Abstract
Purpose
This study aimed to determine the incidence of male breast cancer (MBC) and its survival outcomes in Korea, and to compare these results to those for female breast cancer (FBC).
Materials and Methods
We searched the Korea Central Cancer Registry and identified 227,122 breast cancer cases that were diagnosed between 1999 and 2016. Demographic and clinical characteristics and overall survival (OS) rates were estimated according to sex, age, histological type, and cancer stage.
Results
The 227,122 patients included 1,094 MBC cases and 226,028 FBC cases. Based on the age-standardized rate, the male: female ratio was 0.0055:1. The most common ages at diagnosis were 60-69 years for MBC and 40-49 years for FBC (p < 0.001). Male patients were less likely than female patients to receive adjuvant radiotherapy (7.5% vs. 21.8%, p < 0.001) or adjuvant chemotherapy (40.1% vs. 55.4%, p < 0.001). The 5-year OS rates after diagnosis were 88.8% for all patients, although it was significantly lower for MBC than for FBC (76.2% vs. 88.9%, p < 0.001). In both groups, older age (≥ 60 years) was associated with shorter survival. The 5-year OS rates for the invasive histological types were 75.8% for men and 89.0% for women. The 5-year OS rates in both groups decreased with increasing cancer stage.
Conclusion
MBC was diagnosed at older ages than FBC, and male patients were less likely to receive radiotherapy and chemotherapy. The survival outcomes were worse for MBC than for FBC, with even poorer outcomes related to older age, the inflammatory histological types, and advanced stage. It is important that clinicians recognize the differences between FBC and MBC when treating these patients.
Keywords: Breast neoplasms, Male breast neoplasms, Korea
Introduction
Male breast cancer (MBC) is a very rare disease [1], while female breast cancer (FBC) is the most common type of cancer globally [2]. The incidence of breast cancer has also been increasing over the last 25 years in both male and female patients [3]. Treatment outcomes and overall survival (OS) in FBC cases have improved as a result of increased mammography screening and the development of adjuvant chemotherapy, human epidermal growth factor receptor 2 therapy, and endocrine therapy [4]. However, the rarity of MBC cases makes it difficult to confirm the differences between FBC and MBC, as most previous studies have been retrospective and generated insufficient evidence regarding the efficacy of radiation therapy, adjuvant chemotherapy, and hormone therapy for MBC. Some reports have indicated that MBC has a poorer prognosis than FBC [5,6], although other studies have failed to detect sex-related differences [4], which have generated conflicting opinions regarding treatment and survival outcomes. Moreover, there are no treatment guidelines for MBC, which is generally managed based on the guidelines for FBC [7]. Therefore, the present study aimed to determine the incidence and survival outcomes for MBC using the population-based Korea Central Cancer Registry (KCCR) database. We also aimed to identify differences between MBC and FBC according to age, cancer stage, histological type, treatment, and follow-up.
Materials and Methods
1. Study population and data collection
The KCCR was searched to identify all patients with breast cancer who were diagnosed between 1999 and 2016. Primary breast cancer (C50) was defined according to the International Classification of Diseases, 10th edition (ICD-10) [8]. The KCCR is a population-based cancer registry that was launched in 1999 and collects information regarding approximately 98% of cancer cases in Korea, which is used in the Korea National Cancer Incidence Database [9].
2. Statistical analyses
Patients were grouped according to sex, age (< 40 years, 40-49 years, 50-59 years, 60-69 years, 70-79 years, and ≥ 80 years), the Surveillance, Epidemiology, and End Results (SEER) summary stage, and the first course of treatment within 4 months after the diagnosis. Histological types were first classified according to the ICD-10 [8], and then classified into four types (invasive, Paget, inflammatory, other). We defined tumors histologically as invasive, lobular, tubular, mucinous, medullary, metaplastic, or papillary. They were classified as invasive if they corresponded to invasive carcinoma of no special type. For simplicity, the OS analyses were performed using three larger age groups (< 40 years, 40-59 years, ≥ 60 years). The age-standardized rate (ASR) was used to determine the male: female ratios.
