Abstract
A 19‐year‐old woman with no previous cardiac history was admitted to the hospital with third‐degree atrioventricular block and left ventricular dysfunction. Her condition quickly deteriorated to severe biventricular failure and cardiogenic shock requiring mechanical circulatory support. An endomyocardial biopsy revealed lymphocytic myocarditis with no PCR‐detectable viral genomes, with CD8 T‐cell predominance and pro‐inflammatory macrophage expansion shown by myocardial flow cytometry. The therapy consisted of immunosuppression (high‐dose methylprednisolone) and temporary mechanical circulatory support with enhanced ability to achieve left ventricular unloading by combination of extracorporeal membrane oxygenation with Impella (ECMELLA). After 2 weeks of support, complete and sustained recovery from myocarditis was observed.
Keywords: Fulminant myocarditis, Immunosuppression, Mechanical circulatory support, Extracorporeal membrane oxygenation (ECMO)
Introduction
Fulminant myocarditis (FM) is a rare and severe form of myocarditis that presents with sudden onset of acute heart failure, cardiogenic shock, and/or life‐threatening arrhythmias. FM may be viral, bacterial, toxic, or autoimmune in origin. 1 , 2 The damage of the myocardium may result from direct cardiomyocyte insult or from indirect injury by immune‐mediated cytotoxicity or tissue oedema. The diagnosis and treatment of FM are still a clinical challenge owing to variability of clinical presentation and limited use of myocardial biopsy. The management often requires intensive care, inotropic and mechanical circulatory support (MCS), and sometimes immunosuppressive therapy. Besides supporting circulation, unloading of the left ventricle by MCS may lead to additional disease‐modifying effects over time that can be important for enhancing myocardial recovery. 3
Case report
We report a case of a 19‐year‐old woman with past medical history of atopic eczema and asthma (chronically treated with desloratadine, montelukast, and salmeterol/fluticasone) who presented to a regional hospital after 2 days of gastrointestinal symptoms (diarrhoea and vomiting) with third‐degree atrioventricular block, left ventricular (LV) systolic dysfunction (LV ejection fraction 40%), elevated cardiac biomarkers (high‐sensitivity troponin T 13 850 ng/L and N terminal pro brain natriuretic peptide 19 686 ng/L). The condition progressed quickly to cardiogenic shock requiring inotropes, temporary transvenous pacing, and tracheal intubation. Coronary angiography showed patent arteries. Within the next day, her status further deteriorated with development of severe biventricular dysfunction, widening of QRS complex (Figure 1A ), and multiple organ failure. She was transferred to our centre for further management. Urgent transfemoral veno‐arterial extracorporeal membrane oxygenation (VA ECMO) was introduced. Global LV ejection fraction dropped to <10% with further QRS widening (Figure 1B ).
Figure 1.
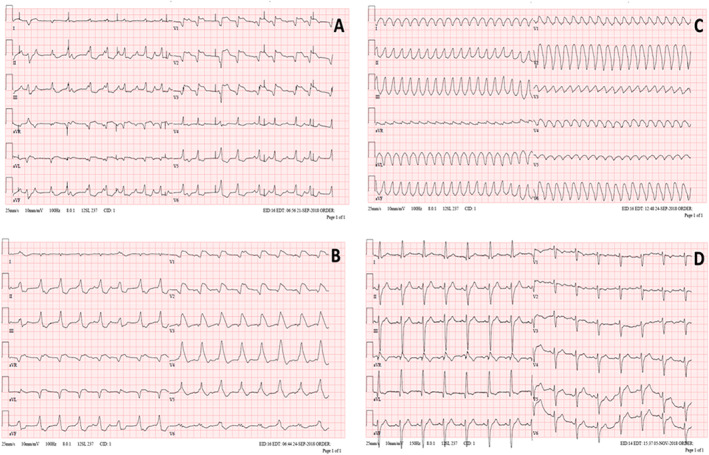
Twelve‐lead electrocardiograms (ECG) (A) at presentation to the hospital, with temporary transvenous pacemaker spikes; (B) prior to ECMO implantation; (C) sustained monomorphic ventricular tachycardia during further deterioration; (D) prior discharge from the hospital.
