ABSTRACT
Background
Vitamin E α-, γ-, or δ-tocopherol (αT, γT, δT) and γ- or δ-tocotrienol (γTE, δTE) are metabolized to hydroxychromanols and carboxychromanols including 13′-carboxychromanol (13′-COOH), 11′-COOH, and carboxyethyl hydroxychroman (CEHC), some of which have unique bioactivities compared with the vitamers. However, the bioavailability of these metabolites has not been well characterized.
Objective
We investigated the pharmacokinetics (PK) of vitamin E forms and metabolites in rats.
Methods
Six-week-old male Wistar rats received 1-time gavage of γT-rich tocopherols (50 mg/kg) containing γT/δT/αT (57.7%, 21.9%, and 10.9%, respectively) or δTE-rich tocotrienols (35 mg/kg) containing δTE/γTE (8:1). We quantified the time course of vitamin E forms and metabolites in the plasma and their 24-h excretion to the urine and feces. The general linear model repeated measure was used for analyses of the PK data.
Results
In the rats’ plasma, Cmax of γT or δTE was 25.6 ± 9.1 μM (Tmax = 4 h) or 16.0 ± 2.3 μM (Tmax = 2 h), respectively, and sulfated CEHCs and sulfated 11′-COOHs were the predominant metabolites with Cmax of 0.4–0.5 μM (Tmax ∼5–7 h) or ∼0.3 μM (Tmax at 4.7 h), respectively. In 24-h urine, 2.7% of γT and 0.7% of δTE were excreted as conjugated CEHCs. In the feces, 17–45% of supplemented vitamers were excreted as unmetabolized forms and 4.9–9.2% as unconjugated carboxychromanols, among which 13′-COOHs constituted ∼50% of total metabolites and the amount of δTE-derived 13′-COOHs was double that of 13′-COOH derived from γT.
Conclusions
PK data of vitamin E forms in rats reveal that γT, δT, γTE, and δTE are bioavailable in the plasma and are mainly excreted as unmetabolized forms and long-chain metabolites including 13′-COOHs in feces, with more metabolites from tocotrienols than from tocopherols.
Keywords: carboxychromanol, γ-tocopherol, δ-tocotrienol, vitamin E, excretion, pharmacokinetic, metabolism, antioxidant, inflammation
Introduction
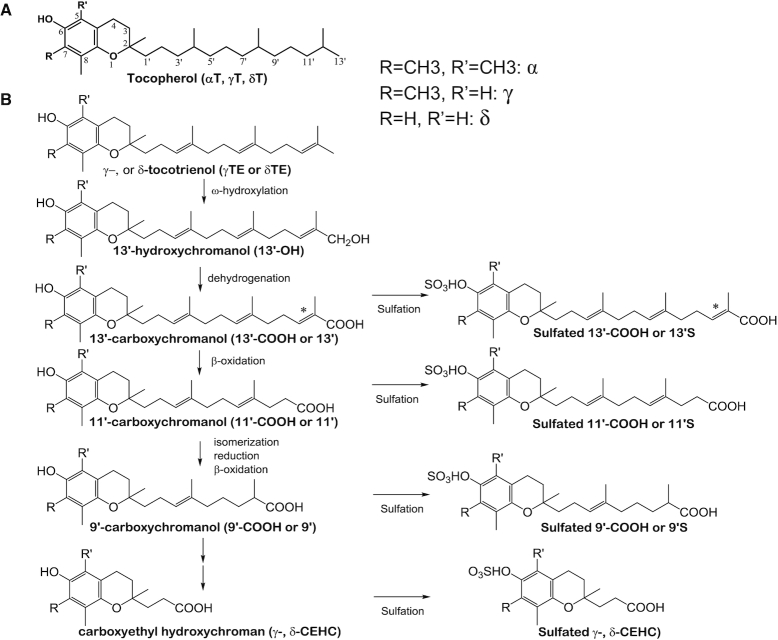
The vitamin E family has 8 naturally occurring fat-soluble antioxidants including α-, γ-, and δ-tocopherol (αT, γT, and δT) and γ-, and δ-tocotrienol (γTE and δTE) (1) (Figure 1). All the vitamin E forms possess a chromanol ring and a 13-carbon-length side chain. Tocopherols have a saturated side chain (Figure 1A), whereas tocotrienols have an unsaturated side chain (2) (Figure 1B). Among the different isoforms, αT is the predominant vitamin E in the plasma and tissues owing to its high binding affinity to hepatic α-tocopherol transfer protein, which transports αT and, to a lesser extent, other vitamin E forms (2–4). In contrast, other vitamin E forms are substantially metabolized in the liver by cytochrome P450-4F2 via ω-hydroxylation and dehydrogenation to generate 13′-hydroxychromanol (13′-OH) and 13′-carboxychromanol (13′-COOH), which is further degraded via β-oxidation to medium- or short-chain carboxychromanols including the terminal metabolite, 3′-COOH or 2-(β-carboxyethyl)-6-hydroxychroman (CEHC) (2, 5) (Figure 1B). Although tocotrienols and tocopherols are similarly metabolized, catabolism of tocotrienols likely involves additional enzymes such as enoyl-CoA isomerases and dienoyl-CA reductases for the metabolism of the double bonds (Figure 1B) (6, 7). In addition, conjugation of carboxychromanols occurs parallel with β-oxidation to generate sulfated long-chain and short-chain carboxychromanols (6, 8, 9) (Figure 1). The terminal metabolite CEHCs and conjugated metabolites are mainly excreted in the urine, whereas longer-chain carboxychromanols appear to be excreted in feces after supplementation of γT and δT (10–15).
FIGURE 1.
Molecular structures of vitamin E forms and vitamin E metabolism. (A) Structures of αT, γT, and δT. (B) Structures of γTE and δTE, and their metabolism. Tocopherols and tocotrienols are metabolized by ω-hydroxylation and dehydrogenation to form 13′-OH and 13′-COOH, which is then further metabolized by β-oxidation to form 11′-COOH, 9′-COOH, and the terminal metabolite CEHC as well as conjugated carboxychromanols. Compared with tocopherols, catabolism of tocotrienols involves isomerization and reduction, in addition to β-oxidation. *This position may have either a double bond or a saturated bond. CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; COOH, carboxychromanol; 13′-OH, 13′-hydroxychromanol; αT, α-tocopherol; γT, γ-tocopherol; γTE, γ-tocotrienol; δT, δ-tocopherol; δTE, δ-tocotrienol.