Analysis of variance testing was used to evaluate continuous variables, and the chi-squared test was used to evaluate categorical variables. Survival curves were created using the Kaplan-Meier method [10] and compared using the log-rank test according to age, histological type, and SEER stage. All statistical tests were two-tailed and results were considered statistically significant at p-values of < 0.05. All statistical analyses were performed using SAS software ver. 9.4 (SAS Institute Inc., Cary, NC) and STATA software ver. 15.1 (StataCorp LLC, College Station, TX).
3. Ethical statement
The study protocol was approved by our institutional review board (NCC 2018-0224) and the requirement for informed consent was waived because it is secondary analysis of de-identified data.
Results
1. Characteristics of MBC in Korea
Between 1999 and 2016, the KCCR included 227,122 patients with newly diagnosed breast cancer, including 1,094 MBC patients and 226,028 FBC patients. Based on the ASR, the male:female ratio was 0.0055. Since 1999, the overall number of patients diagnosed with breast cancer has steadily increased, although different patterns were observed according to sex, with the annual percent change being –0.69% for MBC (p=0.397) and +5.61% for FBC (p < 0.001) (Table 1). Table 2 shows the clinicopathological characteristics according to sex, with male patients being diagnosed at a significantly older age than female patients (61.71±13.97 years vs. 50.82±11.49 years, p < 0.001). The most common age groups at the MBC diagnosis were 60-69 years (24.9%) and 70-79 years (23.5%), while the most common age group was 40-49 years for FBC (36.8%, p < 0.001). In addition, we detected significant differences between MBC and FBC according to disease stage, use of radiotherapy, and use of chemotherapy. The localized stage was the most common in both groups (MBC 44.1% vs. FBC 55.2%, p < 0.001). Surgery was performed in 81.6% of MBC cases and 86.9% of FBC cases (p < 0.001). Furthermore, male patients were significantly less likely than female patients to receive adjuvant radiotherapy (7.5% vs. 21.8%, p < 0.001) or adjuvant chemotherapy (40.1% vs. 55.4%, p < 0.001). The mean follow-up times were 75.91± 58.09 months for male patients and 84.11±56.68 months for female patients (p < 0.001).
Table 1.
Age-standardized rates of breast cancer among men and women according to year
| Year | Diagnosis of breast cancer patients |
|||||
|---|---|---|---|---|---|---|
| Male |
Female |
Male/Female ratio (ASR) | Male/Female ratio (cases) | |||
| ASR | No. | ASR | No. | |||
| 1999 | 0.17 | 39 | 20.69 | 5,640 | 0.0082 | 0.0065 |
| 2000 | 0.23 | 57 | 20.67 | 5,782 | 0.0126 | 0.0084 |
| 2001 | 0.22 | 49 | 24.44 | 7,032 | 0.0090 | 0.0063 |
| 2002 | 0.25 | 56 | 26.88 | 7,936 | 0.0093 | 0.0066 |
| 2003 | 0.23 | 57 | 27.58 | 8,373 | 0.0083 | 0.0055 |
| 2004 | 0.21 | 51 | 29.06 | 9,064 | 0.0072 | 0.0056 |
| 2005 | 0.21 | 55 | 31.83 | 10,124 | 0.0066 | 0.0054 |
| 2006 | 0.19 | 50 | 33.11 | 10,812 | 0.0056 | 0.0046 |
| 2007 | 0.13 | 35 | 35.40 | 11,855 | 0.0036 | 0.0030 |
| 2008 | 0.26 | 75 | 37.08 | 12,693 | 0.0071 | 0.0056 |
| 2009 | 0.20 | 61 | 38.56 | 13,502 | 0.0051 | 0.0044 |
| 2010 | 0.21 | 69 | 40.53 | 14,481 | 0.0051 | 0.0047 |
| 2011 | 0.22 | 72 | 43.88 | 15,976 | 0.0049 | 0.0045 |
| 2012 | 0.18 | 65 | 44.79 | 16,562 | 0.0040 | 0.0038 |
| 2013 | 0.16 | 58 | 45.79 | 17,274 | 0.0035 | 0.0034 |
| 2014 | 0.21 | 77 | 47.45 | 18,236 | 0.0044 | 0.0042 |
| 2015 | 0.20 | 77 | 48.99 | 19,076 | 0.0040 | 0.0040 |
| 2016 | 0.22 | 91 | 54.60 | 21,610 | 0.0040 | 0.0042 |
| Total | 0.20 | 1,094 | 37.38 | 226,028 | 0.0055 | 0.0047 |
| APC | –0.69% (p=0.397) | 5.61% (p < 0.001) | ||||
ASR, age-standardized rate (world standard population); APC, annual percent change.
