also been identified in NF1 (18, e30). Sporadic schwannomas and meningiomas also arise through changes in these signal pathways (19, e31– e34).
NF2 mutations can be demonstrated in the blood of approximately 91% of all patients with a positive family history (20). In patients with de novo mutations, mutation analysis yields less clear results (59%) (20, e35– e36). Genetic mosaics are found in 30–60% of all de novo cases. The mutation is then clearly demonstrable only in tumor tissue. NF2 genetic mosaics can also be passed on to the offspring of affected patients, with a elevated risk of 8–12% (14). The regulatory framework for prenatal diagnosis and PID in NF2 is the same as in NF1.
Clinical grades of severity
Historically, a distinction was made between the Wishart phenotype of NF2 (early onset, rapid progression, neuropathy, poor prognosis) and the (Feiling-)Gardner phenotype (initial diagnosis after age 20, low tumor burden). Nowadays, mild, moderate, and severe phenotypes are distinguished, and radiological criteria (clinician-rated severity, ClinSev) are also applied that are, in part, correlated with genetic severity (GenSev) (e37). The assessment of disease severity in everyday clinical practice is a complex matter (etable 3).
eTable 3. Grades of clinical severity in type 2 neurofibromatosis (NF2) The disease is categorized as moderate whenever its classification as either mild or severe seems clinically inappropriate.*1.
| Mild | Severe |
| Age at onset >30 years + no more than two further tumors (symptomatic or >1.5 cm)*2 |
Age at onset ≤ 20 years + at least two further tumors (symptomatic or > 1.5 cm) aside from the bilateral vestibular nerve schwannomas or demonstration of a tumor of the central nervous system before the 11th birthday and at least one further symptomatic tumor with positive genetic findings for NF2 |
| – Retained good functional hearing – No dizziness – No impairment of verbal expression – No more than mild bilateral visual impairment – No more than mild gait impairment – No more than mild pain |
– Total deafness – Incapacitating vertigo – Dysphagia necessitating tube feeding – Phonation disturbance – Severe visual impairment – Facial nerve palsy, HB grade 3 or worse – Wheelchair-bound because of paraparesis/paraplegia or severe ataxia – Incontinence necessitating catheterization – Severe orientation disturbance – Severe memory impairment – Additional conditions with moderate or severe manifestations (e.g., postoperative syringomyelia, pseudotumor cerebri due to venous sinus occlusion by a parasagittal meningioma, medically intractable depression) – Severe chronic pain |
*1 The listed disease manifestations are to be considered a starting point for the categorization of clinical severity. The assessment should be performed by an experienced physician and updated at each follow-up examination.
*2 Including vestibular nerve schwannomas and tumors that have already been resected
HB, House and Brackmann
Clinical manifestations
Vestibular nerve schwannoma
Bilateral vestibular nerve schwannomas (VS) arise in 90–95% of patients with NF2 (figure 3). Histologically they resemble sporadic vestibular nerve schwannomas (90 % of all VS, lifetime risk 1 in 1000), but they often grow multifocally (21). Each of these tumors or tumor lobules can contain a mixed cell population with different somatic NF2 mutations (polyclonality) (22).
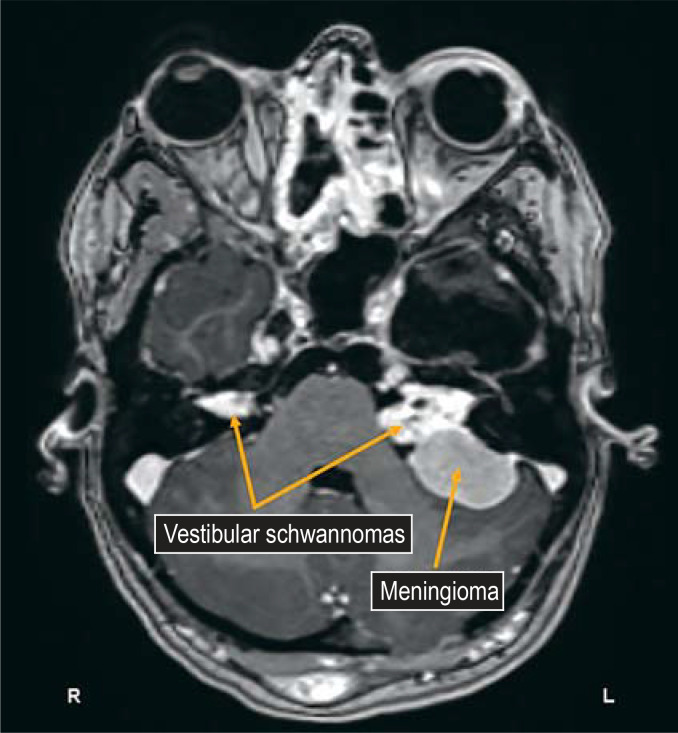
Figure 3.
Typical MRI findings: bilateral vestibular nerve schwannomas (VS) in a patient with type 2 neurofibromatosis. Collision of a VS with a meningioma, as seen here on the patient’s left side, is also not unusual.
Management options: The main goal of the treatment of bilateral vestibular schwannoma is to prevent impending loss of function, especially hearing loss (efigure). Surgical interventions are more challenging than in the management of sporadic VS. The opportunity to spare the patient’s hearing on the side of a small, favorably located lesion in a patient with good hearing in both ears should not be missed (23, 24). If adequate residual hearing is present after surgery, auditory rehabilitation can be carried out with a conventional hearing aid at first, and then later with a cochlear implant or an auditory brainstem implant (ABI) (e38, e39).
