Abstract
Aims
The alarmin S100A8/S100A9 (S100A8/A9) is released by activated monocytes/macrophages and neutrophils in the setting lymphocytic myocarditis (MC). We recently demonstrated its therapeutic potential in experimental acute MC. Now, we investigated the diagnostic relevance of S100A8/A9 serum levels in patients with suspected acute and chronic MC and in patients with heart failure without cardiac inflammation.
Methods and Results
Serum S100A8/A9 levels were analysed in patients with a recent onset of MC [≤ 30 days, n = 32; ejection fraction (EF): 45.4 ± 12.9%], dilated cardiomyopathy patients with inflammation (n = 112; EF: 29.0 ± 11.4%), or without inflammation (n = 58; EF: 26.6 ± 9.3%), and controls (n = 25; EF: 68.5 ± 4.6%), by using specific ELISAs. Blood samples were collected at Time Point 1 (T1), where also endomyocardial biopsies (EMBs) were withdrawn. Patients with a recent onset of MC showed a 4.6‐fold increase in serum S100A8/A9 levels vs. controls (MC: 1948 ± 1670 ng/mL vs. controls: 426 ± 307 ng/mL; P < 0.0001). Serum S100A8/A9 correlated with the disease activity, represented by EMB‐derived counts of inflammatory cells (CD3: r = 0.486, P = 0.0047, lymphocyte function‐associated antigen‐1: r = 0.558, P = 0.0009, macrophage‐1 antigen: r = 0.434, P = 0.013), the EMB mRNA levels of S100A8, S100A9 (r = 0.541, P = 0.002), and left ventricular ejection fraction (LVEF: r = 0.498, P = 0.0043). EMB immunofluorescence co‐stainings display macrophages/monocytes and neutrophils as the main source of S100A8 and S100A9 in recent onset MC. The diagnostic value of serum alarmin levels (cut‐off 583 ng/mL) was characterized by a specificity of 92%, a sensitivity of 90.6%, positive predictive value of 93.5%, negative predictive value of 88.5%, and an accuracy of 0.949 (95% confidence interval [0.89–1]). In a subgroup of MC patients, S100A8/A9 serum levels and EMBs at T1 (n = 12) and a follow‐up visit (T2, n = 12, mean follow‐up 8.5 months) were available. A fall of serum S100A8/A9 (T1: 2208 ± 1843 ng/mL vs. T2: 888.8 ± 513.7 ng/mL; P = 0.00052) was associated with a reduced cardiac inflammation (CD3 T1: 70.02 ± 107.4 cells per square millimetre vs. T2: 59.18 ± 182.5 cells per square millimetre; P = 0.0342, lymphocyte function‐associated antigen‐1 T1: 133.5 ± 187.1 cells per square millimetre vs. T2: 74.12 ± 190.5 cells per square millimetre; P = 0.0186, and macrophage‐1 antigen T1: 132.6 ± 129.5 cells per square millimetre vs. T2: 54.41 ± 65.16 cells per square millimetre; P = 0.0015). Serum S100A8/A9 levels were only slightly increased in patients within the chronic phase of MC and in heart failure patients without inflammation vs. controls.
Conclusions
Serum S100A8/A9 might serve as an additional tool in the diagnostic workup of suspected acute MC patients.
Keywords: S100A8/S100A9, Myocarditis, Endomyocardial biopsy
Introduction
Lymphocytic myocarditis (MC) is defined as an inflammatory cardiac disorder mainly caused by viral infections and is an important cause of heart failure (HF) in young adults. 1 Despite classical pharmacological treatment, acute MC can progress to a dilated chronic phenotype [inflammatory dilated cardiomyopathy (DCMi)] with an impaired prognosis. 2
The diagnosis of ongoing cardiac inflammation remains a clinical challenge and depends on special imaging tools [cardiac magnetic resonance imaging (cMRI)] or the performance of endomyocardial biopsies (EMB). 3 The establishment of a novel biomarker that allows one to identify the appropriate patient to biopsy and then to monitor those patients who are treated for inflammation or viral infection would be a great advance. Several biomarkers have been studied including high‐sensitivity C‐reactive protein (hsCRP), B‐type natriuretic peptides (BNPs), troponin T, soluble ST2, or different microRNAs. However, these markers lack specificity or are still under investigation. 1 , 4 , 5 Until now, no MC‐specific biomarkers have been established, which are able to support the diagnosis in patients with suspected MC and to determine the presence or absence of active myocardial inflammation. 1 , 5 The pro‐inflammatory alarmin, S100A8/S100A9 (S100A8/A9), could be considered as a possible tool to close this diagnostic gap. In contrast to acute phase proteins like hsCRP, which are mainly of hepatic origin, S100A8/A9, as a damage‐associated molecular pattern, 6 , 7 is released predominantly by monocytes and neutrophils and diffuses easily because of its low molecular weight. 8 Accumulating evidence demonstrates an involvement of S100A8/A9 in cardiovascular disorders, such as coronary artery disease, 9 and during viral infections. 10 Clinical evidence highlights that high plasma levels of S100A8/A9 are a risk factor for future myocardial infarcts and cardiovascular death. 9 Furthermore, several studies in other diseases such as Crohn's disease have examined the potential role of S100A8/A9 serum levels as a therapy‐monitoring tool, resulting in its inclusion into the appropriate guidelines. 11 We recently demonstrated that in cardiac tissue, S100A8/A9 plays a pivotal role in human and experimental Coxsackievirus B3 (CVB3)‐induced MC. 12 However, the relevance of S100A8/A9 serum levels in MC has thus far not been unravelled. To investigate the role of serum S100A8/A9 levels in MC, we measured S100A8/A9 serum levels of MC patients with a recent onset of MC (acute ≤30 days) and compared them with controls, DCMi (chronic >30 days, characterized by a cardiac low‐grade inflammation), and dilated cardiomyopathy (DCM, without cardiac inflammation) patients, in the absence of coronary heart disease and cardiac inflammation, respectively.