Table 2.
Clinicopathological characteristics of breast cancer patients in Korea
| Characteristic | Men | Women | p-valuea) |
|---|---|---|---|
| Total patients | 1,094 | 226,028 | |
| Age at diagnosis with breast cancer (yr) | |||
| Mean±SD | 61.71±13.97 | 50.82±11.49 | < 0.001 |
| Median (range) | 63 (24-97) | 49 (6-102) | |
| No. of breast cancer diagnoses according to age (yr) | < 0.001 | ||
| < 40 | 69 (6.4) | 32,708 (14.5) | |
| 40-49 | 166 (15.2) | 83,179 (36.8) | |
| 50-59 | 229 (20.9) | 62,335 (27.6) | |
| 60-69 | 272 (24.9) | 30,882 (13.7) | |
| 70-79 | 257 (23.5) | 13,385 (5.9) | |
| ≥ 80 | 101 (9.2) | 3,539 (1.6) | |
| Histology | < 0.001 | ||
| Invasive | 1,061 (97.0) | 223,561 (98.9) | |
| Paget | 14 (1.3) | 1,288 (0.6) | |
| Inflammatory | 1 (0.1) | 201 (0.1) | |
| Others | 18 (1.6) | 978 (0.4) | |
| Stage (2006-2016) | 730 | 172,077 | < 0.001 |
| Localized | 322 (44.1) | 95,017 (55.2) | |
| Regional | 284 (38.9) | 58,371 (33.9) | |
| Distant | 50 (6.8) | 8,463 (4.9) | |
| Unknown | 74 (10.1) | 10,226 (5.9) | |
| Surgery | < 0.001 | ||
| Yes | 893 (81.6) | 196,516 (86.9) | |
| No | 201 (18.4) | 29,512 (13.1) | |
| Radiation therapy | < 0.001 | ||
| Yes | 82 (7.5) | 49,367 (21.8) | |
| No | 1,012 (92.5) | 177,673 (78.6) | |
| Chemotherapy | < 0.001 | ||
| Yes | 439 (40.1) | 125,247 (55.4) | |
| No | 655 (59.9) | 100,781 (44.6) | |
| Follow-up time (mo) | |||
| Mean±SD | 75.91±58.09 | 84.11±56.68 | < 0.001 |
| Median (range) | 64 (0-227) | 72 (0-227) |
Values are presented as number (%). SD, standard deviation.
ANOVA tests were performed to evaluate differences by factor for continuous variables, and chi-square tests were performed to evaluate differences by factor for categorical variables.