Right ventricular endomyocardial biopsy (EMB) revealed diffuse lymphocytic myocarditis, resembling severe cellular allograft rejection, with interstitial oedema and mild myocyte necrosis (Figure 2 ). PCR for detection of viral pathogens in the EMB specimen was negative (adenoviruses, enteroviruses, coxsackieviruses, parvoviruses, influenza A and B, herpes simplex virus 1 and 2, human herpes virus 6, varicella zoster virus, Epstein–Barr virus, and cytomegalovirus).
Figure 2.
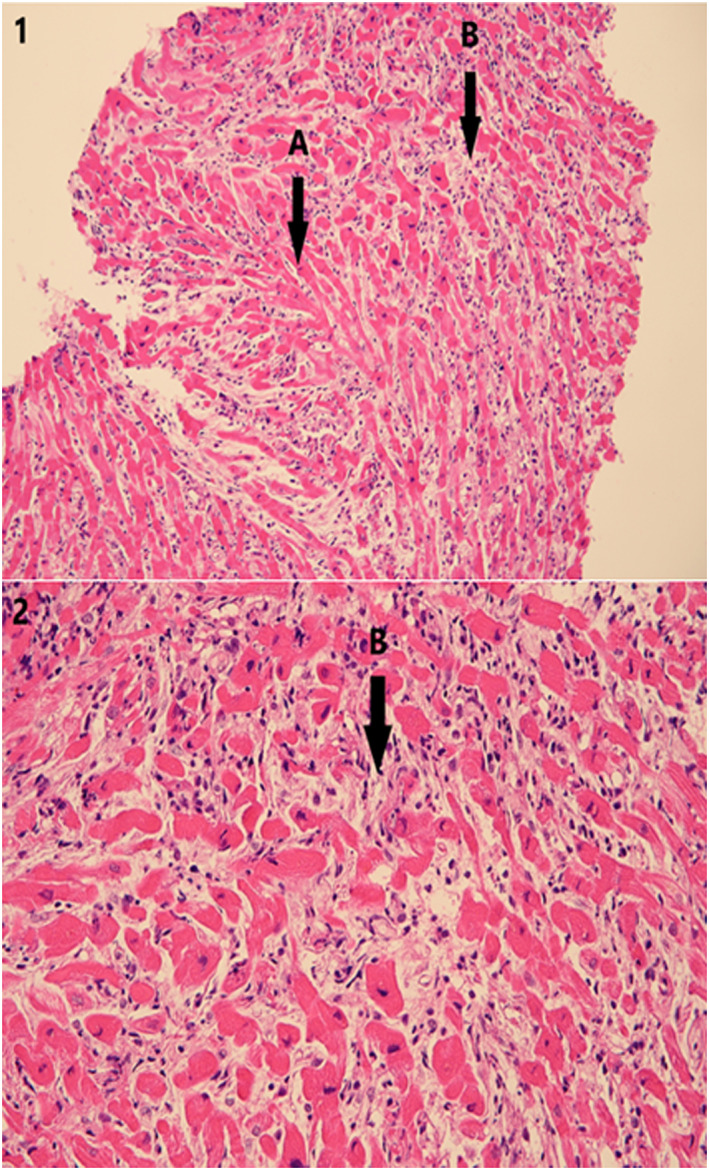
Lymphocytic myocarditis. Haematoxylin–eosin, original magnification (i) ×200 and (2) ×400. (A) Normal myocardial fibres. (B) Myocardial necrosis with interstitial inflammatory oedema and infiltrate, mostly lymphocytic.