Materials and methods
Patient characteristics
In this retrospective analysis of serum S100A8/A9, the study group comprised patients with a recent onset of MC (acute, ≤30 days, n = 32), patients with DCMi (n = 112), and DCM (n = 58). Twenty‐five patients with suspected MC, where a cardiac cause for exercise limitation and chest complaints could be excluded after several investigations, including exclusion of inflammation in their EMBs, served as controls (see Table 1).
Table 1.
Patient characteristics
| Controls | DCM | DCMi | Resent onset of MC | |
|---|---|---|---|---|
| N | 25 | 58 | 112 | 32 |
| Male | 6 (24%) | 47 (81%) | 84 (75%) | 27 (84.3%) |
| Age (years ± SD) | 37.1 ± 16.6 | 51.2 ± 13.3* | 49.4 ± 13.5 | 41.4 ± 18.8* |
| NYHA (I/II/III/IV) | 2/7/1/0 | 12/44/29/14 | 12/44/29/14 | 0/18/8/1 |
| LVEF (% ± SD) | 68.5 ± 4.6 | 26.6 ± 9.3***,### | 29.0 ± 11.4***,### | 45.4 ± 12.9 |
| Medications | ||||
| ACE/AT1 n (%) | 4 (16) | 17 (29) | 46 (41) | 9 (26.4) |
| ß‐blocker n (%) | 2 (0.5) | 16 (27) | 4 (3) | 9 (26.4) |
| Aldosteron antagonist n (%) | 1 (4) | 13 (22) | 38 (34) | 7 (20.6) |
| Diuretics n (%) | 2 (8) | 11 (19) | 33 (29) | 5 (14.7) |
| Glycosides n (%) | 0 | 0 | 4 (3) | 0 |
| Antiarrhythmics n (%) | 1 (4) | 3 (5) | 7 (6) | 0 |
| Virus‐PCR | ||||
| Enterovirus n (%) | 0 | 0 | 1 (0.8) | 0 |
| B19V n (%) | 4 (16) | 11 (19) | 57 (51) | 14 (43.8) |
| HHV n (%) | 0 | 0 | 1 (0.8) | 2 (7) |
| EBV (%) | 0 | 0 | 2 (1.8) | 1 (2.9%) |
| Immunohistology/cut‐off | ||||
| CD3 (cells/mm2)/14 cells/mm2 | 3.8 ± 2.9 | 4.4 ± 6.2###, §§§ | 20.3 ± 63.1 | 35.3 ± 51.4** |
| Mac‐1 (cells/mm2)/40 cells/mm2 | 19.7 ± 10.6 | 20.3 ± 16.6###,§§§ | 50.3 ± 45.3* | 101.8 ± 119.8*** |
| LFA‐1 (cells/mm2)/14 cells/mm2 | 12.8 ± 5.0 | 12.7 ± 10.2###,§§§ | 35.5 ± 72.3 | 93.0 ± 1870.1* |
| CD45R0 (cells/mm2)/40 cells/mm2 | 17.5 ± 9.2 | 30.8 ± 16.5###,§§§ | 72.2 ± 108.0*** | 112.6 ± 106.2*** |
| CTL (perforin) (cells/mm2)/2.9 cells/mm2 | 1.7 ± 1.7 | 0.2 ± 0.6*,§ | 8.5 ± 61.6 | 3.1 ± 9.9 |
| Interstitial fibrosis n (%) | 2 (8) | 36 (62) | 40 (35) | 11 (34.4) |
All data are reported as the mean ± SD. For the comparisons a one way ANOVA with a Dunn's multiple comparison test was performed with * P < 0.05, ** P < 0.005, *** P < 0.0001 vs. control, ### P < 0.0001 vs. MC, § P < 0.05, §§§ P < 0.0001 vs. DCMi. ACE/AT1, angiotensin‐coverting‐enzyme inhibitor/angiotensin II type 1 receptor antagonists; B19V, parvovirus B19; CD3, infiltrating CD3‐positive lymphocytes; CD45R0, infiltrating CD45R0‐positive lymphocytes; DCM, dilated cardiomyopathy; DCMi, inflammatory dilated cardiomyopathy; EBV, Epstein–Barr virus; HHV, human herpes virus; LFA‐1, lymphocyte function‐associated antigen 1; LFEV, left ventricular ejection fraction; Mac‐1, macrophage‐1 antigen; MC, myocarditis.
Blood samples were collected at the first visit [Time Point 1, (T1)], where also EMB were derived for additional clinical examinations, including histology, immunohistochemistry, and viral diagnosis (EMB of acute MC n = 32, DCMi n = 107, and DCM n = 57). From all latter numerated patients, only extra EMB of n = 15 MC, n = 39 DCMi, and n = 15 DCM patients were available for the analysis of S100A8, S100A9 mRNA expression. In a subgroup of acute MC patients (n = 12), we could study serum S100A8/A9 levels and appropriate EMBs at T1 and a follow‐up investigation (T2), with a mean follow‐up time of 8.5 months. The change in EMB inflammation rate between T1 and T2 was calculated by T2 − T1, which was displayed as Δ. Coronary artery disease was angiographically excluded in all patients. Other causes for systemic or local inflammation had been excluded. All diagnostic procedures and evaluations were obtained by using standardized protocols. 13 All patients provided written consent for the procedures (local ethic vote number: EA2/140/16). The investigation conforms with the principles outlined in the Declaration of Helsinki. 14 Detailed patient's characteristics are summarized in Table 1.