Although most research has focused on vitamin E forms, short- and long-chain metabolites have been shown to have stronger bioactivities than the vitamers (2, 16). For instance, γ-CEHC (or 3′-COOH), but not tocopherols, appears to have natriuretic activity (17). We have demonstrated that 13′-COOHs are potent dual inhibitors of cyclooxygenases (18) and 5-lipoxygenase, and for these activities, 13′-COOHs are much stronger than vitamin E forms (19, 20). Further, 13′-COOHs induce apoptosis and autophagy in human cancer cells, but not normal cells, and suppress colon tumor development in mice (16, 20). Despite these interesting activities of vitamin E metabolites, the amounts of long-chain metabolites formed in vivo have not been well characterized. In this study, using our own developed LC-tandem MS (LC/MS/MS) method that allows simultaneous quantification of hydroxychromanols, short- and long-chain carboxychromanols, and sulfated carboxychromanols (14), we investigated the pharmacokinetics (PK) including excretion of vitamin E forms and the formation of their metabolites in response to a single gavage of γT-rich mixed tocopherol (γTmT) and δTE-rich tocotrienol (δTE/γTE) in rats.
Methods
Materials
γTmT that contains αT (10.9%), γT (57.7%), and δT (21.7%) was a gift from BASF. The δTE/γTE (∼8:1) that contains total tocotrienols at 70% was provided by American River Nutrition. γ-CEHC (≥98%), α-CEHC, and (±)-αT-5′-COOH [2-(4-carboxy-4-methylbutyl)-6-hydroxy-2,5,7,8-tetramethylchroman (α-CMBHC)] were purchased from Cayman Chemicals. δT-13′-COOH and δTE-13′-COOH, which are long-chain metabolites from δT and δTE, respectively, were synthesized according to a published procedure (21). All other chemicals were purchased from Sigma.
Animal experiments and sample collection
All the animal experiments were approved by the Purdue Animal Care and Use Committee. Male Wistar rats (6 wk old) were purchased from Charles River. Rats were fed ad libitum with Teklad rodent diet (8604, Envigo), which on average contains ∼40–60 mg αT/kg diet. We conducted 2 independent studies. In each study, there were 2 groups of rats (n = 3 per group) receiving either γTmT or δTE/γTE via gavage. In the first study, we focused on the PK along with urine and fecal excretion between 8 and 24 h. In the second study, we included collection of urine and feces at 0–8 h, 8–24 h, and 24–48 h. Specifically, after 1 wk adaptation, rats were grouped by body-weight match. Plasma and 24-h fecal and urinary samples were collected for baseline measures. Twenty-four hours after baseline collection, rats were given a single gavage of γTmT (γT/δT/αT at 57.7%, 21.9%, and 10.9%) at 50 mg/kg body weight or δTE/γTE (8:1, wt:wt) at 35 mg/kg body weight, which are equivalent to 483 mg γTmT or 338 mg δTE/γTE for a human of 60 kg body weight, respectively (22). These vitamin E forms were delivered in 0.5 mL tocopherol-stripped corn oil (Dyets Inc.) as the vehicle. In the first study, plasma samples were collected repeatedly at 1, 2, 4, 6, and 8 h via a saphenous vein after gavage of γTmT or δTE/γTE. Fecal and urine samples were collected between 8 and 24 h using metabolic cages. Twenty-four hours postgavage, rats were killed and the plasma was collected. To monitor the excretion of vitamin E forms and metabolites at different times, we performed the second study with the same design and focused on collecting urine and fecal samples at baseline and postsupplementation at 0–8, 8–24, and 24–48 h. Our analyses showed that excretion of vitamin E forms or metabolites was at its minimum at 0–8 h or after 24 h. Therefore, we reported the mean of both studies (n = 6) with respect to 24-h excretion of vitamin E forms and metabolites in feces and urine samples.
Extraction of vitamin E forms and metabolites in the plasma and feces
Vitamin E forms and metabolites in the plasma and feces were extracted with methanol and hexane as previously described (14).
Enzyme digestion of extracted vitamin E metabolites including conjugated CEHCs in the urine
One hundred microliters of urine samples were added with δT-13′-COOH or δTE-13′-COOH (1 μM) and extracted by 500 μL working methanol (0.2 mg/mL ascorbic acid). The extraction was repeated 1 more time with 200 μL working methanol. The combined methanol layer was dried under nitrogen. As for samples subjected to enzyme hydrolysis, extracted metabolites were dissolved in 5 μL ethanol and reconstituted in 0.1 M sodium acetate at pH 5 containing 30 U sulfatase (Sigma S9626) and 40 U glucuronidase (Sigma, G0751) (6, 23). Samples were incubated with periodic mixing on a vortex for 18–24 h at 37°C, and then extracted with 5 parts of working methanol (containing 0.2 mg/mL ascorbic acid). The methanol layer was subsequently dried in nitrogen gas. Before LC/MS/MS analysis, samples were reconstituted in ethanol, and α-CMBHC (5 μM) was added as an additional internal standard for injection. It should be noted that, in most studies, injections were consistent between samples and, therefore, α-CMBHC was not used for calculation of concentrations of analytes.
Analysis of vitamin E forms by HPLC with electrochemical detection
Tocopherols and tocotrienols were analyzed by HPLC with electrochemical detection as previously described (8, 13).
Analysis of vitamin E metabolites by LC/MS/MS
The LC/MS/MS analysis was done with an Agilent 1200 LC system coupled to an Agilent 6460 QQQ mass spectrometer equipped with a jet stream ESI source, as previously described (14).
PK analysis
PK parameters were estimated based on the plasma concentration–time data using standard noncompartmental methods (24). AUC was calculated using the log-linear trapezoidal rule to determine the degree of exposure after the administration of vitamin E forms. Other PK parameters determined in this study included observed maximum plasma concentration (Cmax), time at which Cmax was observed (Tmax), and elimination half-life (T1/2) or time at which the plasma concentration was reduced by half after reaching Cmax (25). The decline of vitamin E and metabolites is nonlinear; thus, polynomial fitting curves were applied to estimate T1/2. The recovery rate in the plasma of a specific analyte = AUC × V/total supplement, where V = total blood (16 mL for 250 g of rat, https://www.nc3rs.org.uk/rat-decision-tree-blood-sampling). Percentage excretion of vitamin E forms and metabolites = [(total vitamin E forms and corresponding metabolites in feces and urine of supplemented animals) − baseline]/total supplement.
Statistical analysis
The general linear model repeated measure with Bonferroni post hoc test was used for comparing baseline and subsequent time points of plasma samples in the rat study. Student's t test was used for comparing γTmT or δTE/γTE supplement with the respective baseline for urine and fecal samples. The normality of the data was evaluated by the Shapiro–Wilk test. If data were not normally distributed, log transformation was performed to normalize unequal variances between groups. All the statistical analyses were performed using SPSS version 24 (IBM) and values of P < 0.05 were considered to be statistically significant. All results are expressed as mean ± SEM.
Results
PK of tocopherols and tocotrienols in rat plasma
After a single gavage with γTmT, plasma concentrations of γT and δT elevated to reach Cmax and then decreased at a relatively rapid pace initially followed by a slow decline phase, as shown in Figure 2A, B. In contrast, plasma concentrations of αT did not significantly change (not shown) compared with the baseline (21.0 ± 2.2 μM), probably because of the relatively low amount of αT (1.2 mg) in the supplement but high baseline intake due to high contents of αT in the diet, which contained ≤60 mg/kg (≤0.6 mg/d) of this vitamin E. Upon supplementation, δTE and γTE reached Cmax quickly within 2 h and declined rapidly without a slow decrease phase (Figure 2C, D). Considering similar administering of δTE (31.1 mg/kg) and γT (29.5 mg/kg), the bioavailability of γT is much higher than δTE in the plasma, as indicated by the Cmax and AUCs (Tables 1 and 2).