To comprehensively characterize infiltrating leucocyte subsets, multiparameter flow cytometry (FACS) analysis of EMB was performed in Dr. Čiháková's lab at Johns Hopkins University in Baltimore, MD, USA. Detailed description of the method is provided in the Supporting Information. Samples were shipped deep‐frozen and were processed by cutting, digestion (collagenase II and DNase I), and mechanical dissociation using gentleMACS (Miltenyi Biotec). Cells were filtered, re‐suspended, and counted. Surface immunostaining was performed using standard protocols using fluorochrome‐labelled antibodies (eBiosciences, BD Biosciences, and BioLegend). Markers used for immunostaining were as follows: CD45, TCRαβ, CD4, CD8, CXCR3, CCR6, CCR7, CCR2, IL23R, CD11b, CD66b, HLA‐DR, CD16, CD14, CD68, and CD19. FACS data were acquired with an LSRFortessa and analysed with FlowJo v10.4 (Tree Star). Viability of cells based on competence of plasma membrane was ~12%. As a control, a similar analysis was performed in an autopsy specimen obtained from an individual without cardiac disease.
In the myocarditis EMB, we found a shift in the CD4+/CD8+ T‐cell ratio of 1:3 as compared with 3:1 in the control, suggesting a predominant cytotoxic CD8 response (Figure 3A ). In both CD4 and CD8 compartments, there was a change from the predominant naïve/central memory T‐cell status (CCR7+CXCR3+) observed in the control toward a predominant effector/effector memory phenotype (CCR7negCXCR3+ CD4 T cells and CCR7negCXCR3neg CD8 T cells) (Figure 3B, C ), demonstrating activation of myocardium‐infiltrating T‐cell clones. We also observed expansion and functional changes in the myocardial myeloid compartment. In the autopsy control, the macrophages were almost exclusively CD14+CD16+ macrophages, whereas in myocarditis EMB sample, we found mainly pro‐inflammatory CD14int/+CD16neg macrophages (Figure 3D ). The latter suggests a pro‐inflammatory role of macrophages in supporting the T‐cell response. Because of clear evidence of immune‐mediated origin, immunosuppression with 1 g/day (in 3 days) of methylprednisolone (Solu‐Medrol, Pfizer) and intravenous immunoglobulin G (0.5 g/kg of body weight; Privigen, Behring) was instituted.
Figure 3.
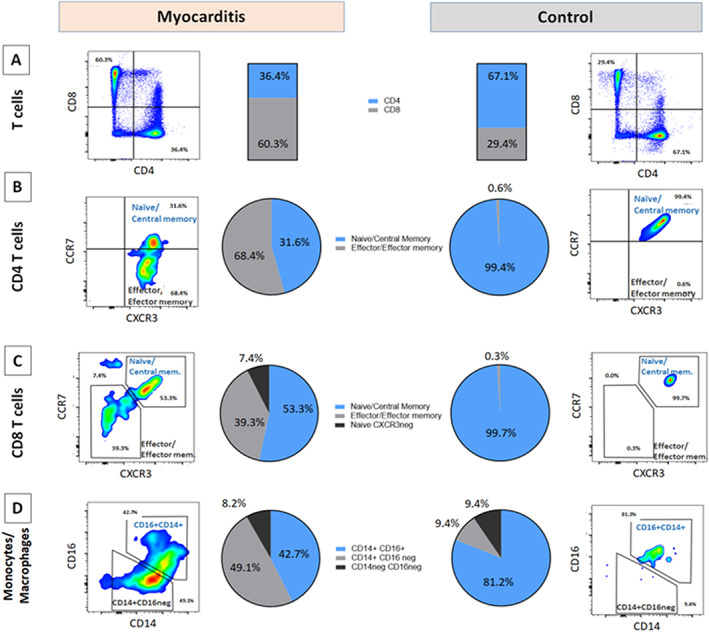
Composition and activation status of intramyocardial leucocyte compartments. Flow cytometry from endomyocardial biopsy (EMB) tissue shows (A) CD4/CD8 balance in myocarditis EMB vs. autopsy control (gated on CD3+CD11bnegCD19neg viable cells), showing a reversed CD4/CD8 ratio in myocarditis. (B, C) Activation status of CD4 T cells and CD8 T cells, respectively, based on CCR7/CXCR3 status, showing in both subsets a shift toward a predominant CCR7neg effector status in myocarditis EMB. (D) Functional subsets of heart infiltrating macrophage/monocytes (gated on CD11b+CD19neg CD3 should not be in normal case (not in superscript)negCD66bneg viable cells), showing a predominant pro‐inflammatory phenotype CD14int/+CD16neg in myocarditis EMB.