2. Survival outcomes for MBC and FBC
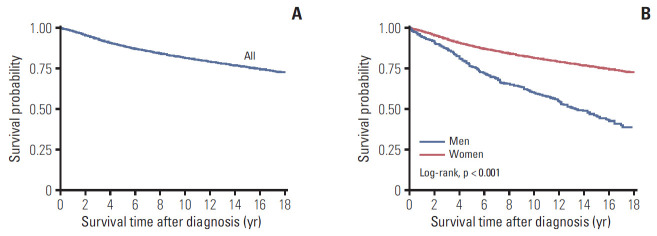
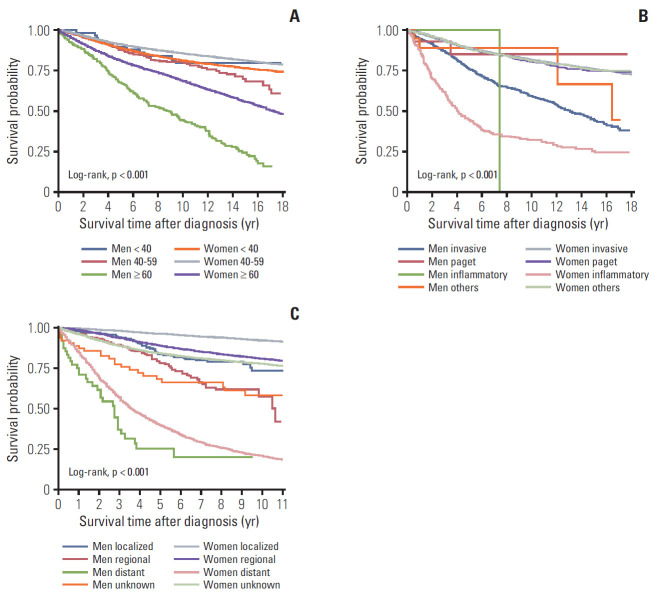
The 5-year OS rates after diagnosis were 88.8% for all patients, 76.2% for male patients, and 88.9% for female patients (p < 0.001). The 10-year OS rates were 81.6% for all patients, 60.1% for male patients, and 81.7% for female patients (Fig. 1). Relative to the younger age groups, MBC patients who were ≥ 60 years old had poorer rates of 5-year OS (67.1%) and 10-year OS (44.0%), and FBC patients who were ≥ 60 years old also had poorer rates of 5-year OS (81.2%) and 10-year OS (68.6%) (Fig. 2A). Among cases with an invasive histological type, the 5-year OS rates were 75.8% for MBC and 89.0% for FBC, while the 10-year OS rates were 59.3% for MBC and 81.8% for FBC. Female patients with inflammatory histological types had significantly poorer OS rates than female patients with other histological types (Fig. 2B). Localized breast cancer was associated with 5-year OS rates of 83.5% for MBC and 96.2% for FBC, while the 10-year OS rates were 73.2% for MBC and 92.0% for FBC. Distant metastasis was associated with poor prognoses in the MBC and FBC groups, with a 5-year OS rate of 25.2% in the MBC group, and rates of 39.5% for 5-year OS and 20.9% for 10-year OS in the FBC group (Fig. 2C).
Fig. 1.
Overall survival among all Korean breast cancer patients (A) and according to sex (B).
Fig. 2.
Overall survival according to sex, age, histological type, and breast cancer stage. (A) Overall survival was evaluated for male patients who were < 40 years old (blue line), men who were 40-59 years old (red line), men who were ≥ 60 years old (green line), female patients who were < 40 years old (orange line), women who were 40-59 years old (gray line), and women who were ≥ 60 years old (purple line). (B) Overall survival was also evaluated for male patients with invasive carcinoma (blue line), Paget disease (red line), inflammatory type (green line), and other cancer types (orange line), as well as for female patients with invasive carcinoma (gray line), Paget disease (purple line), inflammatory type (pink line), and other cancer types (light green line). (C) Overall survival was also evaluated for male patients with localized breast cancer (blue line), regional breast cancer (red line), distant breast cancer (green line), and unknown breast cancer (orange line), as well as for female patients with localized breast cancer (gray line), regional breast cancer (purple line), distant breast cancer (pink line), and unknown breast cancer (light green line).
The univariate analyses revealed that mortality was significantly associated with male sex (p < 0.001), older age (p < 0.001), the inflammatory histological types (p < 0.001), and advanced cancer stage (p < 0.001). The multivariate analyses also revealed that mortality was independently associated with male sex (hazard ratio [HR], 1.68; 95% confidence interval [CI], 1.45 to 1.96; p < 0.001), age of ≥ 60 years (HR, 2.19; 95% CI, 2.09 to 2.30; p < 0.001), the inflammatory histological types (HR, 2.93; 95% CI, 2.22 to 3.87; p < 0.001), and especially distant metastasis (HR, 22.05; 95% CI, 21.16 to 22.98; p < 0.001) (Table 3).
Table 3.