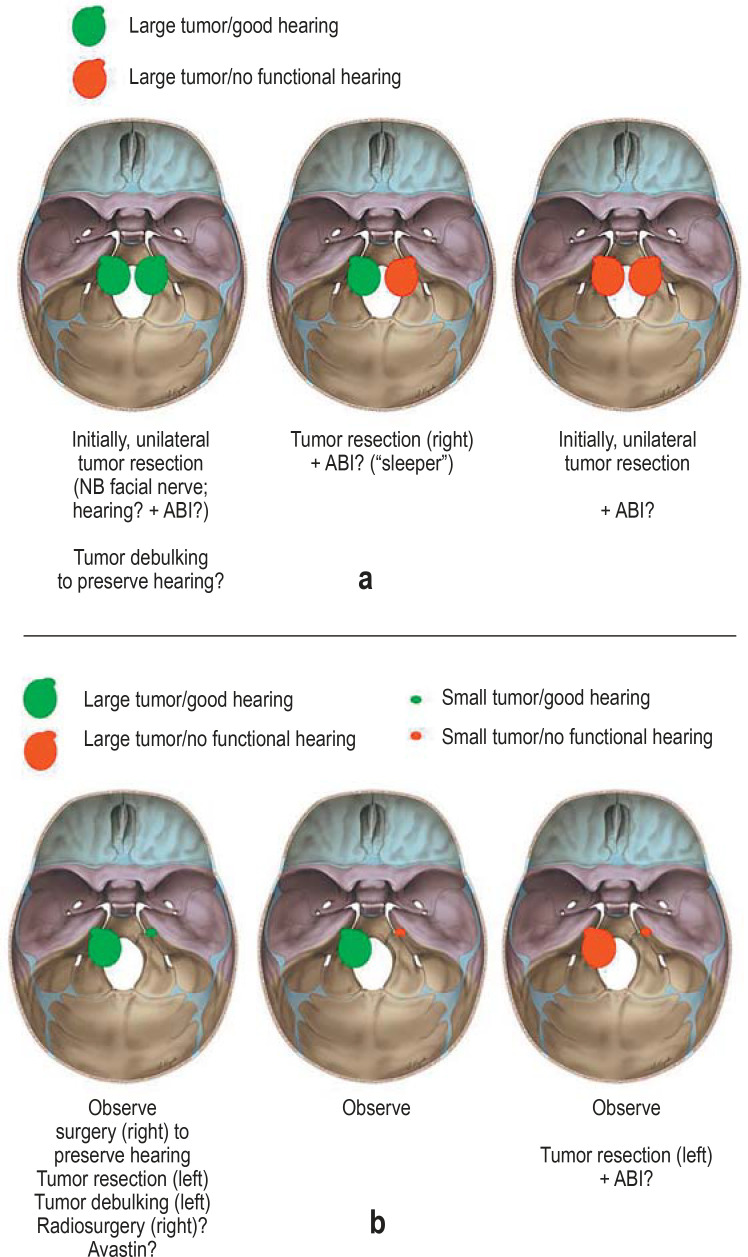
eFigure.
Treatment options for vestibular nerve schwannomas (VS) in type 2 neurofibromatosis (NF2). Possible constellations in the management of VS in NF2, depending on tumor size and residual auditory function:
a) large tumor on both sides
b) asymmetrical tumor distribution
A “sleeper” is an inactive brainstem implant that is only activated in the event that the patient also loses hearing in the other ear.
ABI, Auditory brainstem implant
Growth arrest of vestibular nerve schwannomas by means of stereotactic radiosurgery is less commonly achieved in NF2 patients than in sporadic tumors, and higher radiation doses can accelerate hearing loss (25). The reported 5-year tumor control and hearing preservation rates vary from 66% to 100% and from 33% to 57%, respectively (e40– e46).
Off-label treatment with the vascular endothelial growth factor (VEGF) inhibitor bevacizumab has now become an important therapeutic option. At least over the intermediate term (according to retrospective studies with follow-up intervals of 1–3 years), it is associated with hearing improvement in 57% and tumor shrinkage in 55% of patients, although there is a risk of side effects including hypertension and proteinuria (26, e47).
Other schwannomas
Schwannomas of other cranial nerves are found in 24–51% of NF2 patients (13). Tumors affecting the lower cranial nerves are most likely to cause severe disease with marked impairment of the patient’s quality of life, as they are associated with vocal cord paralysis (35%) and dysphagia (50%) (27). Schwannomas of peripheral nerves (most commonly paraspinal and subcutaneous schwannomas) are found in 70% of NF2 patients (etable 2) (13, e48). In contrast to schwannomatosis, they generally become symptomatic with sensory or motor disturbances, rather than pain. Unlike PNF in NF1, schwannomas in NF2 hardly ever undergo malignant transformation. Resection is the treatment of choice for symptomatic peripheral schwannomas; preservation of function is very important here as well (e49).
Meningiomas
Approximately 50% of patients with NF2 have meningiomas; these tumors are associated with high morbidity (28). One-fifth of all children with meningiomas have NF2 (29). Convexity meningiomas can generally be totally resected without complication, but this is indicated only when their further growth would threaten neurologic function or cerebral perfusion. The risk of postoperative morbidity is much higher with meningiomas of the skull base. The progression-free 5-year survival rate after stereotactic radiosurgery for meningioma is approximately 86% (30). A combined microsurgical and radiosurgical strategy is helpful primarily in the treatment of parasagittal meningiomas and of petroclival tumors that extend into the cavernous sinus. Patients with a heavy meningioma burden may develop malresorptive hydrocephalus.
