Abstract
Aims
Although tolvaptan has been reported to prevent worsening renal function (WRF) in patients with advanced acute heart failure (AHF), evidence regarding the effect of tolvaptan on renal function in patients with new‐onset AHF is not available. This study aimed to investigate the renoprotective effect of tolvaptan in patients hospitalized with new‐onset AHF.
Methods and results
A total of 122 consecutive patients hospitalized with new‐onset AHF between May 2015 and December 2018 were retrospectively evaluated. WRF was defined as an absolute increase in serum creatinine ≥0.3 mg/dL (≥26.4 μmol/L) within 48 h or a 1.5‐fold increase in serum creatinine after hospitalization. The furosemide group (n = 75) and the tolvaptan add‐on group (n = 47) were compared. The tolvaptan group consists of patients who received tolvaptan as an individual physicians' decision. The incidence of WRF was significantly lower in the tolvaptan add‐on group (8.5%) than in the furosemide group (24.0%, P = 0.03). Multivariate logistic regression analysis revealed that tolvaptan treatment was an independent variable related to the prevention of WRF [odds ratio (OR), 0.20; 95% confidence interval (CI), 0.05–0.85]. Furthermore, subgroup analysis revealed a more favourable effect of tolvaptan in patients with serum creatinine ≥1.1 mg/dL on admission (OR, 0.23; 95% CI, 0.06–0.98) and an ejection fraction <50% (OR, 0.19; 95% CI, 0.04–0.90).
Conclusions
A lower incidence of WRF was observed in patients with new‐onset AHF who were treated with the tolvaptan add‐on therapy, specifically those with left ventricular systolic dysfunction and renal impairment on admission.
Keywords: Acute heart failure, Worsening renal function, Tolvaptan
Introduction
Fluid retention and congestion are major causes of acute heart failure (AHF). Loop diuretics have been used as the first line of medication for AHF; however, their use leads to high prevalence of worsening renal function (WRF).1, 2 WRF is associated with a poor long‐term prognosis. According to a meta‐analysis, the occurrence of WRF during AHF hospitalization was reported to contribute to a 1.75‐fold increase in all‐cause mortality.3 The underlying mechanisms of WRF in AHF include a reduction in renal blood flow and an elevation in the central venous pressure.4 However, it is difficult to lower the central venous pressure without reducing the renal blood flow. Tolvaptan, an oral vasopressin type 2 receptor antagonist, has emerged as a new therapeutic option for AHF, and its favourable effects on renal function have been reported in patients with advanced AHF.5, 6, 7, 8, 9, 10 However, little is known regarding the effects of tolvaptan on renal function in the early stage of AHF. Accordingly, we investigated the role of tolvaptan in preventing WRF in patients with new‐onset AHF.
Methods
Study population
A total of 122 consecutive patients hospitalized due to new‐onset AHF between May 2015 and December 2018 were retrospectively studied. Patients with the following characteristics were included in the study: patients with New York Heart Association functional class III or IV on admission, patients aged ≤85 years, and patients receiving less than a 40 mg dose of furosemide before hospitalization. According to the Framingham criteria, AHF was diagnosed with at least two major criteria or one major criterion in conjunction with two minor criteria was met.11 Patients with acute coronary syndrome or those already receiving tolvaptan before hospitalization were excluded in this study. All patients were initially treated with intravenous furosemide on admission. Subsequently, tolvaptan add‐on treatment was initiated in patients who did not meet the following criteria within 3 days after hospitalization: improvement of dyspnoea, decrease in pleural effusion by X‐ray, or decrease in body weight (Figure 1 ). The dose of tolvaptan and timing of initiation of drug therapy after hospitalization were dependent on the physician's decision. Standard medical therapy for AHF including spironolactone, beta‐blockers, angiotensin‐converting enzyme inhibitors, or angiotensin II receptor blockers was prescribed according to the patient's condition during hospitalization.12 Additionally, multidisciplinary care management including cardiac rehabilitation, patient education, medical treatment optimization, and psychosocial support was provided during hospitalization. WRF was defined as an absolute increase in serum creatinine ≥0.3 mg/dL (≥26.4 μmol/L) within 48 h or a 1.5‐fold increase in serum creatinine after hospitalization.13 Patients were divided into the following two groups: the tolvaptan add‐on group (the tolvaptan group) and the non‐tolvaptan group (the furosemide group). The primary endpoint was defined as the presence of WRF during hospitalization.
Figure 1.