Univariate and multivariate Poisson regression analyses according to risk factors between 1999 and 2016
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Sex | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 2.52 | 2.27-2.80 | < 0.001 | 1.68 | 1.45-1.96 | < 0.001 |
| Age group at diagnosis (yr) | ||||||
| < 40 | 1.00 | 1.00 | ||||
| 40-59 | 0.79 | 0.77-0.82 | < 0.001 | 0.89 | 0.85-0.93 | < 0.001 |
| ≥ 60 | 2.05 | 1.98-2.12 | < 0.001 | 2.19 | 2.09-2.30 | < 0.001 |
| Histology | ||||||
| Invasive | 1.00 | 1.00 | ||||
| Paget | 0.96 | 0.87-1.14 | 0.941 | 1.12 | 0.92-1.36 | 0.276 |
| Inflammatory | 5.70 | 4.93-6.59 | < 0.001 | 2.93 | 2.22-3.87 | < 0.001 |
| Others | 0.95 | 0.83-1.10 | 0.513 | 1.22 | 0.95-1.55 | 0.115 |
| Stage (2006-2016) | ||||||
| Localized | 1.00 | 1.00 | ||||
| Regional | 2.78 | 2.68-2.89 | < 0.001 | 2.85 | 2.75-2.96 | < 0.001 |
| Distant | 22.29 | 21.39-23.23 | < 0.001 | 22.05 | 21.16-22.98 | < 0.001 |
| Unknown | 3.50 | 3.31-3.69 | < 0.001 | 3.46 | 3.28-3.65 | < 0.001 |
HR, hazard ratio; CI, confidence interval.
Discussion
The present study revealed that, based on KCCR data from an 18-year period, MBC accounted for 0.48% of all newly diagnosed breast cancers in Korea, with a slightly decreasing trend each year since 1999. In this context, MBC is known to be a rare disease, accounting for approximately 0.6%-1% of newly diagnosed breast cancer cases in Western countries [11,12] and approximately 0.5% of newly diagnosed cases in Japan [13]. In the present study, the median age at diagnosis of MBC was 63 years, which was similar to the reported median age in Western countries (67 years) [14]. Interestingly, MBC was diagnosed approximately 10 years later than FBC in Korea, which may be related to delayed screening and diagnosis, given the lack of awareness regarding MBC [15]. Nevertheless, social awareness and screening events have been actively promoted and appear to be changing the perception of breast cancer in Korea. Moreover, the differences in age at diagnosis may be related to differences in sex hormone changes, rather than a delayed diagnosis caused by a lack of awareness. For example, previous studies have indicated that MBC patients had higher hormone receptor positivity than FBC patients [3,16], and the present study revealed that cancers were typically diagnosed at a localized stage for both MBC and FBC cases.
Relative to FBC, adjuvant radiotherapy is less commonly performed for MBC patients [17], and we also found that radiotherapy was significantly less likely to be performed for MBC than for FBC. Considering the patterns of cancer in men and women according to stage, radiotherapy was performed significantly less frequently in MBC overall, and within each stage (S1 Table). Some studies have suggested that MBC has a more centralized location (vs. FBC), which leads to a higher rate of mastectomy, while other studies have attributed the less frequent use of radiotherapy to older age and cardiovascular or pulmonary comorbidities [7,18]. Furthermore, some studies have concluded that additional radiotherapy does not provide a survival benefit [19,20]. However, other studies have indicated that adjuvant radiotherapy after mastectomy helps reduce the rate of local recurrence [21,22]. Therefore, the use of adjuvant radiotherapy for MBC remains a controversial issue.
This study also found that adjuvant chemotherapy was used significantly less frequently in MBC overall, and within each stage (S2 Table). Since hormone-positive type is relatively common in MBC [16], it may be inferred that anti-hormonal therapy plays a more important role than chemotherapy in MBC. However, a limitation of the KCCR data is that they only included treatment within the first four months after diagnosis.
Improvements in early detection and treatment strategies have improved the survival rate for breast cancer. For example, KCCR data from 2002-2013 revealed that the 5-year relative survival rates were 87% for men and 90.8% for women [23], while data from Statistics Korea (http://kosis.kr) during 2012–2016 revealed that the 5-year relative survival rates were 89.3% for men and 92.7% for women. Interestingly, some previous studies have suggested that MBC has a poorer prognosis and lower survival rate than FBC [24,25], while other reports have suggested that there were no significant sex-related differences in survival [17,26]. The present study revealed that MBC has poorer OS rates than FBC in Korea, as well as several sex-related differences in the age of diagnosis, histological type, and cancer stage. For example, the best survival for MBC was observed in cases that were diagnosed at the age of < 40 years, while an FBC diagnosis at the age of < 40 years was associated with only moderate survival outcomes, which could be related to advanced stage, higher grade, and hormone receptor-negative status [27,28]. In addition, an FBC diagnosis at the age of ≥ 60 years had the poorest outcomes among female patients, which might be related to greater comorbidities at older ages affecting treatment decisions and mortality rates [29].