Spinal tumors
Spinal schwannomas and meningiomas should be resected if they compress the spinal cord or become symptomatic.
Ependymomas
Nearly all intramedullary tumors in patients with NF2 are ependymomas (31, e50); these arise in 33–53% of all patients with NF2, and 85% are located in the cervical spine (32). If they progress, they should be microsurgically excised. Treatment with bevacizumab is an alternative (33).
The growth behavior of NF2-associated tumors
Intracranial vestibular nerve schwannomas and meningiomas have similar annual growth rates, ranging from 0.30 to 2.57 cm3 per year (e51– e54). Non-vestibular schwannomas grow much more slowly (34). As a rule, the growth is “saltatory,” with periods of growth separated by pauses that may last for years (34, e52). In their spontaneous course, VS and meningiomas display a median progression-free interval of 18–21 months (34). For intramedullary ependymomas, the median progression-free interval is 24 months (35).
Peripheral neuropathy
Approximately 47% of patients with NF2 suffer from neuropathy (e55). The peripheral nerve lesions are due to non-compressive microlesions (<5 mm) (36). It is unclear whether these microlesions represent a tumor precursor stage (37, e56). Merlin loss in peripheral axons also plays a role (38).
Ocular complications
Subcapsular cataracts arise in nearly 80% of patients with NF2 (e57). Other ocular complications include epiretinal membranes, retinal hamartoma, optic nerve glioma and meningioma, and intraocular schwannoma. Facial nerve palsy puts the cornea at risk of ulceration.
Schwannomatosis (SWN)
Only since about 2005 has schwannomatosis been considered to constitute a separate entity. It has a similar clinical presentation to NF2, without fulfilling the diagnostic criteria. It is said to be present in approximately 1 in 69 000 births (39), but its prevalence may well be much higher, as many cases are oligosymptomatic. Chronic pain is generally the most prominent symptom.
Diagnostic criteria
This entity is defined by the occurrence of multiple schwannomas without any other stigmata of NF2 (bilateral vestibular nerve schwannomas, subcapsular cataract, NF2 mutation in the blood) (table).
Genetics and molecular pathophysiology
In schwannomatosis, various NF2 mutations can be found in tumor tissue that are not seen in the DNA of blood cells. A small proportion of patients have mutations in the SMARCB1 gene (e58). Mutations in the LZTR1 gene are more common (2, e59); it seems that somatic NF2 mutations arise secondarily (40). Schwannomatosis affects multiple members of a family in fewer than 20% of cases. The inheritance pattern is unclear. Genetic NF2 mosaics and segmental courses of SWN are common.
Clinical manifestations
Schwannomas
Histologically, the schwannomas of schwannomatosis are hardly distinguishable from those seen in sporadic disease or in other tumor syndromes (NF2, Carney complex). In SWN, just as in NF2, there are intrafascicular microlesions in the peripheral nerves.
Pain
Chronic neuropathic pain is a further central feature of SWN. The severity of the pain is not correlated with either the quantitative tumor burden or the distribution of the tumors. SWN, unlike NF2, rarely causes sensorimotor deficits.
Other tumors
Spinal schwannomas are seen in nearly two-thirds of patients (e60). Meningiomas and unilateral vestibular schwannomas can be seen as well (e61, e62). There is debate over whether malignancies are more common in patients with SWN (e63).
Treatment
Painful or functionally limiting schwannomas can be surgically resected. Genetic investigation of the resected tumor material is of high prognostic relevance, in particular when the diagnosis is initially unclear. Co-analgetic drugs (amitriptyline, gabapentin, pregabalin) have been found especially useful in the treatment of the associated neuropathic pain.
Key Messages.
The neurofibromatoses are genetically and clinically distinct diseases with variable course.
Their clinical severity is primarily a function of the time of appearance of the first symptoms, the overall tumor burden, the symptoms, the underlying genetic mutation, and the patient’s individual coping strategy.
Most tumors affecting the central nervous system in type 2 neurofibromatosis grow irregularly, with interspersed growth pauses.
The life expectancy of patients with neurofibromatosis is improved by early detection of disease and by the coordination of medical care at a specialized center, in collaboration with the family physician.
The key elements of medical management are the preservation of neural function and the treatment of pain (schwannomatosis), together with the reinforcement of cognitive resources and self-image in patients with type 1 neurofibromatosis.
The Neurofibromatoses.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 14 May 2021. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What accompanying diseases are mainly responsible for the markedly reduced life expectancy of persons with type 1 neurofibromatosis?
Type 2 diabetes and peripheral arterial occlusive disease
Gastrointestinal tumors and generalized epilepsy
Malignant tumors, heart attack, and stroke
Melanoma and non-Hodgkin lymphoma
Hypertriglyceridemia and hypertension
Question 2
What is the function of the neurofibromatosis 1 gene?
It activates MAP kinase signal transduction and thereby regulates cellular proliferation.
It inhibits JAK kinase, which regulates the division of Schwann cells.
It is primarily expressed during fetal development and leads postnatally to an accumulation of p53 protein.
It regulates the activity of the EGFR gene and thereby triggers the development of cutaneous and glial tumors.