FIGURE 2.
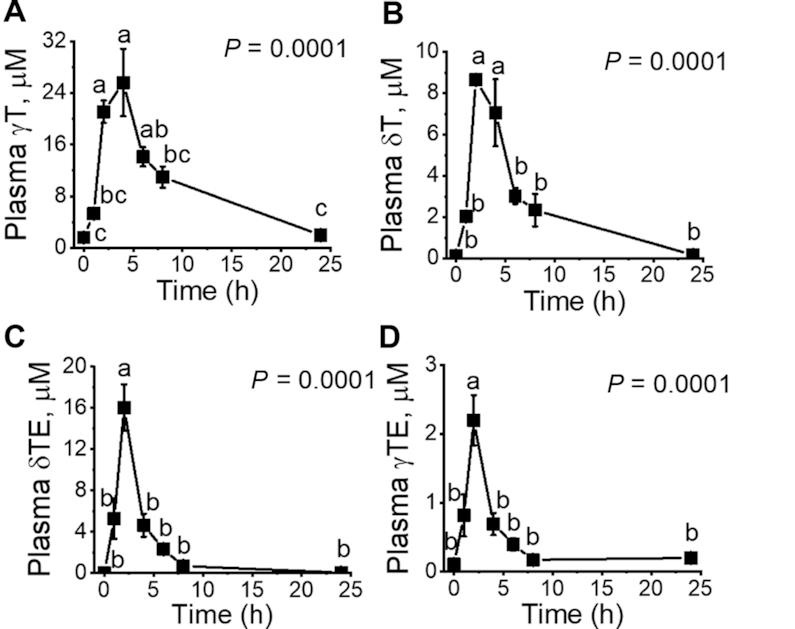
Pharmacokinetics of vitamin E forms in rat plasma after supplementation with a single dose of γTmT (28.9 mg γT/kg; 11 mg δT/kg; 5.5 mg α-tocopherol/kg) or δTE/γTE (31.1 mg δTE/kg; 3.9 mg γTE/kg). (A, B) The time course of plasma γT and δT in response to γTmT. (C, D) The time course of plasma δTE and γTE in response to δTE/γTE. General linear model repeated measure with Bonferroni post hoc test was used for data analyses. Data are expressed as mean ± SEM, n = 3. Labeled means without a common letter differ, P < 0.05. Abbreviations of vitamin E forms and metabolites are shown in Figure 1. γT, γ-tocopherol; γTE, γ-tocotrienol; γTmT, γ-tocopherol-rich mixed tocopherol; δT, δ-tocopherol; δTE, δ-tocotrienol; δTE/γTE, δ-tocotrienol-rich tocotrienol.
TABLE 1.
Pharmacokinetic parameters of plasma γT, δT, and their metabolites after 1-time supplement with γTmT (28.9 mg γT/kg; 11 mg δT/kg; 5.5 mg α-tocopherol/kg) in rats1
| AUC, μM × h | Cmax, μM | Tmax, h | T1/2,2 h | |
|---|---|---|---|---|
| γT | 207.5 ± 24.4 | 25.6 ± 5.2 | 4.0 ± 0.0 | 6.4 ± 0.3 |
| δT | 48.9 ± 8.1 | 8.64 ± 0.20 | 2.7 ± 0.7 | 4.3 ± 0.2 |
| γ-CEHC | 2.24 ± 0.02 | 0.14 ± 0.02 | 4.7 ± 1.8 | 30.1 ± 12 |
| SO3-γ-CEHC | 5.47 ± 0.75 | 0.43 ± 0.07 | 7.3 ± 0.7 | 8.2 ± 1.1 |
| SO3-δ-CEHC | 1.99 ± 0.26 | 0.20 ± 0.01 | 6.0 ± 1.2 | 6.9 ± 1.0 |
| γT-9′S | 2.21 ± 0.44 | 0.24 ± 0.04 | 6.0 ± 1.2 | 3.6 ± 0.3 |
| δT-9′S | 0.21 ± 0.02 | 0.036 ± 0.00 | 7.3 ± 0.7 | 1.4 ± 0.0 |
| γT-11′S | 3.52 ± 0.52 | 0.34 ± 0.04 | 6.0 ± 2.0 | 4.6 ± 0.3 |
| δT-11′S | 0.23 ± 0.03 | 0.036 ± 0.01 | 4.7 ± 0.7 | 4.0 ± 2.6 |
| γT-13′S | 1.02 ± 0.24 | 0.26 ± 0.07 | 4.0 ± 0.0 | 6.1 ± 2.1 |
| δT-13′S | 0.24 ± 0.04 | 0.10 ± 0.01 | 4.7 ± 0.7 | 3.3 ± 1.1 |
| γT-13′ | 0.032 ± 0.021 | 0.010 ± 0.01 | 4.7 ± 0.7 | 1.9 ± 0.2 |
| δT-13′ | 0.11 ± 0.04 | 0.021 ± 0.0 | 4.0 ± 0.0 | 3.3 ± 0.5 |
| γT-13′-OH | 0.044 ± 0.020 | 0.019 ± 0.0 | 4.0 ± 0.0 | 1.7 ± 0.0 |
| δT-13′-OH | 0.041 ± 0.011 | 0.014 ± 0.0 | 4.0 ± 0.0 | 1.9 ± 0.3 |
Values are mean ± SEM, n = 3. Abbreviations of vitamin E forms and metabolites are shown in Figure 1. CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; Cmax, maximum plasma concentration; COOH, carboxychromanol; Tmax, time at which Cmax was observed; 9′S, sulfated 9′-COOH; 11′S, sulfated 11′-COOH; 13′-OH, 13′-hydroxychromanol; 13′S, sulfated 13′-COOH; γT, γ-tocopherol; δT, δ-tocopherol.
Elimination half time (T1/2) is the time taken for the plasma concentration to fall by half after reaching Cmax.
TABLE 2.