Two days after ECMO implantation, the patient developed episodes of refractory sustained ventricular tachycardia (rate 224 b.p.m.) requiring cardioversion (Figure 1C ), accompanied by complete LV akinesis and worsening pulmonary gas exchange. In order to achieve better venting of stagnating LV cavity, Impella 2.5 (Abiomed) pump was introduced transfemorally across the aortic valve. With such ‘ECMELLA’ (ECMO + Impella) configuration, 3 prompt stabilization was achieved. Immediately after ECMELLA, ventricular tachycardia ceased and QRS complex narrowed (Figure 1D ). Owing to continued LV dysfunction after several days, Impella 2.5 was exchanged for Impella 5.0 device introduced via a graft sewn on right subclavian artery (Figure 4 ), a device that has longer durability and full‐flow support capability. Immunosuppression was continued with oral prednisone 1 mg/kg/day. On Day 10, echocardiogram showed marked improvement of systolic function of ventricles (left ventricular ejection fraction > 60%). ECMO was discontinued on the Day 11, Impella was explanted on the Day 13, and the patient remained haemodynamically stable. Further recovery was uneventful, and the patient was discharged after 44 days. At the discharge, patient had normal cardiac function by echocardiography and cardiac magnetic resonance imaging, without evidence of late gadolinium enhancement, with normal BNP and troponin levels, and with incomplete right bundle branch block in electrocardiogram. Corticosteroids were gradually tapered off, and 360 days after discharge, the patient is in stable condition, back to school, with 2.5 mg/day of bisoprolol as the only cardiac medication.
Figure 4.
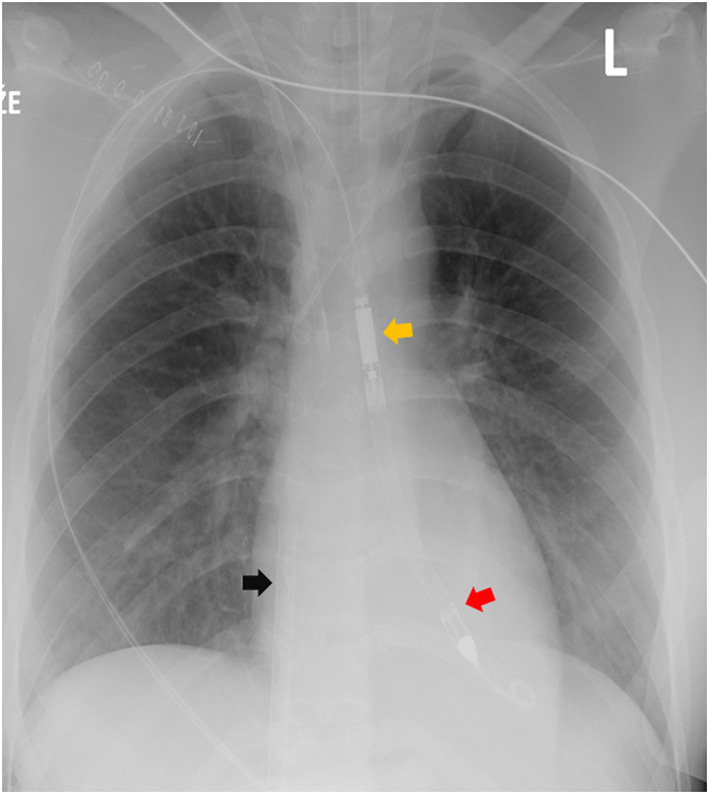
Chest radiogram showing venous cannula of extracorporeal membrane oxygenation (ECMO) circuit (black arrow), Impella 5.0 inflow segment in the left ventricle (red arrow), and outflow segment in aorta (yellow arrow).