It encodes the tumor suppressor protein neurofibromin and suppresses the activity of the proto-oncogene Ras.
Question 3
What are the diagnostic criteria for type 1 neurofibromatosis?
Six or more café-au-lait spots and first-degree relatives with NF1
Radiological demonstration of a non-vestibular schwannoma and of at least two non-cutaneous schwannomas
At least one grand mal seizure and ≥ 2 meningiomas before puberty
Polyneuropathy and cataract in young adulthood
Cardiac valvular disease and eighth cranial nerve dysfunction
Question 4
What type of hematopoietic malignancy is more commonly diagnosed in persons with type 1 neurofibromatosis?
Acute lymphocytic leukemia
Chronic lymphocytic leukemia
Prolymphocytic leukemia
Myeloid leukemia
Hairy-cell leukemia
Question 5
Which of the following findings secures the diagnosis of type 2 neurofibromatosis?
Sphenoid wing dysplasia
Optic nerve glioma
Bilateral vestibular schwannomas
Two or more plexiform neurofibromas
Juvenile posterior lens opacification
Question 6
What type of tumor can arise from a plexiform neurofibroma?
Glioma
Malignant peripheral nerve sheath tumor
Meningioma
Ependymoma
Medulloblastoma
Question 7
Which of the following is a sign of schwannomatosis?
Chronic neuropathic pain and non-vestibular schwannomas
Bilateral vestibular vertigo and hearing loss
Astrocytomas and epileptic seizures
Neurofibromas and organ compression
Meningiomas and anosmia
Question 8
Persons with type 1 neurofibromatosis are at increased risk of developing a glioma. What type of tumor most often affects the optic nerve?
Subependymal giant-cell astrocytoma
Anaplastic astrocytoma
Pleomorphic xanthoastrocytoma
Pilocytic astrocytoma
Gliosarcoma
Question 9
Which of the following substances has been found useful to relieve pain caused by a schwannoma?
Acetylsalicylic acid
Metamizole
Ibuprofen
Oxycodone
Gabapentin
Question 10
Approximately what proportion of patients with type 2 neurofibromatosis have neuropathic symptoms?
10%
30%
50%
70%
90%
►Participation is possible only via the Internet: cme.aerzteblatt.de
Acknowledgments
Received on 18 April 2019, revised version accepted on 20 March 2020
Translated from the original German by Ethan Taub, M.D.
Acknowledgments
The authors thank everyone working with them in the neurofibromatosis center at the Helios Hospital in Erfurt, the outpatient neurofibromatosis clinic at the University Medical Center Hamburg-Eppendorf, NYU Langone Medical Center, the Neurofibromatosis Working Group (Arbeitsgemeinschaft Neurofibromatosen) and the German Neurofibromatosis Association (Bundesverband Neurofibromatose e. V.).
Footnotes
Conflict of interest statement
The authors state that no conflicts of interest exist.
References
- 1.Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18:624–638. doi: 10.1093/neuonc/nov200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kehrer-Sawatzki H, Farschtschi S, Mautner VF, Cooper DN. The molecular pathogenesis of schwannomatosis, a paradigm for the co-involvement of multiple tumour suppressor genes in tumorigenesis. Hum Genet. 2017;136:129–148. doi: 10.1007/s00439-016-1753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–843. doi: 10.1016/S1474-4422(14)70063-8. [DOI] [PubMed] [Google Scholar]
- 4.DeBella K, Szudek J, Friedman JM. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105:608–614. doi: 10.1542/peds.105.3.608. [DOI] [PubMed] [Google Scholar]
- 5.Mautner V-F, Lindenau M, Kaufmann D. Klinik und Genetik der Neurofibromatose. Dtsch Arztebl. 1995;92:A1759–A1764. [Google Scholar]
- 6.Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen‘s disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012;5:852–862. [PMC free article] [PubMed] [Google Scholar]
- 7.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 8.Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26:704–711. doi: 10.1136/jmg.26.11.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine TM, Materek A, Abel J, O‘Donnell M, Cutting LE. Cognitive profile of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:8–20. doi: 10.1016/j.spen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Tucker T, Schnabel C, Hartmann M, et al. Bone health and fracture rate in individuals with neurofibromatosis 1 (NF1) J Med Genet. 2009;46:259–265. doi: 10.1136/jmg.2008.061895. [DOI] [PubMed] [Google Scholar]
- 12.Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375:2550–2560. doi: 10.1056/NEJMoa1605943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin AL, Gutmann DH. Advances in the treatment of neurofibromatosis-associated tumours. Nat Rev Clin Oncol. 2013;10:616–624. doi: 10.1038/nrclinonc.2013.144. [DOI] [PubMed] [Google Scholar]
- 14.Kresak JL, Walsh M. Neurofibromatosis: a review of NF1, NF2, and schwannomatosis. J Pediatr Genet. 2016;5:98–104. doi: 10.1055/s-0036-1579766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hexter J, Jones A, Joe H, et al. Clinical and molecular predictors of mortality in neurofibromatosis 2: a UK national analysis of 1 192 patients. J Med Genet. 2015;52:699–705. doi: 10.1136/jmedgenet-2015-103290. [DOI] [PubMed] [Google Scholar]