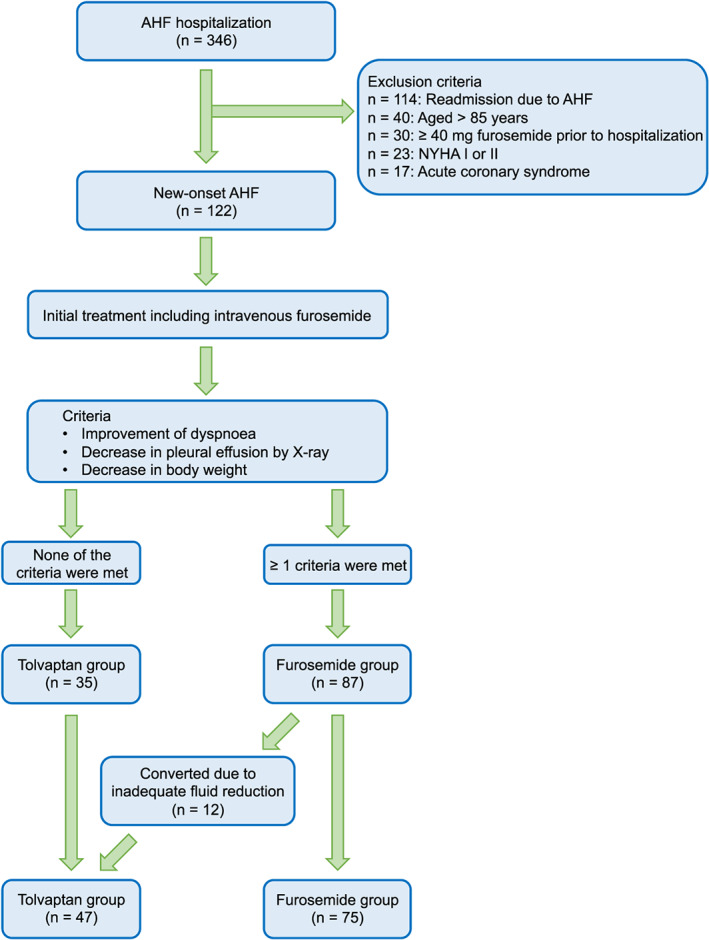
Study flow chart. AHF, acute heart failure; NYHA, New York Heart Association functional.
Data collection
Data on baseline patient characteristics, previous medical history, and pre‐hospitalization medications and discharge medications were obtained from the patients' medical records. Transthoracic echocardiography was performed by expert sonographers on admission, and left ventricular ejection fraction was calculated by using the modified Simpson method.14, 15 Image quality of transthoracic echocardiography was comparable in both groups.16 The loop diuretic doses were converted to furosemide equivalents according to the previous reports.17, 18 Systolic and diastolic blood pressures were recorded at admission, 24 h after admission and on Day 7. Body weight on admission and at discharge was measured to assess the effectiveness of the treatment on fluid reduction. The investigation conformed with the principles outlined in the Declaration of Helsinki.19 All patients provided written informed consent for inclusion in the study. The study protocol was approved by the Ethics Committee of Kansai Medical University Medical Center and was registered at the University Hospital Medical Information Network clinical trial registry (ID: UMIN000036417).
Statistical analyses
Continuous variables are presented as the means ± standard deviations, and categorical variables are presented as numbers and percentages. Differences between the two groups were analysed using the Student's t‐test for continuous variables and the χ2 test for categorical variables. A P value <0.05 was considered significant. Body weight between the two groups was compared using the analysis of covariance adjusted for body weight on admission. Multivariate logistic regression analysis was performed to detect important variables related to WRF. Subgroup analysis was performed for subgroups pre‐specified before the initiation of the study. JMP 14.2.0 software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patient characteristics on admission
The flow chart of studied patients is shown in Figure 1 . Forty‐seven patients were included in the tolvaptan group, and 75 patients were included in the furosemide group. None of the patients in this study died in the hospital. There was no significant difference in the mean hospital stay between the tolvaptan group (24 ± 12 days) and the furosemide group (20 ± 12 days, P = 0.09). Thirty‐five patients in the tolvaptan group initiated tolvaptan treatment within 3 days of hospitalization, whereas 12 patients who had initially good response to furosemide initiated tolvaptan >3 days after hospitalization because of subsequent inadequate fluid reduction. The maximum daily dose of tolvaptan was 7.0 ± 3.4 mg. Patient characteristics on admission are shown in Table 1. Blood urea nitrogen, creatinine, and B‐type natriuretic peptide levels were significantly higher in the tolvaptan group than in the furosemide group. There were no significant differences in the dose of furosemide before hospitalization and transthoracic echocardiographic parameters between the two groups. Although there were no significant differences in the systolic and diastolic blood pressures on admission between the two groups, systolic blood pressure at 24 h after admission and on Day 7 and diastolic blood pressure on Day 7 were significantly lower in the furosemide group than in the tolvaptan group (24 h after admission: 115 ± 18 mmHg vs. 123 ± 23 mmHg, respectively, P = 0.039; Day 7: 113 ± 15 mmHg vs. 119 ± 17 mmHg, respectively, P = 0.049; and diastolic blood pressure on Day 7: 67 ± 14 mmHg vs. 72 ± 11 mmHg, respectively, P = 0.046) (Figure 2 ). Discharge medications are shown in Supporting Information, Table S1 . There were no significant differences in the medications except tolvaptan. In contrast, the dose of furosemide was significantly higher in the furosemide group than in the tolvaptan group.