Discussion
Older studies suggested that patients with more severe presentation of myocarditis are more likely to improve cardiac function over time, 1 but recent reports contradicted the finding. 4 Even in patients with lymphocytic myocarditis, which is considered as more benign variant of FM, event rates are still high especially if associated with LV dysfunction or ventricular tachyarrhythmia. 5 Early institution of MCS and early myocardial biopsy to ascertain prognosis and to guide immunosuppression is critical to successful management of FM. 3 , 6
While the role of immunosuppression in lymphocytic FM is not yet fully defined due to lack of randomized human studies, it may be considered if acute viral infection in the biopsy is ruled out and if there is high likelihood of autoimmune aetiology, for example in patients with pre‐existing autoimmune disease. 2 In addition to conventional histology, we used multichannel flow cytometry of myocardial sample to better the nature of myocardial inflammation in our patient. We found striking evidence of CD8 T‐cell predominance, accompanied by activation status of the entire T lymphoid compartment. The finding resembled acute cellular allograft rejection, a condition responsive to high‐dosed course of methylprednisolone, which was also successfully used in our case. Interestingly, CD14int/+CD16neg macrophages were also involved in the inflammatory milieu that may contribute to the self‐sustained inflammatory process in situ, supporting the important role of macrophages in the development of myocarditis. 7 The presence of normal cardiovascular magnetic resonance (CMR) image in biopsy‐proven myocarditis can be explained by an interval between the biopsy and CMR imaging that were performed already after administration of immunosuppressive therapy and after resolution of LV dysfunction. The absence of late gadolinium enhancement on CMR is consistent with lack of scar development in some patients with myocarditis. 8
Temporary MCS using ECMO increases the chances of recovery/long‐term survival for patients with the most dramatic presentations. However, ECMO is often not enough to achieve decompression of stagnating left ventricle, 4 , 9 which may lead to lung oedema, LV intracavitary thrombus, and impaired myocardial tissue drainage. 3 From the methods for unloading of ECMO‐supported left ventricle, the choice of intraaortic balloon pump, Impella, or direct surgical LV venting depends on the individual clinical settings, risk of the intervention, local experience, and the time expected for recovery. 10 We chose Impella pump because of its feasibility, effective lowering of LV intracavitary pressures, 3 and capability of almost complete support. The dislodgment and intravascular haemolysis are the potential risks of this device.
Our case suggests that adding Impella to ECMO (ECMELLA concept) might enhance LV cavity drainage, which can be important for enhancing myocardial recovery/remission. 11 Rapid resolution of QRS prolongation, followed by improvement of LV dysfunction after adding Impella to ECMO support, suggests a possible beneficial role of low end‐diastolic LV pressure, with diminished wall tension, enhanced interstitial fluid clearance, and higher perfusion gradient for resolution of myocardial inflammation. 3 , 6 High‐flow Impella 5.0 is also very useful for weaning VA‐ECMO circuit, and it allows patient rehabilitation in case of longer support.
In summary, our case illustrates that intense haemodynamic unloading with ECMO and Impella combined with immunosuppressive therapy may offer exciting new therapeutic ability to successfully manage even life‐threatening myocardial inflammation.
Conflict of interest
None declared.
Funding
This work was supported by the Ministry of Health of the Czech Republic (grant AZV 17‐28784A awarded to V.M.). All rights reserved.