Pharmacokinetic parameters for plasma tocotrienols and metabolites after a single gavage of δTE/γTE (31.1 mg δTE/kg; 3.9 mg γTE/kg) in rats1
| AUC, μM × h | Cmax, μM | Tmax, h | T1/2,2 h | |
|---|---|---|---|---|
| δTE | 43.6 ± 7.72 | 16.0 ± 2.3 | 2.0 ± 0.0 | 1.4 ± 0.1 |
| γTE | 8.84 ± 1.59 | 2.20 ± 0.37 | 2.0 ± 0.0 | 1.7 ± 0.1 |
| SO3-δ-CEHC | 4.57 ± 0.79 | 0.51 ± 0.09 | 3.3 ± 0.7 | 5.1 ± 1.5 |
| SO3-γ-CEHC | 1.25 ± 0.33 | 0.084 ± 0.01 | 4.0 ± 2.0 | 14.1 ± 3.7 |
| δTE-9′ | 0.044 ± 0.01 | 0.01 ± 0.00 | 4.0 ± 1.6 | 4.7 ± 2.8 |
| δTE-9′S | 0.34 ± 0.05 | 0.05 ± 0.01 | 5.3 ± 0.7 | 3.4 ± 0.7 |
| γTE-9′S | 0.58 ± 0.10 | 0.05 ± 0.0 | 6.0 ± 1.2 | 6.6 ± 2.4 |
| δTE-11′S | 2.12 ± 0.24 | 0.32 ± 0.06 | 4.7 ± 0.7 | 4.1 ± 1.2 |
| γTE-11′S | 2.62 ± 0.30 | 0.18 ± 0.0 | 7.3 ± 0.7 | 14 ± 4.0 |
| δTE-13′S | 0.10 ± 0.0 | 0.03 ± 0.03 | 4.0 ± 0.0 | 2.6 ± 0.4 |
| δTE-13′ | 0.10 ± 0.05 | 0.018 ± 0.0 | 2.0 ± 0.0 | 3.5 ± 0.2 |
| δTE-13′-OH | 0.24 ± 0.06 | 0.04 ± 0.01 | 3.3 ± 0.7 | 1.9 ± 0.2 |
Values are mean ± SEM, n = 3. Abbreviations of vitamin E forms and metabolites are shown in Figure 1. CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; Cmax, maximum plasma concentration; COOH, carboxychromanol; Tmax, time at which Cmax was observed; 9′S, sulfated 9′-COOH; 11′S, sulfated 11′-COOH; 13′-OH, 13′-hydroxychromanol; 13′S, sulfated 13′-COOH; γTE, γ-tocotrienol; δTE, δ-tocotrienol; δTE/γTE, δ-tocotrienol-rich tocotrienol.
Elimination half time (T1/2) is the time taken for the plasma concentration to fall by half after reaching Cmax.
Time-course formation of vitamin E metabolites in rat plasma
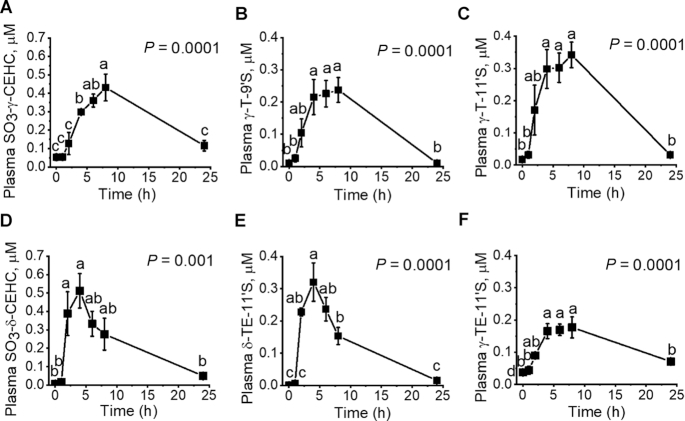
Using our own developed LC/MS/MS methodology (14), we were able to characterize PK profiles of unconjugated and sulfated vitamin E metabolites after administration of γTmT and δTE/γTE, as summarized in Tables 1 and 2, respectively. Sulfated CEHCs and sulfated 9′- and 11′-COOHs were the predominant metabolites from tocopherols and tocotrienols, and it took 3–7 h for these compounds to reach Cmax (Tables 1 and 2, Figure 3). Upon reaching Cmax, most of these metabolites showed relatively slow decline (Figure 3). Unlike other vitamin E forms, the metabolites of αT were not detectable in the plasma.
FIGURE 3.
Pharmacokinetics of major vitamin E metabolites in rat plasma after supplementation with a single dose of γTmT (28.9 mg γT/kg; 11 mg δT/kg; 5.5 mg α-tocopherol/kg) or δTE/γTE (31.1 mg δTE/kg; 3.9 mg γTE/kg). (A, B, C) The time course of sulfated γ-CEHC, γT-9’S, and γT-11’S in response to the supplementation of γTmT. (D, E, F) The time course of sulfated δ-CEHC, δTE-11’S, and γTE-11’S in response to the supplementation of δTE/γTE. General linear model repeated measure with Bonferroni post hoc test was used for data analyses. Data are expressed as mean ± SEM, n = 3. Labeled means without a common letter differ, P < 0.05. Abbreviations of vitamin E forms and metabolites are shown in Figure 1. CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; COOH, carboxychromanol; 9′S, sulfated 9′-COOH; 11′S, sulfated 11′-COOH; γT, γ-tocopherol; γTE, γ-tocotrienol; γTmT, γ-tocopherol-rich mixed tocopherol; δT, δ-tocopherol; δTE, δ-tocotrienol; δTE/γTE, δ-tocotrienol-rich tocotrienol.
Excretion of vitamin E forms and metabolites in rat feces
We monitored 24-h excretion of vitamin E forms and their metabolites in feces before (baseline) and after supplementation. Supplementation of γTmT resulted in 12-, 14-, and 2.1-fold elevation of γT, δT, and αT, respectively, in the feces compared with the baseline amounts (Table 3). Due to extremely low δTE in the basal diet, fecal excretion of δTE and γTE increased >380- and 4.7-fold, respectively, in response to δTE/γTE supplementation compared with the baseline (Table 4). Our calculation of excretion percentage showed that 45%, 37%, 34%, 36%, and 17% of αT, γT, δT, γTE, and δTE, respectively, were excreted as the intact vitamers in feces during the 24-h period (Tables 3 and 4).
TABLE 3.