- 16.Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974–1986. doi: 10.1016/S0140-6736(09)60259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz A, Büttner R, Hagel C, et al. The importance of nerve microenvironment for schwannoma development. Acta Neuropathol. 2016;132:289–307. doi: 10.1007/s00401-016-1583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–527. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- 19.Petrilli AM, Fernández-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016;35:537–548. doi: 10.1038/onc.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans DGR, Ramsden RT, Shenton A, et al. Mosaicism in neurofibromatosis type 2: an update of risk based on uni/bilaterality of vestibular schwannoma at presentation and sensitive mutation analysis including multiple ligation-dependent probe amplification. J Med Genet. 2007;44:424–428. doi: 10.1136/jmg.2006.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stivaros SM, Stemmer-Rachamimov AO, Alston R, et al. Multiple synchronous sites of origin of vestibular schwannomas in neurofibromatosis Type 2. J Med Genet. 2015;52:557–562. doi: 10.1136/jmedgenet-2015-103050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewan R, Pemov A, Kim HJ, et al. Evidence of polyclonality in neurofibromatosis type 2-associated multilobulated vestibular schwannomas. Neuro Oncol. 2015;17:566–573. doi: 10.1093/neuonc/nou317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samii M, Matthies C. Management of 1 000 vestibular schwannomas (acoustic neuromas): hearing function in 1 000 tumor resections. Neurosurgery. 1997;40:248–260. doi: 10.1097/00006123-199702000-00005. discussion 260-2. [DOI] [PubMed] [Google Scholar]
- 24.Slattery WH, Fisher LM, Hitselberger W, Friedman RA, Brackmann DE. Hearing preservation surgery for neurofibromatosis Type 2-related vestibular schwannoma in pediatric patients. J Neurosurg. 2007;106:255–260. doi: 10.3171/ped.2007.106.4.255. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen R, Claesson M, Stangerup SE, et al. Fractionated stereotactic radiotherapy of vestibular schwannomas accelerates hearing loss. Int J Radiat Oncol Biol Phys. 2012;83:e607–e611. doi: 10.1016/j.ijrobp.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin SR, Merker VL, Halpin C, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012;33:1046–1052. doi: 10.1097/MAO.0b013e31825e73f5. [DOI] [PubMed] [Google Scholar]
- 27.Best SR, Ahn J, Langmead S, et al. Voice and swallowing dysfunction in neurofibromatosis 2. Otolaryngol Head Neck Surg. 2018;158:505–510. doi: 10.1177/0194599817741839. [DOI] [PubMed] [Google Scholar]
- 28.Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–618. [PubMed] [Google Scholar]
- 29.Evans DG, Birch JM, Ramsden RT. Paediatric presentation of type 2 neurofibromatosis. Arch Dis Child. 1999;81:496–499. doi: 10.1136/adc.81.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentworth S, Pinn M, Bourland JD, et al. Clinical experience with radiation therapy in the management of neurofibromatosis-associated central nervous system tumors. Int J Radiat Oncol Biol Phys. 2009;73:208–213. doi: 10.1016/j.ijrobp.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 31.Hagel C, Stemmer-Rachamimov AO, Bornemann A, et al. Clinical presentation, immunohistochemistry and electron microscopy indicate neurofibromatosis type 2-associated gliomas to be spinal ependymomas. Neuropathology. 2012;32:611–616. doi: 10.1111/j.1440-1789.2012.01306.x. [DOI] [PubMed] [Google Scholar]
- 32.Plotkin SR, O‘Donnell CC, Curry WT, Bove CM, MacCollin M, Nunes FP. Spinal ependymomas in neurofibromatosis type 2: a retrospective analysis of 55 patients. J Neurosurg Spine. 2011;14:543–547. doi: 10.3171/2010.11.SPINE10350. [DOI] [PubMed] [Google Scholar]
- 33.Farschtschi S, Merker VL, Wolf D, et al. Bevacizumab treatment for symptomatic spinal ependymomas in neurofibromatosis type 2. Acta Neurol Scand. 2016;133:475–480. doi: 10.1111/ane.12490. [DOI] [PubMed] [Google Scholar]
- 34.Lawson McLean AC, Rosahl SK. Growth dynamics of intracranial tumors in patients with neurofibromatosis type 2. World Neurosurg. 2017;98:152–161. doi: 10.1016/j.wneu.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 35.Lawson McLean AC, Rosahl SK. Growth dynamics of intramedullary spinal tumors in patients with neurofibromatosis type 2. Clin Neurol Neurosurg. 2016;146:130–137. doi: 10.1016/j.clineuro.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Hagel C, Lindenau M, Lamszus K, Kluwe L, Stavrou D, Mautner VF. Polyneuropathy in neurofibromatosis 2: clinical findings, molecular genetics and neuropathological alterations in sural nerve biopsy specimens. Acta Neuropathol. 2002;104:179–187. doi: 10.1007/s00401-002-0535-7. [DOI] [PubMed] [Google Scholar]