Supporting information
Data S1. Supporting Information
Jurcova, I. , Rocek, J. , Bracamonte‐Baran, W. , Zelizko, M. , Netuka, I. , Maluskova, J. , Kautzner, J. , Cihakova, D. , Melenovsky, V. , and Maly, J. (2020) Complete recovery of fulminant cytotoxic CD8 T‐cell‐mediated myocarditis after ECMELLA unloading and immunosuppression. ESC Heart Failure, 7: 1976–1981. 10.1002/ehf2.12697.
Ivana Jurcova, Jan Rocek, Vojtech Melenovsky, and Jiri Maly contributed equally to this work.
References
- 1. McCarthy RE 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long‐term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. New Engl J Med 2000; 342: 690–695. [DOI] [PubMed] [Google Scholar]
- 2. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648. [DOI] [PubMed] [Google Scholar]
- 3. Tschope C, Van Linthout S, Klein O, Mairinger T, Krackhardt F, Potapov EV, Schmidt G, Burkhoff D, Pieske B, Spillmann F. Mechanical unloading by fulminant myocarditis: LV‐IMPELLA, ECMELLA, BI‐PELLA, and PROPELLA concepts. J Cardiovasc Transl Res 2019; 12: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ammirati E, Cipriani M, Lilliu M, Sormani P, Varrenti M, Raineri C, Petrella D, Garascia A, Pedrotti P, Roghi A, Bonacina E, Moreo A, Bottiroli M, Gagliardone MP, Mondino M, Ghio S, Totaro R, Turazza FM, Russo CF, Oliva F, Camici PG, Frigerio M. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation 2017; 136: 529–545. [DOI] [PubMed] [Google Scholar]
- 5. Ammirati E, Veronese G, Brambatti M, Merlo M, Cipriani M, Potena L, Sormani P, Aoki T, Sugimura K, Sawamura A, Okumura T, Pinney S, Hong K, Shah P, Braun O, Van de Heyning CM, Montero S, Petrella D, Huang F, Schmidt M, Raineri C, Lala A, Varrenti M, Foa A, Leone O, Gentile P, Artico J, Agostini V, Patel R, Garascia A, Van Craenenbroeck EM, Hirose K, Isotani A, Murohara T, Arita Y, Sionis A, Fabris E, Hashem S, Garcia‐Hernando V, Oliva F, Greenberg B, Shimokawa H, Sinagra G, Adler ED, Frigerio M, Camici PG. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2019; 74: 299–311. [DOI] [PubMed] [Google Scholar]
- 6. Tschope C, Cooper LT, Torre‐Amione G, Van Linthout S. Management of myocarditis‐related cardiomyopathy in adults. Circ Res 2019; 124: 1568–1583. [DOI] [PubMed] [Google Scholar]
- 7. Hou X, Chen G, Bracamonte‐Baran W, Choi HS, Diny NL, Sung J, Hughes D, Won T, Wood MK, Talor MV, Hackam DJ, Klingel K, Davogustto G, Taegtmeyer H, Coppens I, Barin JG, Cihakova D. The cardiac microenvironment instructs divergent monocyte fates and functions in myocarditis. Cell Rep 2019; 28: 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdel‐Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz‐Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005; 45: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 9. Ginsberg F, Parrillo JE. Fulminant myocarditis. Crit Care Clin 2013; 29: 465–483. [DOI] [PubMed] [Google Scholar]
- 10. Donker DW, Brodie D, Henriques JPS, Broome M. Left ventricular unloading during veno‐arterial ECMO: a simulation study. ASAIO J 2019; 65: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spillmann F, Van Linthout S, Schmidt G, Klein O, Hamdani N, Mairinger T, Krackhardt F, Maroski B, Schlabs T, Soltani S, Anker S, Potapov EV, Burkhoff D, Pieske B, Tschope C. Mode‐of‐action of the PROPELLA concept in fulminant myocarditis. Eur HeartJ 2019; 40: 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