Twenty-four-hour fecal excretion of tocopherols and metabolites in rats after a single gavage of γTmT in comparison with baseline excretion1
| Baseline, nmol | γTmT supplement, nmol | Total excretion,2 % | |
|---|---|---|---|
| δT | 173 ± 43 | 2450 ± 290* | 33.9 ± 6.3 |
| γT | 583 ± 200 | 7030 ± 960* | 37.4 ± 4.0 |
| αT | 1260 ± 290 | 2680 ± 320* | 45.1 ± 5.3 |
| γ-CEHC | 36.7 ± 4.7 | 53.5 ± 9.5* | |
| δ-CEHC | 3.26 ± 0.60 | 12.9 ± 1.7* | |
| γT-5′ | 0.41 ± 0.12 | 2.70 ± 0.37* | |
| γT-7′ | 10.0 ± 2.2 | 62.5 ± 10.8* | |
| δT-7′ | 0.80 ± 0.04 | 11.0 ± 1.8* | |
| γT-9′ | 3.38 ± 0.39 | 19.7 ± 2.1* | |
| δT-9′ | 1.01 ± 0.13 | 10.7 ± 2.7* | |
| γT-11′ | 6.46 ± 1.20 | 101.2 ± 13.4* | |
| δT-11′ | 2.86 ± 0.64 | 48.6 ± 12.1* | |
| γT-13′ | 23.7 ± 3.5 | 421 ± 71* | |
| δT-13′ | 14.0 ± 1.6 | 291 ± 86* | |
| γT-13′-OH | 4.24 ± 0.86 | 126 ± 28* | |
| δT-13′-OH | 1.02 ± 0.15 | 57.4 ± 21.8* | |
| γT-11′S | 1.46 ± 0.35 | 46.1 ± 15.4* | |
| δT-11′S | n.d.3 | 14.2 ± 6.3* | |
| γT-13′S | 14.3 ± 1.2 | 120 ± 12* | |
| δT-13′S | 3.50 ± 0.20 | 35.6 ± 4.4* | |
| Total δ-metabolites | 26.4 ± 3.3 | 482.3 ± 140.2* | 6.76 ± 1.99 |
| Total γ-metabolites | 100.6 ± 15.1 | 952.4 ± 160.3* | 4.92 ± 0.85 |
Values are mean ± SEM, n = 6. Student's t test was used for comparing γTmT supplement with baseline. *P < 0.05, difference between baseline and γTmT supplement. Abbreviations of vitamin E forms and metabolites are shown in Figure 1.CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; COOH, carboxychromanol; n.d., nondetectable; 11′S, sulfated 11′-COOH; 13′-OH, 13′-hydroxychromanol; 13′S, sulfated 13′-COOH; αT, α-tocopherol; γT, γ-tocopherol; δT, δ-tocopherol.
Total excretion % = (24-h fecal excretion in response to γTmT supplement minus baseline excretion)/intake.
Detection limit: 0.04–0.2 pmol on column.
TABLE 4.
Twenty-four-hour fecal excretion of δTE, γTE, and their metabolites in rats after a single gavage of δTE/γTE in comparison with baseline1
| Baseline, nmol | δTE/γTE supplement, nmol | Total excretion,2 % | |
|---|---|---|---|
| γTE | 243 ± 74 | 1110 ± 75* | 36.3 ± 0.9 |
| δTE | 8.92 ± 1.91 | 3430 ± 530* | 17.4 ± 2.9 |
| γ-CEHC | 9.22 ± 1.55 | 15.9 ± 3.3* | |
| δ-CEHC | 1.63 ± 0.22 | 28.6 ± 6.3* | |
| γTE-7′ | 4.92 ± 0.12 | 15.5 ± 1.3 | |
| δTE-7′ | n.d.3 | 9.39 ± 0.97* | |
| γTE-9′ | 4.89 ± 0.54 | 21.3 ± 3.6* | |
| δTE-9′ | n.d.3 | 27.0 ± 3.1* | |
| γTE-11′ | 17.7 ± 2.0 | 65.2 ± 9.8* | |
| δTE-11′ | 0.81 ± 0.08 | 161 ± 16* | |
| γTE-13′ (2DB) | 29.4 ± 4.4 | 116 ± 23* | |
| γTE-13′ (3DB) | 6.72 ± 1.52 | 24.8 ± 4.3* | |
| δTE-13′ (2DB) | 1.42 ± 0.21 | 452 ± 57* | |
| δTE-13′ (3DB) | 0.54 ± 0.21 | 451 ± 67* | |
| γTE-13′OH | 6.54 ± 0.89 | 23.9 ± 3.5* | |
| δTE-13′OH | 0.66 ± 0.07 | 245 ± 28* | |
| γTE-13′S4 | 1.60 ± 0.32 | 18.0 ± 3.3* | |
| δTE-13′S4 | n.d.3 | 102 ± 15* | |
| Total δ-metabolites | 5.14 ± 0.80 | 1480 ± 192* | 7.49 ± 0.98 |
| Total γ-metabolites | 80.9 ± 11.4 | 301 ± 52* | 9.21 ± 1.32 |
Values are mean ± SEM, n = 6. Student's t test was used for comparing δTE/γTE supplement with baseline. *P < 0.05, difference between baseline and δTE/γTE supplement. Abbreviations of vitamin E forms and metabolites are shown in Figure 1. CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; COOH, carboxychromanol; DB, double bonds on the side chain of carboxychromanol; n.d., nondetectable; 13′-OH, 13′-hydroxychromanol; 13′S, sulfated 13′-COOH; γTE, γ-tocotrienol; δTE, δ-tocotrienol.
Total excretion % = (24-h fecal excretion in response to δTE/γTE minus 24-h baseline excretion)/intake.
Detection limit: 0.04–0.2 pmol on column.
γTE-13′S and δTE-13′S were presented as the sum of 2DB and 3DB.
As for the metabolites, unlike the observation in the plasma where sulfated metabolites dominated, the main metabolites found in feces were unconjugated carboxychromanols and hydroxychromanols, which were markedly elevated in response to supplementation of vitamin E forms. 13′-COOHs were the most abundant metabolites in feces and accounted for ∼50% of total metabolites found in feces in response to the supplements (Tables 3 and 4). Interestingly, total excretion of δTE-13′-COOHs (combining isoforms with 2 or 3 double bonds) was twice as much as that of γT-13′-COOH (P < 0.05), and total fecal metabolites from δTE tended to be ∼50% higher than those from γT (P = 0.08), despite similar amounts of supplementation of these 2 isoforms. Overall, we estimated that 4.9%, 6.8%, 9.2%, and 7.5% of γT, δT, γTE, and δTE, respectively, were excreted as metabolites in feces (Tables 3 and 4).
Excretion of metabolites in rat urine
Unlike the prevalent presence of unconjugated long-chain metabolites in feces, conjugated CEHCs constituted the major metabolites in the urine and were markedly elevated in response to supplementation of γTmT and δTE/γTE (Table 5). Total excreted CEHCs (conjugated or unconjugated) from tocopherols were >3-fold higher than those from tocotrienols (P < 0.05). Urinary excretion of conjugated CEHCs from γT or δT, as measured after glucuronidase and sulfatase digestion, was much higher than that of sulfated CEHCs, suggesting that glucuronide but not sulfated CEHCs was the predominant form of metabolites. However, the amounts of “conjugated” CEHCs and sulfated CEHCs that resulted from δTE/γTE supplementation appeared to be comparable. Overall, 2.7% of γT, 2.2% of δT, 1.9% of γTE, and 0.7% of δTE were excreted as metabolites through the urine. These data together with the aforementioned fecal data indicate that most metabolites of vitamin E forms were excreted via feces instead of urine.
TABLE 5.