Clinical definition and disease entities
Cardiac inflammation was defined as the evidence of infiltrating lymphocytes (CD3) > 14.0 cells per square millimetre (median cell count), lymphocyte function‐associated antigen 1 (LFA‐1)‐positive cells >14.0 cells per square millimetre (median cell count), CD45R0 > 40.0 cells per square millimetre (median cell count), CTL (perforin) > 2.9 cells per square millimetre (median cell count), and macrophages (Mac‐1) >40.0 cells per square millimetre (median cell count), and positive/negative Dallas criteria in the EMB analyses. 1 , 15 Fifty three percent showed a viral persistence in the MC, 54.5% in the DCMi, and 19% in the DCM group, which included parvovirus B19, human herpes virus 6, enteroviruses, and Epstein–Barr virus. Patients with acute MC were clinically presented with acute onset of angina, ST‐ segment elevation, elevated creatinine kinase or troponin T, or sudden onset of HF or arrhythmias mimicking acute myocardial infarction. Predominantly, focal inflammatory cell infiltrates were confirmed via histology and/or immunohistology. 16 The DCMi group comprised patients with a suspected disease onset >30 days prior to clinical presentation, a DCM phenotype, and an EMB‐proven infiltration of lymphocytes or macrophages. DCM patients presented symptoms of HF, left ventricular (LV) enlargement and global wall motion abnormalities of unknown origin with a documented reduced systolic LV ejection fraction (LVEF) below 45% without any history or clinical signs of MC or myocardial inflammation in EMB. In the controls, EMB failed to elucidate any specific reason of the clinically subjective complaints.
Endomyocardial biopsy
After routine non‐invasive diagnostic work‐up and angiography had failed to elucidate any specific cause of HF, all patients underwent EMB and left heart catheterization in a standardized manner, as previously described. 17 A minimum of eight EMBs was obtained from the left ventricle with a flexible bioptome (Westmed, Germany). Two specimens were fixed in 5% buffered formalin and embedded in paraffin for histologic evaluation while immunohistochemical analyses (2 EMB) were carried out in TissueTec© embedded frozen specimens. 18 , 19 Inflammatory cells [lymphocytes (CD3), LFA‐1, Mac‐1] were counted by quantitative digital imaging analysis. The remaining four to six biopsy specimens were immediately frozen in liquid nitrogen and used for the evaluation of RNA or DNA viruses by real‐time polymerase chain reaction. 20 An additional EMB was used for the evaluation of S100A8 and S100A9 mRNA expression. The EMB diagnosis of MC was based on histomorphologic criteria according to the Dallas classification supported by quantitative immunohistological staining techniques. 18 Patients with focal lymphocytic infiltrations or numbers of CD3‐positive lymphocytic cells exceeding 14 CD3‐positive lymphocytes per square millimetre in the presence of inflammatory cell‐associated myocytolysis were classified as MC patients according to the World Heart Federation, respectively. 1 , 21
RNA isolation, cDNA synthesis, and gene expression analysis
For the analysis of S100A8 and S100A9 mRNA expression, total RNA isolation was performed, followed by cDNA synthesis with the High Capacity Kit (Life Technologies GmbH, Darmstadt, Germany). Because of limited amounts of EMB cDNA, a pre‐amplification technique was applied preceding gene expression analysis, as described before. 22 To assess the relative mRNA expression, real‐time polymerase chain reaction was performed using gene expression assays from Life Technologies for S100A8 (Hs00374264_g1) and S100A9 (Hs00610058_m1). Data were normalized to the housekeeping gene CDKN1b (Hs00153277_m1) as endogenous control and are expressed using the formula 2−Δ Ct.
Immunofluorescence investigations of S100A8 and S100A9 expressing cells
For the identification of S100A8 or S100A9 specific expressing cells, a double staining was performed. Successive Tissue‐Tek OCT embedded human EMB samples were stained with a specific human S100A8 or S100A9 antibody (S100A8 or S100A9 anti‐human rabbit). Subsequently, all tissue samples were stained with a secondary goat anti‐rabbit DyLight 549 antibody (goat anti‐rabbit + DyLight 549, Dianova, Hamburg, Germany). With the help of specific characteristics, congruent areas were identified on the successive slides and enabled the demonstration of co‐expressed S100A8 and S100A9. Furthermore, for the identification of specific S100A8‐expressing cells and S100A9‐expressing cells, the slides were co‐stained with primary antibodies: CD68 anti‐human mouse, Bio SB, Santa Barbara, USA, CD11b anti‐human mouse, Immuno Tools, Friesoythe, Germany, and Ly6g anti‐human mouse, Genetex, Irvive, USA; and secondary antibody goat anti‐mouse + Biotin Dianova.
Serum S100A8/S100A9 levels
Serum S100A8/A9 levels were determined by specific ELISA as described elsewhere. 23
Statistical analyses
A Mann–Whitney U test for non‐normal distributed data comparisons was used. Normal distribution was visually checked by using box plots. For pairwise comparisons, the nonparametric Wilcoxon matched‐pairs signed rank test was used. In case of correlation analyses, the nonparametric Spearman test was utilized with the resulting correlation parameter r, the P value, and the appropriate XY pairs. Univariate linear regression was used in order to calculate the relation between an independent and a dependent ordinal parameter, with the resulting parameters including the regression coefficient r 2 and the P value. To distinguish between several groups, ideal cut‐offs for serum S100A8/A9 were determined by using receiver operating curve analysis. Resulting parameters are sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), the area under the curve (AUC), false positive, and false negative values. A P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA), IBM SPSS Statistics, for Windows (version 22.0; IBM Corp, Armonk, NY), and the statistical programme r (version R 3.5.1, Lucent Technologies, USA).