- 37.Bäumer P, Mautner VF, Bäumer T, et al. Accumulation of non-compressive fascicular lesions underlies NF2 polyneuropathy. J Neurol. 2013;260:38–46. doi: 10.1007/s00415-012-6581-8. [DOI] [PubMed] [Google Scholar]
- 38.Schulz A, Baader SL, Niwa-Kawakita M, et al. Merlin isoform 2 in neurofibromatosis type 2-associated polyneuropathy. Nat Neurosci. 2013;16:426–433. doi: 10.1038/nn.3348. [DOI] [PubMed] [Google Scholar]
- 39.Evans DG, Bowers NL, Tobi S, et al. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018;89:1215–1219. doi: 10.1136/jnnp-2018-318538. [DOI] [PubMed] [Google Scholar]
- 40.Piotrowski A, Xie J, Liu YF, et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet. 2014;46:182–187. doi: 10.1038/ng.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152:327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- E2.Poyhonen M, Kytölä S, Leisti J. Epidemiology of neurofibromatosis type 1 (NF1) in northern Finland. J Med Genet. 2000;37:632–636. doi: 10.1136/jmg.37.8.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Evans DGR, Ingham SL. Reduced life expectancy seen in hereditary diseases which predispose to early-onset tumors. Appl Clin Genet. 2013;6:53–61. doi: 10.2147/TACG.S35605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Koontz NA, Wiens AL, Agarwal A, Hingtgen CM, Emerson RE, Mosier KM. Schwannomatosis: the overlooked neurofibromatosis? AJR Am J Roentgenol. 2013;200:W646–W653. doi: 10.2214/AJR.12.8577. [DOI] [PubMed] [Google Scholar]
- E5.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using US. death certificates. Am J Hum Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1— mutations in NF1gene as a cause of disease. Dev Period Med. 2014;18:297–306. [PubMed] [Google Scholar]
- E7.Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1–16. doi: 10.1016/j.jaad.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Upadhyaya M, Osborn MJ, Maynard J, Kim MR, Tamanoi F, Cooper DN. Mutational and functional analysis of the neurofibromatosis type 1 (NF1) gene. Hum Genet. 1997;99:88–92. doi: 10.1007/s004390050317. [DOI] [PubMed] [Google Scholar]
- E9.Sung L, Anderson JR, Arndt C, Raney RB, Meyer WH, Pappo AS. Neurofibromatosis in children with rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma study IV. J Pediatr. 2004;144:666–668. doi: 10.1016/j.jpeds.2004.02.026. [DOI] [PubMed] [Google Scholar]
- E10.Boulanger JM, Larbrisseau A. Neurofibromatosis type 1 in a pediatric population: Ste-Justine‘s experience. Can J Neurol Sci. 2005;32:225–231. doi: 10.1017/s0317167100004017. [DOI] [PubMed] [Google Scholar]
- E11.Curless RG. Use of „unidentified bright objects“ on MRI for diagnosis of neurofibromatosis 1 in children. Neurology. 2000;55:1067–1068. doi: 10.1212/wnl.55.7.1067-a. [DOI] [PubMed] [Google Scholar]
- E12.Gill DS, Hyman SL, Steinberg A, North KN. Age-related findings on MRI in neurofibromatosis type 1. Pediatr Radiol. 2006;36:1048–1056. doi: 10.1007/s00247-006-0267-2. [DOI] [PubMed] [Google Scholar]
- E13.Goh WHS, Khong P-L, Leung CSY, Wong VCN. T2-weighted hyperintensities (unidentified bright objects) in children with neurofibromatosis 1: their impact on cognitive function. J Child Neurol. 2004;19:853–858. doi: 10.1177/08830738040190110201. [DOI] [PubMed] [Google Scholar]
- E14.Lopes Ferraz Filho JR, Munis MP, Soares Souza A, Sanches RA, Goloni-Bertollo EM, Pavarino-Bertelli EC. Unidentified bright objects on brain MRI in children as a diagnostic criterion for neurofibromatosis type 1. Pediatr Radiol. 2008;3:305–310. doi: 10.1007/s00247-007-0712-x. [DOI] [PubMed] [Google Scholar]
- E15.Coudé FX, Mignot C, Lyonnet S, Munnich A. Academic impairment is the most frequent complication of neurofibromatosis type-1 (NF1) in children. Behav Genet. 2006;36:660–664. doi: 10.1007/s10519-005-9040-9. [DOI] [PubMed] [Google Scholar]
- E16.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- E17.Krab LC, Aarsen FK, Goede-Bolder de A, et al. Impact of neurofibromatosis type 1 on school performance. J Child Neurol. 2008;23:1002–1010. doi: 10.1177/0883073808316366. [DOI] [PubMed] [Google Scholar]
- E18.Watt SE, Shores A, North KN. An examination of lexical and sublexical reading skills in children with neurofibromatosis type 1. Child Neuropsychol. 2008;14:401–418. doi: 10.1080/09297040701595505. [DOI] [PubMed] [Google Scholar]
- E19.Eijk S, Mous SE, Dieleman GC, et al. Autism spectrum disorder in an unselected cohort of children with neurofibromatosis type 1 (NF1) J Autism Dev Disord. 2018;48:2278–2285. doi: 10.1007/s10803-018-3478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Rosai J, editor. Elsevier. 10. Philadelphia: 2011. Rosai and Ackerman‘s surgical pathology. [Google Scholar]
- E21.Crowe FW, Schull WJ, Neel JV. A clinical, pathological, and genetic study of multiple neurofibromatosis. Springfield, Illinois: Charles C. Thomas. 1956;181 [Google Scholar]