Major metabolites detected in rat urine in response to a single gavage of γTmT or δTE/γTE in comparison with their respective baseline (in nanomoles unless otherwise indicated)1
| Baseline2 | γTmT supplement | Baseline3 | δTE/γTE supplement | |
|---|---|---|---|---|
| δ-CEHC | 0.101 ± 0.02 | 2.29 ± 0.32* | 0.120 ± 0.037 | 6.89 ± 2.28* |
| SO3-δ-CEHC | 0.679 ± 0.101 | 19.8 ± 0.58* | 0.751 ± 0.396 | 55.7 ± 0.7* |
| Conjugated-δ-CEHC | 2.23 ± 0.71 | 126 ± 16* | 3.77 ± 0.90 | 80.6 ± 18* |
| γ-CEHC | 0.091 ± 0.021 | 1.01 ± 0.27* | 0.177 ± 0.103 | 1.39 ± 0.07 |
| SO3-γ-CEHC | 7.89 ± 0.80 | 63.7 ± 9.4* | 12.8 ± 2.3 | 36.3 ± 2.5* |
| Conjugated-γ-CEHC | 9.38 ± 4.01 | 413 ± 65* | 16.2 ± 5.2 | 36.3 ± 9.4 |
| Total δ-metabolites4 | 3.01 ± 0.69 | 148 ± 17* | 4.6 ± 1.4 | 143 ± 21* |
| Excretion,5 % | 2.21 ± 0.26 | 0.71 ± 0.11 | ||
| Total γ-metabolites4 | 17.4 ± 5.1 | 478 ± 75* | 29.6 ± 8.7 | 74.1 ± 12* |
| Excretion,5 % | 2.67 ± 0.43 | 1.89 ± 0.31 |
Values are mean ± SEM, n = 6. Student's t test was used for comparing γTmT or δTE/γTE supplement with the respective baseline. *P < 0.05, difference between γTmT or δTE/γTE supplement and the respective baseline. Abbreviations of vitamin E forms and metabolites are shown in Figure 1. CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; COOH, carboxychromanol; γTE, γ-tocotrienol; γTmT, γ-tocopherol-rich mixed tocopherol; δTE, δ-tocotrienol; δTE/γTE, δ-tocotrienol-rich tocotrienol.
Baseline for γTmT-supplement rats.
Baseline for δTE/γTE-supplement rats.
Total metabolites: sum of δ- or γ-series metabolites.
Excretion % = (24-h urine excretion of total metabolites in response to supplements minus 24-h baseline excretion)/intake.
Based on plasma AUCs and fecal/urinary excretion of unmetabolized vitamers and total metabolites, we estimated total recovery of γT and δTE, which were 2 of the major forms of vitamin E in the supplements (Table 6). The result indicates that the overall recovery rate of γT was twice as much as that of δTE (Table 6).
TABLE 6.
Twenty-four-hour recovery of γT, δTE, and their metabolites in rat plasma, urine, and feces in response to a single gavage of γTmT or δTE/γTE1
| % | Plasma vit. E forms2 | Plasma metabolites2 | Fecal vit. E forms3 | Fecal metabolites3 | Urine metabolites4 | Total excretion5 | Total recovery6 |
|---|---|---|---|---|---|---|---|
| γT | 19.0 ± 2.2 | 1.40 ± 0.18 | 37.4 ± 4.0 | 4.92 ± 0.85 | 2.67 ± 0.43 | 45.0 ± 5.3 | 65.4 ± 7.7 |
| δTE | 3.56 ±0.7 | 0.61 ± 0.11 | 17.4 ± 2.9 | 7.49 ± 0.98 | 0.71 ± 0.11 | 25.6 ± 3.9 | 29.8 ± 4.8 |
Values are mean ± SEM. vit., vitamin; γT, γ-tocopherol; γTE, γ-tocotrienol; γTmT, γ-tocopherol-rich mixed tocopherol; δTE, δ-tocotrienol; δTE/γTE, δ-tocotrienol-rich tocotrienol.
The values were calculated as the ratio of plasma AUC × V to the amount of γT or δTE intake.
The values were the ratio of 24-h fecal excretion of γT (or δTE or the sum of metabolites) to the amount of γT or δTE intake.
The values were the ratio of 24-h urinary excretion of the sum of metabolites to the amount of γT or δTE intake.
Total excretion % = (fecal vitamin E and metabolites + urine metabolites)/intake.
Total recovery % = (plasma AUC × V of γT or δTE and metabolites + fecal and urinary excretion)/intake.
Discussion
Our present study characterizes the time-course formation of short- and long-chain vitamin E metabolites after a single supplement of tocopherols or tocotrienols in rats. According to the data of AUC and Cmax, sulfated CEHCs and sulfated long-chain carboxychromanols, i.e., sulfated 9′-COOH, sulfated 11′-COOH, and sulfated 13′-COOH, are among the major metabolites detected in rat plasma, whereas unconjugated carboxychromanols or hydroxychromanol are present at much lower concentrations than the sulfated analogs. This observation is consistent with our previous findings, where sulfated carboxychromanols appear at higher concentrations in rat plasma than other metabolites in response to γT or γTE supplementation (6, 14). According to Tmax, 13′-COOHs and 13′-OH appear to reach Cmax more quickly than their shorter-chain or conjugated counterparts, which is likely because 13′-COOHs and 13′-OH are the metabolites initially formed as a result of ω-oxidation (2, 5).
Our PK data allow quantitative evaluation of the relative availability of different vitamin E forms and metabolites in the plasma, except for αT, which was not elevated in this study. Based on the ratio of AUC × V to the intake of each vitamer, the relative recovery of intact vitamin E forms in the plasma follows the order of γT (19.1%) >δT (11.6%) >γTE (5.9%) >δTE (3.6%). These data are in agreement with the higher Cmax of γT than that of δTE, despite similar amounts of intake of these 2 vitamin E forms. Thus, γT and δT are more bioavailable than their tocotrienol counterparts. Interestingly, the ratio of total metabolites’ AUC × V to the intake of the corresponding vitamins follows the order of γTE (3%) >γT (1.4%) >δTE ≈δT (0.6–0.7%), which is consistent with our previous observations of higher concentrations of metabolites from γTE than those from γT, and lower γTE than γT in the plasma of rats supplemented with the same dose of these vitamin E forms (6). Furthermore, Sontag and Parker (26) demonstrated that tocopherol-ω-hydrolase metabolizes γTE more effectively than γT or δTE, and γTE can be more rapidly metabolized in HepG2 cells expressing CYP4F2.