Results
S100A8/S100A9 serum levels in patients with a recent onset of myocarditis, inflammatory dilated cardiomyopathy, or dilated cardiomyopathy
Patients with a recent onset of MC, which represents an early stage of the pathogenesis, 5 demonstrated a 4.6‐fold (P < 0.0001) increase in S100A8/A9 serum levels vs. controls. ( Figure 1A). S100A8/A9 serum levels were not significantly different between male and female patients (male: 1542 ± 1142 ng/mL vs. female: 2943 ± 2420 ng/mL, P = 0.221) and no difference with respect to the age (≤40 years: 1902 ± 1566 ng/mL vs. >40 years: 1760 ± 1651 ng/mL, P = 0.472). Serum S100A8/A9 levels were increased by 3.0‐fold (P < 0.0001) and 1.8‐fold (P = 0.0005) in DCMi and DCM patients vs. controls, respectively (Figure 1B,C). However, serum S100A8/A9 levels of DCMi and DCM patients were 1.6‐fold (P = 0.018) and 2.5‐fold (P < 0.0001) lower vs. MC ≤ 30 patients (DCMi: 1252 ± 1327 ng/mL vs. MC ≤ 30: 1948 ± 1670 ng/mL, P = 0.018; DCM: 778.7 ± 479 ng/mL vs. MC ≤ 30: 1948 ± 1670 ng/mL, P < 0.0001). Furthermore, representative immunofluorescence S100A8 or S100A9 co‐stainings with specific cells in EMBs of recent onset MC patients, displayed a co‐expression of S100A8 and S100A9 mainly in macrophages/monocytes (positive for CD68 and CD11b), and Ly6g‐positive neutrophils (Figure 2A). In DCMi and DCM patients, EMBs display co‐expression of S100A8 and S100A9 mostly in CD11b‐positive macrophages/monocytes and neutrophils ( Figure 2B,C). Analysing all groups, alarmin levels were independent of viral persistence and did not differ between virus‐negative or virus‐positive patients.
Figure 1.
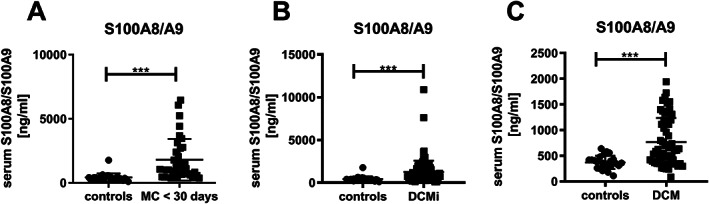
Serum S100A8/S100A9 levels in recent onset myocarditis, and (inflammatory) dilated cardiomyopathy patients. Serum levels of S100A8/A9 (A) in patients with recent onset of myocarditis (n = 32; acute, MC ≤ 30) vs. controls (co; n = 25), (B) in patients with inflammatory dilated cardiomyopathy (n = 112; DCMi) vs. controls (co; n = 25), and (C) in patients with dilated cardiomyopathy (n = 58; DCM) vs. controls (co; n = 25), with ***P < 0.0005 Mann–Whitney test.
Figure 2.
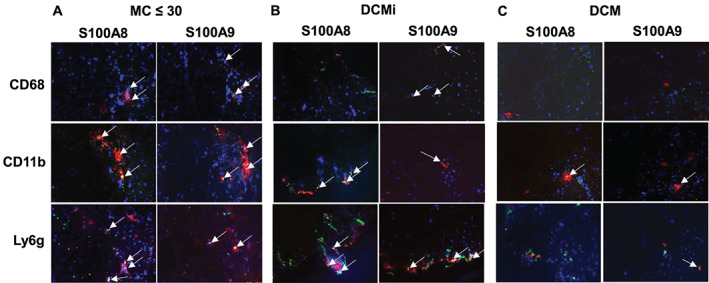
Endomyocardial cell‐specific S100A8 and S100A9 protein co‐expression in recent onset myocarditis, inflammatory dilated cardiomyopathy, and dilated cardiomyopathy. Representative immunofluorescence co‐stainings for S100A8 (red coloured) or S100A9 (red coloured) in macrophages/monocytes (positive for CD68 and CD11b; green coloured), and neutrophils (positive for Ly6g; green coloured) (A) in acute myocarditis patient (MC recent onset <30 days), (B) inflammatory dilated cardiomyopathy (DCMi), and (C) dilated cardiomyopathy (DCM) patient. Arrows highlight co‐stainings.
Correlation between serum levels of S100A8/S100A9, cardiac invaded inflammatory cells, cardiac alarmin expression, and ejection fraction
Only in patients with a recent onset of MC, serum S100A8/A9 levels correlated with the disease activity, represented by EMB‐derived counts of invaded inflammatory cells (CD3: r = 0.486, P = 0.0047, XY pairs = 32, LFA‐1: r = 0.558, P = 0.0009, XY pairs = 32, Mac‐1: r = 0.434, P = 0.013, XY pairs = 32). They also correlated between EMB mRNA S100A8 and S100A9 expression levels (r = 0.541, P = 0.002, XY pairs = 30) as well as with the LVEF (EF: r = 0.498, P = 0.0043, XY pairs = 31) in this group (Figure 3).
Figure 3.
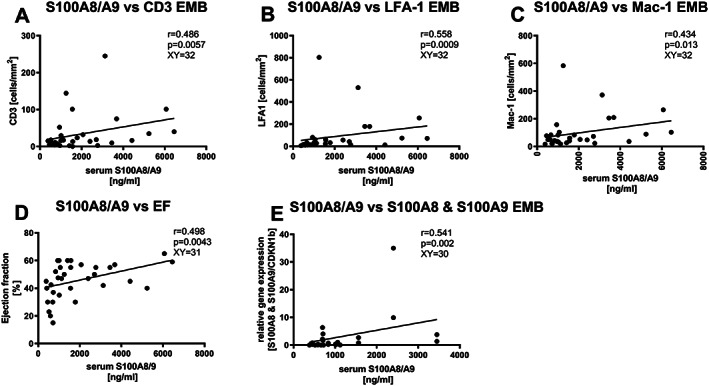
Correlation between serum S100A8/S100A9 and endomyocardial CD3‐, LFA‐1‐, Mac‐1, ejection fraction, S00A8, and S100A9, in patients with a recent onset of myocarditis. Correlation between serum S100A8/A9 levels and endomyocardial biopsy (EMB) presence of (A) CD3‐ (XY pairs n = 32) (B), LFA‐1‐ (XY pairs n = 32), (C) Mac‐1‐positive (XY pairs n = 32) cells, (D) the ejection fraction (EF; XY pairs n = 31), and mRNA expression of (E) S100A8 and S100A9 (XY pairs n = 30). r = Spearman coefficient.