- E22.Yanoff M. Ocular pathology. In: Sassari J, editor. Elsevier-Saunders. Philadelphia: 2009. [Google Scholar]
- E23.Heervä E, Koffert A, Jokinen E, et al. A controlled register-based study of 460 neurofibromatosis 1 patients: increased fracture risk in children and adults over 41 years of age. J Bone Miner Res. 2012;27:2333–2337. doi: 10.1002/jbmr.1685. [DOI] [PubMed] [Google Scholar]
- E24.Lion-François L, Gueyffier F, Mercier C, et al. The effect of methylphenidate on neurofibromatosis type 1: a randomised, double-blind, placebo-controlled, crossover trial. Orphanet J Rare Dis. 2014;9 doi: 10.1186/s13023-014-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Lidzba K, Granstroem S, Leark RA, et al. Pharmacotherapy of attention deficit in neurofibromatosis type 1: effects on cognition. Neuropediatrics. 2014;45:240–246. doi: 10.1055/s-0034-1368117. [DOI] [PubMed] [Google Scholar]
- E26.Mautner VF, Kluwe L, Thakker SD, et al. Treatment of ADHD in neurofibromatosis type 1. Dev Med Child Neurol. 2002;44:164–170. doi: 10.1017/s0012162201001876. [DOI] [PubMed] [Google Scholar]
- E27.Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–618. [PubMed] [Google Scholar]
- E28.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- E29.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- E30.Lim JY, Kim H, Kim YH, et al. Merlin suppresses the SRE-dependent transcription by inhibiting the activation of Ras-ERK pathway. Biochem Biophys Res Commun. 2003;302:238–245. doi: 10.1016/s0006-291x(03)00124-4. [DOI] [PubMed] [Google Scholar]
- E31.Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E32.Stamenkovic I, Yu Q. Merlin, a „magic“ linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11:471–484. doi: 10.2174/138920310791824011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E33.Hanemann CO. Magic but treatable? Tumours due to loss of merlin. Brain. 2008;131:606–615. doi: 10.1093/brain/awm249. [DOI] [PubMed] [Google Scholar]
- E34.Neff BA, Welling DB, Akhmametyeva E, Chang L-S. The molecular biology of vestibular schwannomas: dissecting the pathogenic process at the molecular level. Otol Neurotol. 2006;27:197–208. doi: 10.1097/01.mao.0000180484.24242.54. [DOI] [PubMed] [Google Scholar]
- E35.Kluwe L, Mautner V, Heinrich B, et al. Molecular study of frequency of mosaicism in neurofibromatosis 2 patients with bilateral vestibular schwannomas. J Med Genet. 2003;40:109–114. doi: 10.1136/jmg.40.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E36.Love S, Perry A, Ironside JW. Budka H, editor. Greenfield’s neuropathology. Boca Raton: CRC Press. (9) 2015 [Google Scholar]
- E37.Ferner RE, Shaw A, Evans DG, et al. Longitudinal evaluation of quality of life in 288 patients with neurofibromatosis 2. J Neurol. 2014;261:963–969. doi: 10.1007/s00415-014-7303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Neff BA, Wiet RM, Lasak JM, et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007;117:1069–1072. doi: 10.1097/MLG.0b013e31804b1ae7. [DOI] [PubMed] [Google Scholar]
- E39.Rosahl S, Lenarz T, Matthies C, Samii M, Sollmann WP, Laszig R. Hirnstammimplantate zur Wiederherstellung des Hörvermögens: Entwicklung und Perspektiven. Dtsch Arztebl. 2004;101:A180–A188. [Google Scholar]
- E40.Anderson BM, Khuntia D, Bentzen SM, et al. Single institution experience treating 104 vestibular schwannomas with fractionated stereotactic radiation therapy or stereotactic radiosurgery. J Neurooncol. 2014;116:187–193. doi: 10.1007/s11060-013-1282-4. [DOI] [PubMed] [Google Scholar]
- E41.Han JH, Kim DG, Chung HT, Paek SH, Jung HW. Hearing outcomes after stereotactic radiosurgery for vestibular schwannomas mechanism of hearing loss and how to preserve hearing. Adv Tech Stand Neurosurg. 2016;43:3–36. doi: 10.1007/978-3-319-21359-0_1. [DOI] [PubMed] [Google Scholar]
- E42.Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60:460–468. doi: 10.1227/01.NEU.0000255340.26027.53. discussion 468-70. [DOI] [PubMed] [Google Scholar]
- E43.Meijer OWM, Vandertop WP, Lagerwaard FJ, Slotman BJ. Linear accelerator-based stereotactic radiosurgery for bilateral vestibular schwannomas in patients with neurofibromatosis type 2. Neurosurgery. 2008;62:A37–A42. doi: 10.1227/01.neu.0000325935.23852.9d. discussion A42-3. [DOI] [PubMed] [Google Scholar]
- E44.Phi JH, Kim DG, Chung HT, Lee J, Paek SH, Jung HW. Radiosurgical treatment of vestibular schwannomas in patients with neurofibromatosis type 2: tumor control and hearing preservation. Cancer. 2009;115:390–398. doi: 10.1002/cncr.24036. [DOI] [PubMed] [Google Scholar]
- E45.Vachhani JA, Friedman WA. Radiosurgery in patients with bilateral vestibular schwannomas. Stereotact Funct Neurosurg. 2007;85:273–278. doi: 10.1159/000107359. [DOI] [PubMed] [Google Scholar]