Our study reveals new and interesting aspects of fecal and urinary excretion of tocopherols, tocotrienols, and their metabolites. First, our data indicate that a large portion of tocopherols and tocotrienols, as well as their metabolites, are excreted to feces, and the extent of excretion of these compounds depends on the specific isoform. For instance, ∼37% and 45% of supplemented γT and αT, respectively, were excreted as the unmetabolized form, whereas only ∼17% of δTE was found in the fecal samples. The relatively high fecal excretion of αT observed in this study might be resultant from enhanced bile excretion due to high αT in the baseline diet and generally low catabolism of this vitamin E form. Second, in contrast to the vitamers, more fecal metabolites derived from δTE (7.5%) were detected than those from γT (4.9%). In particular, fecal excretion of δTE-13′-COOH, the predominant metabolite in feces, is double that of γT-13′-COOH despite similar intakes of δTE and γT. Third, unlike feces where metabolites of δTE/γTE are higher than those of γTmT, more urinary metabolites from tocopherols are detected than those from δTE. Interestingly, most conjugated CEHCs from tocopherols are not sulfated CEHCs, suggesting potential extensive glucuronidation. On the other hand, ≤40% of CEHCs derived from tocotrienols are in the sulfated form. Overall, ∼70% of γT or δT metabolites and 80–90% of metabolites from γTE and δTE appear to be excreted via feces as unconjugated ω-oxidation products, whereas only small portions of metabolites, mostly in the conjugated form, are found in the urine. Although the difference in conjugation of fecal compared with urinary metabolites may be explained by enhanced solubility of conjugated compounds for urine excretion, the mechanism related to conjugation and unconjugation of vitamin E metabolites in the liver and gut is not known and warrants investigation in the future. Regardless, our present data are consistent with those by Bardowell et al. (10), who reported higher fecal than urinary excretion of metabolites when mice were supplemented with γT and δT. Because large amounts of tocopherols, tocotrienols, and their metabolites were found in feces of rats supplemented with γTmT or δTE/γTE, we conclude that fecal excretion is the major route of elimination of vitamin E forms and metabolites when large quantities of vitamin E forms are consumed.
Our estimation of total recovery of vitamin E shows that ∼65% of γT was recovered as the unmetabolized form and its metabolites, but the recovery of δTE appeared to be only ∼30% (Table 6). This difference may be partially rooted in the difference in tissue retention. Although it is known that tocotrienols are low in many tissues, previous studies reported that considerable amounts of tocotrienols were found in adipose tissue or skin of rodents after administration (27–29). Future studies should be conducted to evaluate tissue distribution of tocopherols and tocotrienols including skin and adipose tissues. Moreover, it is important to note that relatively low recovery of δTE may also be caused by incomplete detection of metabolites including potential glycine and glucuronide conjugates, which warrants further investigation (30). In addition, other limitations of our present study include a relatively small sample size, data limited to the male gender, using baseline instead of vehicle control for evaluation of supplement outcomes, and using non–isotopically labeled compounds for estimation of the recovery.
Despite some limitations, our current results reveal distinct, and also similar, PK characteristics between rats and humans. For instance, we find that γT and δTE have Tmax of 4 and 2 h in rats, respectively, whereas γT and δTE in humans have been reported to show Tmax of ∼12 h and 5.6 h, respectively (31–33). This is consistent with the notion that laboratory animals usually have a higher rate of drug elimination than humans (34). The present study shows that δTE reached Cmax at 15.6 μM, which is higher than that reported in humans, i.e., mean Cmax of 7–10 μM ranging from 5 to 15 μM with large individual variances (32). In addition, we observed sulfated CEHCs and sulfated 11′-COOHs to be the predominant metabolites in rat plasma, whereas unconjugated γ-CEHC reaches several micromoles per liter in the plasma of humans in response to γT supplementation (35, 36). Mahipal et al. (32) reported that δ-CEHC is the predominant metabolite found in the plasma after multiple-dose supplementation of δTE (200–1600 mg) in human subjects, but the conjugation status was not identified because sulfatase and glucuronidase were employed to remove conjugation during sample preparation. In addition, Giusepponi et al. (37) conducted a study where healthy subjects took 1000 IU RRR-αT/d for 1 wk and reported that α-CEHC, α-13′-COOH, and α-13′-OH were detected in the plasma, although whether these metabolites were in conjugated or unconjugated forms was not clear.
The bioavailability of vitamin E forms and metabolites, together with their bioactivities, determine in vivo beneficial effects of these compounds for disease prevention and therapy. The present study, along with published work (32, 33, 35, 36), show that γT and δTE can reach concentrations of 30 and 10–16 μM, respectively, in the plasma of animals and humans. At these concentrations, these vitamins have been shown to exhibit anti-inflammatory and anticancer effects in mechanistic studies (2, 38–41). Consistent with being bioavailable and bioactive, these vitamin E forms exhibit anti-inflammatory actions in rat inflammation models (42), are protective against asthma in humans (36), and prevent cancer development in cancer models (40, 43). As for the metabolites, 13′-COOHs have been demonstrated to have anti-inflammatory and anticancer activities (2, 18–20, 40, 44, 45). Our current and previous studies (13, 14) show that, although low in the blood, unconjugated 13′-COOHs are the predominant metabolites found in feces and constitute ∼50% of overall fecal metabolites. High amounts of 13′-COOHs in fecal samples suggest that these metabolites may potentially have an impact on gastrointestinal health. Indeed, we have shown that δTE-13′-COOH significantly inhibited colon cancer development in mice (20). In the future, research should be carried out to characterize the PK including excretion of vitamin E forms and metabolites in humans.
Acknowledgments
The authors’ responsibilities were as follows—both authors: designed the research, conducted the research, analyzed the data, performed statistical analysis, have primary responsibility for the final contents, and read and approved the final manuscript.
Notes
Supported in part by NIH grant R01ES023349 (to QJ) , Indiana Clinical and Translational Science Institute pilot grant UL1TR001108 (to QJ), and Purdue University, College of Agriculture grant AgSEED (to QJ).
Author disclosures: KYL and QJ, no conflicts of interest.
Abbreviations used: CEHC, 2-(β-carboxyethyl)-6-hydroxychroman, also called 3′-COOH; Cmax, maximum plasma concentration; COOH, carboxychromanol; LC/MS/MS, LC-tandem MS; PK, pharmacokinetics; Tmax, time at which Cmax was observed; 13′-OH, 13′-hydroxychromanol; α-CMBHC, 2-(4-carboxy-4-methylbutyl)-6-hydroxy-2,5,7,8-tetramethylchroman; αT, α-tocopherol; γT, γ-tocopherol; γTE, γ-tocotrienol; γTmT, γ-tocopherol-rich mixed tocopherol; δT, δ-tocopherol; δTE, δ-tocotrienol; δTE/γTE, δ-tocotrienol-rich tocotrienol.