Diagnostic value of S100A8/S100A9 serum levels in patients with a recent onset of myocarditis, inflammatory dilated cardiomyopathy, or dilated cardiomyopathy
Receiver operating curve analyses of S100A8/A9 in MC at early onset (≤30 days) provided a cut‐off value of 583 ng/mL with a specificity of 92%, sensitivity of 90.6%, a PPV of 93.5%, a NPV of 88.5%, a false positive value of 6.3%, a false negative value of 9.3%, and an AUC = 0.949 vs. controls (95% confidence interval (CI) [0.89–1]) (Figure 4A). Intriguingly, at a cut‐off of 425 ng/mL, we showed a high rule out possibility, displayed by a sensitivity of 96.8% (specificity of 72.0%), and at a cut‐off of 777.5 ng/mL, the rule in value was displayed by a high specificity of 96% (sensitivity of 71.8%). Furthermore, also in DCMi and DCM patients, the diagnostic value of serum S100A8/A9 at a cut‐off of 583 ng/mL was determined vs. controls {DCMi: specificity = 92%, sensitivity = 72.3%, a PPV = 97.6%, a NPV = 42.6%, and an AUC = 0.85 (95% CI [0.771–0.928]), DCM: specificity = 92%, sensitivity = 54.4%, a PPV = 93.9%, a NPV = 46.9%, and an AUC = 0.735 (95% CI [0.621–0.848])} (Figure 4B,C).
Figure 4.
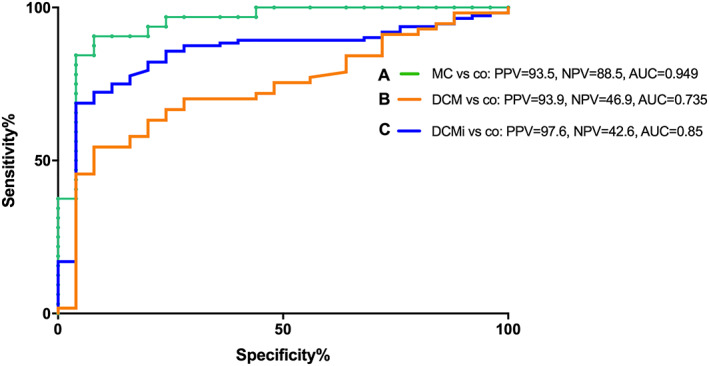
Receiver operating characteristic curves. Receiver operating characteristics (ROC) of serum S100A8/S100A9 levels (cut‐off 583 ng/mL) for A. recent onset myocarditis (MC; n = 32) patients vs. controls (C) for inflammatory dilated cardiomyopathy (DCMi; n = 112) patients vs. controls, and (B) for dilated cardiomyopathy (DCM; n = 58) patients vs. controls. AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value.
Decrease of S100A8/S100A9 serum levels correlates with improvements of endomyocardial inflammation in patients with a recent onset of myocarditis
An additional subgroup analysis of recent onset MC patients, with serum S100A8/A9 and EMB inflammatory marker levels at two different time points T1 and T2 with a mean follow‐up time of 8.5 months (n = 12), allowed us to obtain first evidence regarding serum S100A8/A9 levels as a potential therapy‐monitoring tool. Interestingly, S100A8/A9 serum levels of ≤30 day MC patients decreased significantly after conventional medication treatment (T1: 2208 ± 1843 ng/mL vs. T2: 888.8 ± 513.7 ng/mL; P = 0.0005) (Figure 5A). The decrease of S100A8/A9 was accompanied by a significant decrease of EMB inflammatory marker such as CD3 (T1: 70.02 ± 107.4 cells per square millimetre vs. T2: 59.18 ± 182.5 cells per square millimetre; P = 0.0342), LFA‐1 (T1: 133.5 ± 187.1 cells per square millimetre vs. T2: 74.12 ± 190.5 cells per square millimetre; P = 0.0186), and Mac‐1 (T1: 132.6 ± 129.5 cells per square millimetre vs. T2: 54.41 ± 65.16 cells per square millimetre; P = 0.0015) (Figure 5B–D). Importantly, we found that the changes (Δ: T2 − T1) in serum S100A8/A9 positively correlated with changes of LFA‐1 (r = 0.627, P = 0.04, XY = 11).
Figure 5.
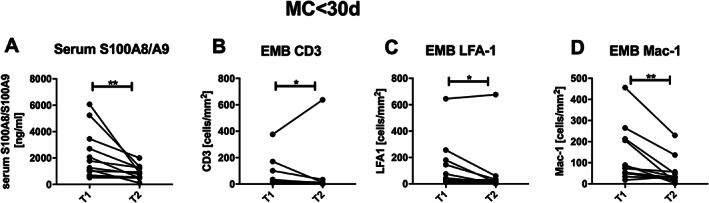
Decrease of serum S100A8/S100A9 is accompanied with the reduction of endomyocardial CD3‐positive cells, LFA‐1‐positive cells, and Mac‐1‐positive cells after conventional heart failure treatment in patients with a recent onset of myocarditis. (A). Serum S100A8/S100A9 levels with n = 12 and endomyocardial biopsy (EMB) present (B) CD3 n = 12, (C) LFA‐1 n = 11, and (D) Mac‐1‐positive cells n = 12, with * P < 0.05 and ** P < 0.005 Wilcoxon test matched pairs. Time Points 1 (T1) and 2 (T2).