This study had several strengths, such as the use of a population-based cancer registry that contained information regarding approximately 98% of Korean cancer cases [9]. Second, to the best of our knowledge, this study evaluated the largest sample of MBC cases in Asia, which might help more clearly identify differences between MBC and FBC, relative to the smaller samples from previous studies. However, the present study also has several limitations, with the most important issue being the lack of information regarding detailed tumor characteristics, such as hormone receptor status, human epidermal growth factor receptor 2 status, and Ki-67 status, which would have influenced treatment selection. Second, we did not have detailed information regarding the surgical procedures that were performed. Third, we only evaluated the treatment status up to 4 months after diagnosis, which might explain why we observed a lower rate of adjuvant radiotherapy relative to the rates in previous studies. Other limitations include a lack of information regarding family history, social history, BRCA1/BRCA2 mutations, comorbidities, and personal history of cancer. For example, male carriers of pathological BRCA variants are known to have increased risks of breast, prostate, pancreatic, stomach, and hematological cancers, with MBC being more closely associated with BRCA2 mutations than BRCA1 mutations [30]. Therefore, it is possible that there were secondary cancers related to BRCA mutations, which might have influenced the survival differences bet-ween male and female patients with breast cancer. Further studies are needed to consider the influence of BRCA mutations on the outcomes of MBC, and to provide a more detailed investigation regarding the characteristics of MBC.
When comparing OS rates, we divided the histological type into four groups because of differences in treatment and prognosis. In this study, we were defined as an invasive type, which accounts for the majority, and the limitation was that the group was not compared for each type.
In conclusion, the present study revealed noticeable differences between MBC and FBC cases, with MBC having an older peak age at diagnosis. In addition, MBC had poorer survival outcomes than FBC, and we observed different OS rates patterns according to age, histological type, and cancer stage. Therefore, clinicians need to recognize the differences between FBC and MBC when treating these patients, and additional studies are needed to develop specific treatment guidelines for MBC.
Acknowledgments
This work was supported by National Cancer Center Grants (NCC-1911274-1 and NCC-1910132-1).
Footnotes
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
The status of adjuvant radiotherapy according to disease stage
The status of adjuvant chemotherapy according to disease stage
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51–7. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 4.Hong JH, Ha KS, Jung YH, Won HS, An HJ, Lee GJ, et al. Clinical features of male breast cancer: experiences from seven institutions over 20 years. Cancer Res Treat. 2016;48:1389–98. doi: 10.4143/crt.2015.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28:232–9. doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu N, Johnson KJ, Ma CX. Male breast cancer: an updated Surveillance, Epidemiology, and End Results data analysis. Clin Breast Cancer. 2018;18:e997–1002. doi: 10.1016/j.clbc.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Raposo C, Zambrana Tevar F, Sereno Moyano M, Lopez Gomez M, Casado E. Male breast cancer. Cancer Treat Rev. 2010;36:451–7. doi: 10.1016/j.ctrv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . International statistical classification of diseases and related health problems. 10th rev. Geneva: World Health Organization; 1994. [Google Scholar]
- 9.Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–30. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteve J, Benhamou E, Raymond L. Statistical methods in cancer research. Volume IV. Descriptive epidemiology. IARC Sci Publ. 1994;(128):1–302. [PubMed] [Google Scholar]