- E46.Yeung AH, Sughrue ME, Kane AJ, Tihan T, Cheung SW, Parsa AT. Radiobiology of vestibular schwannomas: mechanisms of radioresistance and potential targets for therapeutic sensitization. Neurosurg Focus. 2009;27 doi: 10.3171/2009.9.FOCUS09185. E2. [DOI] [PubMed] [Google Scholar]
- E47.Slusarz KM, Merker VL, Muzikansky A, Francis SA, Plotkin SR. Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol. 2014;73:1197–1204. doi: 10.1007/s00280-014-2456-2. [DOI] [PubMed] [Google Scholar]
- E48.Hilton DA, Hanemann CO. Schwannomas and their pathogenesis. Brain Pathol. 2014;24:205–220. doi: 10.1111/bpa.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E49.Bendon CL, Furniss D, Giele HP. Comparison of outcomes of peripheral nerve schwannoma excision in neurofibromatosis type 2 patients and non-neurofibromatosis type 2 patients: a case control study. J Plast Reconstr Aesthet Surg. 2015;68:1199–1203. doi: 10.1016/j.bjps.2015.05.026. [DOI] [PubMed] [Google Scholar]
- E50.Mautner VF, Tatagiba M, Lindenau M, et al. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am J Roentgenol. 1995;165:951–955. doi: 10.2214/ajr.165.4.7676998. [DOI] [PubMed] [Google Scholar]
- E51.Abaza MM, Makariou E, Armstrong M, Lalwani AK. Growth rate characteristics of acoustic neuromas associated with neurofibromatosis type 2. Laryngoscope. 1996;106:694–699. doi: 10.1097/00005537-199606000-00007. [DOI] [PubMed] [Google Scholar]
- E52.Dirks MS, Butman JA, Kim HJ, et al. Long-term natural history of neurofibromatosis type 2-associated intracranial tumors. J Neurosurg. 2012;117:109–117. doi: 10.3171/2012.3.JNS111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E53.Goutagny S, Bah AB, Henin D, et al. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: clinical, radiological, and molecular features. Neuro Oncol. 2012;14:1090–1096. doi: 10.1093/neuonc/nos129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E54.Li H, Hao S-Y, Wang L, et al. Factors influencing the growth rate of vestibular schwannoma in patients with neurofibromatosis type 2. Acta Neurochir (Wien) 2015;157:1983–1990. doi: 10.1007/s00701-015-2542-1. [DOI] [PubMed] [Google Scholar]
- E55.Sperfeld AD, Hein C, Schröder JM, Ludolph AC, Hanemann CO. Occurrence and characterization of peripheral nerve involvement in neurofibromatosis type 2. Brain. 2002;125:996–1004. doi: 10.1093/brain/awf115. [DOI] [PubMed] [Google Scholar]
- E56.Stemmer-Rachamimov AO, Ino Y, Lim ZY, et al. Loss of the NF2 gene and merlin occur by the tumorlet stage of schwannoma development in neurofibromatosis 2. J Neuropathol Exp Neurol. 1998;57:1164–1167. doi: 10.1097/00005072-199812000-00008. [DOI] [PubMed] [Google Scholar]
- E57.McLaughlin ME, Pepin SM, MacCollin M, Choopong P, Lessell S. Ocular pathologic findings of neurofibromatosis type 2. Arch Ophthalmol. 2007;125:389–394. doi: 10.1001/archopht.125.3.389. [DOI] [PubMed] [Google Scholar]
- E58.Hulsebos TJM, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80:805–810. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E59.Hutter S, Piro RM, Reuss DE, et al. Whole exome sequencing reveals that the majority of schwannomatosis cases remain unexplained after excluding SMARCB1 and LZTR1 germline variants. Acta Neuropathol. 2014;128:449–452. doi: 10.1007/s00401-014-1311-1. [DOI] [PubMed] [Google Scholar]
- E60.Merker VL, Esparza S, Smith MJ, Stemmer-Rachamimov A, Plotkin SR. Clinical features of schwannomatosis: a retrospective analysis of 87 patients. Oncologist. 2012;17:1317–1322. doi: 10.1634/theoncologist.2012-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E61.Christiaans I, Kenter SB, Brink HC, et al. Germline SMARCB1 mutation and somatic NF2 mutations in familial multiple meningiomas. J Med Genet. 2011;48:93–97. doi: 10.1136/jmg.2010.082420. [DOI] [PubMed] [Google Scholar]
- E62.Smith MJ, Kulkarni A, Rustad C, et al. Vestibular schwannomas occur in schwannomatosis and should not be considered an exclusion criterion for clinical diagnosis. Am J Med Genet A. 2012;158A:215–219. doi: 10.1002/ajmg.a.34376. [DOI] [PubMed] [Google Scholar]
- E63.Evans DGR, Huson SM, Birch JM. Malignant peripheral nerve sheath tumours in inherited disease. Clin Sarcoma Res. 2012;2 doi: 10.1186/2045-3329-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E64.National Institutes of Health Consensus Development Conference Statement. Neurofibromatosis. Bethesda, Md., USA, July 13-15, 1987. Neurofibromatosis. 1988;1:172–178. [PubMed] [Google Scholar]
- E65.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- E66.Evans DG, Baser ME, O‘Reilly B, et al. Management of the patient and family with neurofibromatosis 2: a consensus conference statement. Br J Neurosurg. 2005;19:5–12. doi: 10.1080/02688690500081206. [DOI] [PubMed] [Google Scholar]
- E67.Plotkin SR, Blakeley JO, Evans DG, et al. Update from the 2011 International Schwannomatosis Workshop: from genetics to diagnostic criteria. Am J Med Genet A. 2013;161:405–416. doi: 10.1002/ajmg.a.35760. [DOI] [PMC free article] [PubMed] [Google Scholar]