Baseline S100A8/S100A9 serum levels as a predictor for changes of endomyocardial inflammation in patients with a recent onset of myocarditis
To assess the predictive capability we performed univariate linear regression analysis. In the model, the Δ of EMB LFA‐1 was used as a dependent variable, while serum S100A8/A9 levels were used as an independent variable. We were able to demonstrate that baseline serum S100A8/A9 levels predicted the change in EMB LFA‐1 (LFA‐1: r 2 = 0.576, P = 0.007, n = 11): the higher the baseline S100A8/A9 serum levels, the higher the change in EMB inflammation.
Discussion
This is the first study to demonstrate the relevance of serum S100A8/A9 levels in patients with a recent onset of MC, as displayed by its association with the disease activity. Furthermore, we have first evidence that serum S100A8/A9 has an additional value as a potential diagnostic and monitoring biomarker in these patients.
The diagnosis of acute MC is a challenge because of its diverse clinical presentation. 1 The position paper of the European Society of Cardiology (ESC) working group on myocardial disease recommends to perform an cMRI in cases of suspected acute MC and to do an EMB in unstable clinical scenarios to initiate an aetiology‐directed therapy. 1 For follow‐up monitoring, a repeated cMRI is discussed as a possible option. The EMB is the gold standard for the diagnosis of patients with suggested MC, allowing characterization of the specific virus and the amount and type of infiltrated immune cells. However, it is invasive, can also be subject to sampling error, and needs experienced operators to perform it safely. 3 Additionally, imaging techniques such as cMRI are expensive and not always available. 3 , 24 Thus, some clinical centres opt out of invasive EMB and/or cost intensive MRI‐based diagnosis, instead utilizing the wait and see strategy. In part, this strategy derives from the notion that approximately 70% of those patients ultimately undergo remission. 25 Caforio et al. 25 and the ESC recommendations 1 stress the need to bridge the gap between clinical and tissue‐based diagnosis of MC, for example by using long‐term prospective data in EMB‐proven acute MC to validate newly proposed non‐invasive biomarkers by multivariable analysis. 25
Serum markers of inflammation, like hsCRP levels, simply signify an inflammatory state and are not cardiac specific, 26 while markers of cardiac dysfunction, like BNP, can be elevated in acute MC, but are also neither sensitive nor specific. 1 , 5 Recently, Liu et al. 27 confirmed the low diagnostic value of hsCRP in suspected MC patients vs. controls, displayed by an AUC of only 0.610, a specificity of 51.85%, and a sensitivity of 58.88%. Cardiac troponin and BNPs are more sensitive markers of myocyte injury and dysfunction in patients with clinically suspected MC than creatinine kinase levels. However, they are non‐specific, and even if levels are normal, they do not exclude MC and bear no relation to the state of cardiac inflammation. 1 Recently, it has been shown that serum soluble ST2 is increased in ≤50 –year‐old male patients but not in female patients with MC and is associated with an increased HF risk in these male patients. 4 However, there are currently no studies describing its diagnostic value in patients with MC. 4 Contemporary cMRI techniques, including T1/T2 mapping, enable a non‐invasive diagnosis of acute MC with high sensitivity, but less‐than‐ideal specificity, which decreases further in chronic settings. 2 , 5 Overall, serum markers of inflammation and cardiac dysfunction can be elevated in acute MC, but are neither sensitive nor specific in terms of determining the presence or absence of active myocardial inflammation. 5
We recently demonstrated that S100A8 and S100A9 mRNA expression is increased in cardiac tissue of human and experimental CVB3‐induced MC. In this study, we demonstrate the significance of measuring serum S100A8/A9 levels for the clinical differential diagnosis of patients with suspected MC. 12 We show a high potential diagnostic value for serum S100A8/A9, which is reflected by the significantly higher serum levels of S100A8/A9 in recent onset MC patients compared with controls. Intriguingly, these patients revealed a positive correlation between alarmin serum levels, EMB inflammation, and cardiac S100A8 and S100A9 mRNA expression. In agreement with the literature, we show that cardiac macrophages/monocytes and neutrophils are the main source of co‐expressed S100A8 and S100A9. We hypothesize that serum S100A8/A9 levels may reflect cardiac inflammation in patients with suspected acute MC, which is most intensive in the early stage of the pathogenesis 5 and is characterized by an ongoing invasion of alarmin‐expressing macrophages, monocytes, and neutrophils. 28 This is in line with findings in patients with active cardiac sarcoidosis. 29 At a cut‐off value of 583 ng/mL, serum S100A8/A9 allowed the correct diagnosis of suspected MC with a high diagnostic accuracy of 94.9%. Only 6.3% were false positive and 9.3% were not detected. Intriguingly, with a high probability of 96% and 96.8%, we can rule in and rule out acute MC patients and thus when a cMRI or an EMB is indicated or not, respectively. The grey zone, which is beyond the probability of 96% and 96.8%, indicates the performance of further testing. In patients with low‐grade cardiac inflammation (DCMi), and in DCM, serum S100A8/A9 levels are lower in comparison with acute MC and may further indicate that the systemic and local inflammatory process is silencing, resolving, and/or not a prominent disease driver. Our first results demonstrate the potential clinical relevance of serum S100A8/A9 in the acute MC phase, which declines in chronic disease states such as DCMi and is not relevant in DCM. In addition, the dynamics of serum alarmin levels indicated by a fall from acute MC to DCMi could serve as an important disease or treatment‐monitoring tool. Intriguingly, in a small patient cohort with a recent onset of MC, our data show that alarmin serum levels and EMB inflammation markers decreased after a mean time of 8.5 months during conventional treatment and were accompanied by a reduction of EMB inflammation. Importantly, the decrease of serum alarmin levels correlated positively with the improvement of myocardial inflammation. The potential value of S100A8/A9 serum levels as a therapy‐monitoring tool is further corroborated by the predictive role of baseline alarmin levels for the change in EMB inflammation. First analyses show that the higher the baseline S100A8/A9 serum levels are the higher the change in EMB inflammation. This relationship between baseline alarmin levels and change in local inflammation has also been shown in patients with a recent onset of rheumatoid arthritis. 30
Strength and limitations
This is the first study to show the relevance of serum S100A8/A9 in EMB‐proven acute MC patients. However, our study has several limitations including the retrospective data acquisition and the small patient number in the MC group. The latter is based on the fact that in patients with acute MC, EMBs are not routinely performed. Furthermore, the small number of cases in our study does not allow for more powerful statistical analysis such as conditional logistic regression, multivariate adjustments, post‐hoc power analysis, and as well as matching for sex and age. Our study is more hypothesis‐generating, and thus, prospective clinical trials with larger cohorts are necessary to verify the importance of serum S100A8/A9 as a diagnostic and monitoring tool. One of the important and unique aims of our study was to investigate the correlation between EMB alarmin expression and its serum levels in well‐characterized MC patients and to learn more about the mode‐of‐action and the involved mechanisms.