References
- 1. Brigelius-Flohe R, Traber M. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 2. Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manor D, Morley S. The α-tocopherol transfer protein. Vitam Horm. 2007;76:45–65. [DOI] [PubMed] [Google Scholar]
- 4. Thakur V, Morley S, Manor D. Hepatic α-tocopherol transfer protein: ligand-induced protection from proteasomal degradation. Biochemistry. 2010;49:9339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sontag TJ, Parker RS. Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism: novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–6. [DOI] [PubMed] [Google Scholar]
- 6. Freiser H, Jiang Q. γ-Tocotrienol and γ-tocopherol are primarily metabolized to conjugated 2-(β-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J Nutr. 2009;139:884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113–8. [DOI] [PubMed] [Google Scholar]
- 8. Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J Lipid Res. 2007;48:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang WC, Regnier FE, Jiang Q, Adamec J. In vitro stable isotope labeling for discovery of novel metabolites by liquid chromatography–mass spectrometry: confirmation of γ-tocopherol metabolism in human A549 cell. J Chromatogr A. 2010;1217:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bardowell SA, Ding X, Parker RS. Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism. J Lipid Res. 2012;53:2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bardowell SA, Duan F, Manor D, Swanson JE, Parker RS. Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism. J Biol Chem. 2012;287:26077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiku S, Hamamura K, Nakamura T. Novel urinary metabolite of d-δ-tocopherol in rats. J Lipid Res. 1984;25:40–8. [PubMed] [Google Scholar]
- 13. Jiang Q, Jiang Z, Jones Hall Y, Jang Y, Snyder PW, Bain C, Huang J, Jannasch A, Cooper B, Wang Y et al.. Gamma-tocopherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice. Free Radic Biol Med. 2013;65:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang Q, Xu T, Huang J, Jannasch AS, Cooper B, Yang C. Analysis of vitamin E metabolites including carboxychromanols and sulfated derivatives using LC/MS/MS. J Lipid Res. 2015;56:2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swanson J, Ben R, Burton G, Parker R. Urinary excretion of 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman is a major route of elimination of γ-tocopherol in humans. J Lipid Res. 1999;40:665–71. [PubMed] [Google Scholar]
- 16. Jiang Q. Natural forms of vitamin E and metabolites—regulation of cancer cell death and underlying mechanisms. IUBMB Life. 2019;71:495–506. [DOI] [PubMed] [Google Scholar]
- 17. Wechter WJ, Kantoci D, Murray ED, D'Amico DC, Jung ME, Wang WH. A new endogenous natriuretic factor: LLU-alpha. Proc Natl Acad Sci U S A. 1996;93:6002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci U S A. 2008;105:20464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunol. 2011;186:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jang Y, Park N-Y, Rostgaard-Hansen AL, Huang J, Jiang Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic Biol Med. 2016;95:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maloney D, Hecht S. A stereocontrolled synthesis of δ-trans-tocotrienoloic acid. Org Lett. 2005;7:4297–300. [DOI] [PubMed] [Google Scholar]
- 22. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freiser H, Jiang Q. Optimization of the enzymatic hydrolysis and analysis of plasma conjugated γ-CEHC and sulfated long-chain carboxychromanols, metabolites of vitamin E. Anal Biochem. 2009;388:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–89. [DOI] [PubMed] [Google Scholar]
- 25. Persky AM, Pollack GM. Foundations in pharmacokinetics. Chapel Hill (NC: ): University of North Carolina Press; 2013;24, 77, 185. [Google Scholar]
- 26. Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their ω-oxidation by tocopherol-ω-hydroxylase. J Lipid Res. 2007;48:1090–8. [DOI] [PubMed] [Google Scholar]
- 27. Okabe M, Oji M, Ikeda I, Tachibana H, Yamada K. Tocotrienol levels in various tissues of Sprague-Dawley rats after intragastric administration of tocotrienols. Biosci Biotechnol Biochem. 2002;66:1768–71. [DOI] [PubMed] [Google Scholar]
- 28. Ikeda S, Niwa T, Yamashita K. Selective uptake of dietary tocotrienols into rat skin. J Nutr Sci Vitaminol (Tokyo). 2000;46:141–3. [DOI] [PubMed] [Google Scholar]
- 29. Deng L, Peng Y, Wu Y, Yang M, Ding Y, Chen Q, Fu Q. Tissue distribution of emulsified γ-tocotrienol and its long-term biological effects after subcutaneous administration. Lipids Health Dis. 2014;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson CH, Slanar O, Krausz KW, Kang DW, Patterson AD, Kim JH, Luecke H, Gonzalez FJ, Idle JR. Novel metabolites and roles for α-tocopherol in humans and mice discovered by mass spectrometry–based metabolomics. Am J Clin Nutr. 2012;96:818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frank J, Lee S, Leonard SW, Atkinson JK, Kamal-Eldin A, Traber MG. Sex differences in the inhibition of γ-tocopherol metabolism by a single dose of dietary sesame oil in healthy subjects. Am J Clin Nutr. 2008;87:1723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahipal A, Klapman J, Vignesh S, Yang CS, Neuger A, Chen DT, Malafa MP. Pharmacokinetics and safety of vitamin E δ-tocotrienol after single and multiple doses in healthy subjects with measurement of vitamin E metabolites. Cancer Chemother Pharmacol. 2016;78:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Springett GM, Husain K, Neuger A, Centeno B, Chen DT, Hutchinson TZ, Lush RM, Sebti S, Malafa MP. A phase I safety, pharmacokinetic, and pharmacodynamic presurgical trial of vitamin E δ-tocotrienol in patients with pancreatic ductal neoplasia. EBioMedicine. 2015;2:1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nau H. Species differences in pharmacokinetics and drug teratogenesis. Environ Health Perspect. 1986;70:113–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burbank AJ, Duran CG, Almond M, Wells H, Jenkins S, Jiang Q, Yang C, Wang T, Zhou HB, Hernandez ML et al.. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. J Allergy Clin Immun. 2017;140:1179–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burbank AJ, Duran CG, Pan Y, Burns P, Jones S, Jiang Q, Yang C, Jenkins S, Wells H, Alexis N et al.. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J Allergy Clin Immunol. 2018;141:1231–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giusepponi D, Torquato P, Bartolini D, Piroddi M, Birringer M, Lorkowski S, Libetta C, Cruciani G, Moretti S, Saluti G et al.. Determination of tocopherols and their metabolites by liquid-chromatography coupled with tandem mass spectrometry in human plasma and serum. Talanta. 2017;170:552–61. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Jiang Q. γ-Tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPβ and NF-κB in macrophages. J Nutr Biochem. 2013;24:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang C, Jiang Q. Vitamin E δ-tocotrienol inhibits TNF-α-stimulated NF-κB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J Nutr Biochem. 2019;64:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang Q. Natural forms of vitamin E as effective agents for cancer prevention and therapy. Adv Nutr. 2017;8:850–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. γ-Tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang Q, Moreland M, Ames BN, Yin X. A combination of aspirin and γ-tocopherol is superior to that of aspirin and α-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. J Nutr Biochem. 2009;20:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen JX, Liu A, Lee MJ, Wang H, Yu S, Chi E, Reuhl K, Suh N, Yang CS. δ- and γ-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Mol Carcinog. 2017;56:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Birringer M, Lington D, Vertuani S, Manfredini S, Scharlau D, Glei M, Ristow M. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free Radic Biol Med. 2010;49:1315–22. [DOI] [PubMed] [Google Scholar]
- 45. Wallert M, Schmolz L, Koeberle A, Krauth V, Glei M, Galli F, Werz O, Birringer M, Lorkowski S. α-Tocopherol long-chain metabolite α-13′-COOH affects the inflammatory response of lipopolysaccharide-activated murine RAW264.7 macrophages. Mol Nutr Food Res. 2015;59:1524–34. [DOI] [PubMed] [Google Scholar]