- 11.Hill TD, Khamis HJ, Tyczynski JE, Berkel HJ. Comparison of male and female breast cancer incidence trends, tumor characteristics, and survival. Ann Epidemiol. 2005;15:773–80. doi: 10.1016/j.annepidem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.White J, Kearins O, Dodwell D, Horgan K, Hanby AM, Speirs V. Male breast carcinoma: increased awareness needed. Breast Cancer Res. 2011;13:219. doi: 10.1186/bcr2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioka A, Tsukuma H, Ajiki W, Oshima A. Survival of male breast cancer patients: a population-based study in Osaka, Japan. Jpn J Clin Oncol. 2006;36:699–703. doi: 10.1093/jjco/hyl095. [DOI] [PubMed] [Google Scholar]
- 14.Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005;10:471–9. doi: 10.1634/theoncologist.10-7-471. [DOI] [PubMed] [Google Scholar]
- 15.Miao H, Verkooijen HM, Chia KS, Bouchardy C, Pukkala E, Laronningen S, et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29:4381–6. doi: 10.1200/JCO.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 16.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137:678–87. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 17.Choi MY, Lee SK, Lee JE, Park HS, Lim ST, Jung Y, et al. Characterization of Korean male breast cancer using an online nationwide breast-cancer database: matched-pair analysis of patients with female breast cancer. Medicine (Baltimore) 2016;95:e3299. doi: 10.1097/MD.0000000000003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367:595–604. doi: 10.1016/S0140-6736(06)68226-3. [DOI] [PubMed] [Google Scholar]
- 19.Erlichman C, Murphy KC, Elhakim T. Male breast cancer: a 13-year review of 89 patients. J Clin Oncol. 1984;2:903–9. doi: 10.1200/JCO.1984.2.8.903. [DOI] [PubMed] [Google Scholar]
- 20.Gough DB, Donohue JH, Evans MM, Pernicone PJ, Wold LE, Naessens JM, et al. A 50-year experience of male breast cancer: is outcome changing? Surg Oncol. 1993;2:325–33. doi: 10.1016/0960-7404(93)90063-5. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarthy A, Kim CR. Post-mastectomy radiation in male breast cancer. Radiother Oncol. 2002;65:99–103. doi: 10.1016/s0167-8140(02)00210-4. [DOI] [PubMed] [Google Scholar]
- 22.Yu E, Suzuki H, Younus J, Elfiki T, Stitt L, Yau G, et al. The impact of post-mastectomy radiation therapy on male breast cancer patients: a case series. Int J Radiat Oncol Biol Phys. 2012;82:696–700. doi: 10.1016/j.ijrobp.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Jung SY, Jung KW, Ha J, Won YJ, Kim YA, Kwon Y, et al. Different patterns of conditional survival of breast cancer patients by age and histologic types: evidence from the Korean nationwide registry. Cancer Epidemiol Biomarkers Prev. 2019;28:1169–76. doi: 10.1158/1055-9965.EPI-18-1072. [DOI] [PubMed] [Google Scholar]
- 24.Gnerlich JL, Deshpande AD, Jeffe DB, Seelam S, Kimbuende E, Margenthaler JA. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol. 2011;18:1837–44. doi: 10.1245/s10434-010-1468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott-Conner CE, Jochimsen PR, Menck HR, Winchester DJ. An analysis of male and female breast cancer treatment and survival among demographically identical pairs of patients. Surgery. 1999;126:775–80. [PubMed] [Google Scholar]
- 26.Marchal F, Salou M, Marchal C, Lesur A, Desandes E. Men with breast cancer have same disease-specific and event-free survival as women. Ann Surg Oncol. 2009;16:972–8. doi: 10.1245/s10434-009-0327-6. [DOI] [PubMed] [Google Scholar]
- 27.Bharat A, Aft RL, Gao F, Margenthaler JA. Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. J Surg Oncol. 2009;100:248–51. doi: 10.1002/jso.21268. [DOI] [PubMed] [Google Scholar]
- 28.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–7. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berglund A, Wigertz A, Adolfsson J, Ahlgren J, Fornander T, Warnberg F, et al. Impact of comorbidity on management and mortality in women diagnosed with breast cancer. Breast Cancer Res Treat. 2012;135:281–9. doi: 10.1007/s10549-012-2176-4. [DOI] [PubMed] [Google Scholar]
- 30.Mohamad HB, Apffelstaedt JP. Counseling for male BRCA mutation carriers: a review. Breast. 2008;17:441–50. doi: 10.1016/j.breast.2008.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The status of adjuvant radiotherapy according to disease stage
The status of adjuvant chemotherapy according to disease stage