Conclusions
Serum S100A8/A9 levels in recent onset MC patients reflect the inflammatory disease activity in cardiac tissue, independent of viral persistence, age, or gender. Additionally, we have first evidence that serum S100A8/A9 could potentially serve as a diagnostic and therapy‐monitoring biomarker in patients with suspected acute MC. The realization of this biomarker in this setting, probably also in combination with others, could improve prognosis by aiding in the rapid diagnosis and treatment of this disease.
Conflict of interest
This research was supported by an independent research grant by Novartis.
MN has been consultant to the IKDT (Institute for Cardiac Diagnosis and Therapy GmbH, Berlin) 2004–2008, and has received honoraria for presentations and/or participated in advisory boards from Abbot, Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Fresenius, Miltenyi Biotech, Novartis, Pfizer, Roche and Zoll.
Funding
This study was supported by the Berlin‐Brandenburg Center for Regenerative Therapies – BCRT (Bundesministerium für Bildung und Forschung – 0313911) to CT, by Novartis, and Berlin Institute of Health.
Acknowledgements
We thank Annika Koschel, who performed the pre‐amplification, Kerstin Puhl, who carried out the immunofluorescence stainings, and Marzena Sosnowski, who was involved in the work‐up of the human tissue. We would like to acknowledge the assistance of the BCRT and Prof. Geraldine Rauch (Institute for Biometry and clinical epidemiology) for the statistical analyses.
Müller, I. , Vogl, T. , Kühl, U. , Krannich, A. , Banks, A. , Trippel, T. , Noutsias, M. , Maisel, A. S. , van Linthout, S. , and Tschöpe, C. (2020) Serum alarmin S100A8/S100A9 levels and its potential role as biomarker in myocarditis. ESC Heart Failure, 7: 1442–1451. 10.1002/ehf2.12760.
Sophie Van Linthout, PhD, and Carsten Tschöpe, MD, contributed equally to the study.
References
- 1. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. European Society of Cardiology Working Group on M and Pericardial D. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 2. Van Linthout S, Tschope C. Viral myocarditis: a prime example for endomyocardial biopsy‐guided diagnosis and therapy. Curr Opin Cardiol 2018; 33: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tschope C, Cooper LT, Torre‐Amione G, Van Linthout S. Management of myocarditis‐related cardiomyopathy in adults. Circ Res 2019; 124: 1568–1583. [DOI] [PubMed] [Google Scholar]
- 4. Coronado MJ, Bruno KA, Blauwet LA, Tschope C, Cunningham MW, Pankuweit S, van Linthout S, Jeon ES, McNamara DM, Krejci J, Bienertova‐Vasku J, Douglass EJ, Abston ED, Bucek A, Frisancho JA, Greenaway MS, Hill AR, Schultheiss HP, Cooper LT Jr, Fairweather D. Elevated sera sST 2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc 2019; 8: e008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis‐‐diagnosis, treatment options, and current controversies. Nat Rev Cardiol 2015; 12: 670–680. [DOI] [PubMed] [Google Scholar]
- 6. Pruenster M, Vogl T, Roth J, Sperandio M. S100A8/A9: from basic science to clinical application. Pharmacol Ther 2016; 167: 120–131. [DOI] [PubMed] [Google Scholar]
- 7. Vogl T, Stratis A, Wixler V, Voller T, Thurainayagam S, Jorch SK, Zenker S, Dreiling A, Chakraborty D, Frohling M, Paruzel P, Wehmeyer C, Hermann S, Papantonopoulou O, Geyer C, Loser K, Schafers M, Ludwig S, Stoll M, Leanderson T, Schultze JL, Konig S, Pap T, Roth J. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest 2018; 128: 1852–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tweehuysen L, den Broeder N, van Herwaarden N, Joosten LAB, van Lent PL, Vogl T, van den Hoogen FHJ, Thurlings RM, den Broeder AA. Predictive value of serum calprotectin (S100A8/A9) for clinical response after starting or tapering anti‐TNF treatment in patients with rheumatoid arthritis. RMD Open 2018; 4: e000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cotoi OS, Duner P, Ko N, Hedblad B, Nilsson J, Bjorkbacka H, Schiopu A. Plasma S100A8/A9 correlates with blood neutrophil counts, traditional risk factors, and cardiovascular disease in middle‐aged healthy individuals. Arterioscler Thromb Vasc Biol 2014; 34: 202–210. [DOI] [PubMed] [Google Scholar]
- 10. Muller F, Froland SS, Aukrust P, Fagerhol MK. Elevated serum calprotectin levels in HIV‐infected patients: the calprotectin response during ZDV treatment is associated with clinical events. J Acquir Immune Defic Syndr 1994; 7: 931–939. [PubMed] [Google Scholar]
- 11. Preiss JC, Bokemeyer B, Buhr HJ, Dignass A, Hauser W, Hartmann F, Herrlinger KR, Kaltz B, Kienle P, Kruis W, Kucharzik T, Langhorst J, Schreiber S, Siegmund B, Stallmach A, Stange EF, Stein J. Hoffmann JC and German Society of G. [Updated German clinical practice guideline on "Diagnosis and treatment of Crohn's disease" 2014]. Z Gastroenterol 2014; 52: 1431–1484. [DOI] [PubMed] [Google Scholar]
- 12. Muller I, Vogl T, Pappritz K, Miteva K, Savvatis K, Rohde D, Most P, Lassner D, Pieske B, Kuhl U, Van Linthout S, Tschope C. Pathogenic role of the damage‐associated molecular patterns S100A8 and S100A9 in Coxsackievirus B3‐induced myocarditis. Circ Heart Fail 2017; 10. [DOI] [PubMed] [Google Scholar]
- 13. Angelow A, Weitmann K, Schmidt M, Schwedler S, Vogt H, Havemann C, Staudt A, Felix SB, Stangl K, Klingel K, Kandolf R, Kuhl U, Lassner D, v Schlippenbach J, Schultheiss HP, Hoffmann W. The German Transregional Collaborative Research Centre 'Inflammatory Cardiomyopathy‐‐Molecular Pathogenesis and Therapy'. Methods and baseline results from a 3‐centre clinical study. Cardiology 2009; 113: 222–230. [DOI] [PubMed] [Google Scholar]
- 14. Rickham PP. Human experimentation. Code of Ethics of the World Medical Association Declaration of Helsinki. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lassner D, Siegismund CS, Kuhl U, Rohde M, Stroux A, Escher F, Schultheiss HP. CCR5del32 genotype in human enteroviral cardiomyopathy leads to spontaneous virus clearance and improved outcome compared to wildtype CCR5. J Transl Med 2018; 16: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noutsias M, Pauschinger M, Poller WC, Schultheiss HP, Kuhl U. Immunomodulatory treatment strategies in inflammatory cardiomyopathy: current status and future perspectives. Expert Rev Cardiovasc Ther 2004; 2: 37–51. [DOI] [PubMed] [Google Scholar]
- 17. Tschope C, Kherad B, Schultheiss HP. How to perform an endomyocardial biopsy? Turk Kardiyol Dern Ars 2015; 43: 572–575. [DOI] [PubMed] [Google Scholar]
- 18. Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987; 18: 619–624. [DOI] [PubMed] [Google Scholar]
- 19. Kuhl U, Noutsias M, Seeberg B, Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart 1996; 75: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pauschinger M, Rutschow S, Chandrasekharan K, Westermann D, Weitz A, Peter Schwimmbeck L, Zeichhardt H, Poller W, Noutsias M, Li J, Schultheiss HP, Tschope C. Carvedilol improves left ventricular function in murine coxsackievirus‐induced acute myocarditis association with reduced myocardial interleukin‐1beta and MMP‐8 expression and a modulated immune response. Eur J Heart Fail 2005; 7: 444–452. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen TS, Nyengaard JR, Moller J, Banner J, Nielsen LP, Baandrup UT. Quantitative diagnosis of lymphocytic myocarditis in forensic medicine. Forensic Sci Int 2014; 238: 9–15. [DOI] [PubMed] [Google Scholar]
- 22. Kuhl U, Lassner D, Dorner A, Rohde M, Escher F, Seeberg B, Hertel E, Tschope C, Skurk C, Gross UM, Schultheiss HP, Poller W. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res Cardiol 2013; 108: 372. [DOI] [PubMed] [Google Scholar]
- 23. Wieland CW, Vogl T, Ordelman A, Vloedgraven HG, Verwoolde LH, Rensen JM, Roth J, Boer J, Hessels J. Myeloid marker S100A8/A9 and lymphocyte marker, soluble interleukin 2 receptor: biomarkers of hidradenitis suppurativa disease activity? Br J Dermatol 2013; 168: 1252–1258. [DOI] [PubMed] [Google Scholar]
- 24. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018; 72: 3158–3176. [DOI] [PubMed] [Google Scholar]
- 25. Caforio ALP, Malipiero G, Marcolongo R, Iliceto S. Clinically suspected myocarditis with pseudo‐infarct presentation: the role of endomyocardial biopsy. J Thorac Dis 2017; 9: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ridker PM, Luscher TF. Anti‐inflammatory therapies for cardiovascular disease. Eur Heart J 2014; 35: 1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Huang X, Liu Y, Li D, Zhang J, Yang L. Application value of hypersensitive C‐reactive protein, lactic acid and myoglobin in the combined detection of myocarditis. Exp Ther Med 2019; 17: 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogl T, Gharibyan AL, Morozova‐Roche LA. Pro‐inflammatory S100A8 and S100A9 proteins: self‐assembly into multifunctional native and amyloid complexes. Int J Mol Sci 2012; 13: 2893–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terasaki F, Fujita M, Shimomura H, Tsukada B, Otsuka K, Otsuka K, Katashima T, Ikemoto M, Kitaura Y. Enhanced expression of myeloid‐related protein complex (MRP8/14) in macrophages and multinucleated giant cells in granulomas of patients with active cardiac sarcoidosis. Circ J 2007; 71: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 30. Andres Cerezo L, Mann H, Pecha O, Plestilova L, Pavelka K, Vencovsky J, Senolt L. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent‐onset rheumatoid arthritis. Arthritis Res Ther 2011; 13: R122. [DOI] [PMC free article] [PubMed] [Google Scholar]