Table 1.
Patient characteristics on admission
| Tolvaptan group (n = 47) | Furosemide group (n = 75) | P value | |
|---|---|---|---|
| Age, years | 70 ± 12 | 69 ± 12 | 0.80 |
| Male | 33 (70) | 47 (63) | 0.39 |
| BMI, kg/m2 | 25.6 ± 4.8 | 23.5 ± 6.1 | 0.05 |
| Hypertension | 29 (62) | 47 (63) | 0.92 |
| Dyslipidaemia | 20 (43) | 35 (47) | 0.66 |
| Diabetes | 19 (40) | 26 (35) | 0.52 |
| Prior smoking | 25 (53) | 39 (52) | 0.90 |
| Prior myocardial infarction | 7 (15) | 6 (8) | 0.23 |
| Atrial fibrillation | 22 (47) | 25 (33) | 0.14 |
| Laboratory data | |||
| Haemoglobin, g/dL | 12.4 ± 2.8 | 12.4 ± 2.7 | 0.97 |
| BUN, mg/dL | 24 ± 11 | 20 ± 8 | 0.02 |
| Creatinine, mg/dL | 1.3 ± 0.7 | 1.1 ± 0.5 | 0.02 |
| Sodium, mEq/L | 139 ± 7 | 139 ± 5 | 0.69 |
| Potassium, mEq/L | 4.2 ± 0.6 | 4.2 ± 0.6 | 0.82 |
| AST, U/L | 30 ± 15 | 43 ± 55 | 0.12 |
| ALT, U/L | 26 ± 19 | 28 ± 27 | 0.55 |
| BNP, pg/mL | 1213 ± 767 | 900 ± 726 | 0.02 |
| Pre‐hospitalization medication | |||
| Loop diuretics | 17 (36) | 15 (20) | 0.05 |
| Furosemide dose, mg/day | 21 ± 4 (n = 17) | 19 ± 4 (n = 15) | 0.18 |
| ACEi or ARB | 14 (30) | 20 (27) | 0.71 |
| Beta‐blocker | 18 (38) | 18 (24) | 0.09 |
| Spironolactone | 2 (4) | 3 (4) | 0.94 |
| Echocardiography | |||
| Left atrial diameter, mm | 47.4 ± 6.8 | 44.9 ± 8.1 | 0.08 |
| LV end‐diastolic diameter, mm | 52.5 ± 7.9 | 51.2 ± 10.5 | 0.45 |
| LV end‐systolic diameter, mm | 41.5 ± 10.7 | 40.5 ± 12.7 | 0.64 |
| EF, % | 41.5 ± 18.8 | 41.5 ± 17.6 | 0.98 |
| EF < 50% | 30 (64) | 51 (68) | 0.64 |
| Cardiac output, L/min | 4.5 ± 1.5 | 4.6 ± 1.5 | 0.99 |
ACEi, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; DBP, diastolic blood pressure; EF, ejection fraction; SBP, systolic blood pressure.
Data presented as mean ± standard deviations, or number (%).
Figure 2.
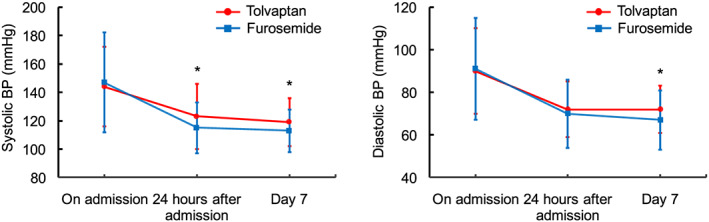
Change in systolic and diastolic blood pressures during hospitalization. * P < 0.05, compared with the furosemide group. BP, blood pressure.
Incidence of worsening renal function
The incidence of WRF during hospitalization was significantly lower in the tolvaptan group than in the furosemide group (Figure 3 ). The maximum daily dose of furosemide during hospitalization was 34 ± 15 mg/day in the tolvaptan group and 37 ± 13 mg/day in the furosemide group (P = 0.21). Body weights on admission and at discharge were 67.0 ± 16.4 and 58.4 ± 13.8 kg, respectively, in the tolvaptan group and 61.5 ± 13.8 and 55.7 ± 11.7 kg, respectively, in the furosemide group (Supporting Information, Figure S1 ). There was no significant difference in the amount of change in body weight between the two groups (−8.6 ± 8.1 and − 5.8 ± 5.3 kg for the tolvaptan and the furosemide groups, respectively). Multivariate logistic regression analysis was performed in the following seven variables: age, sex, maximum dose of furosemide during admission, creatinine at admission, B‐type natriuretic peptide, ejection fraction, and use of tolvaptan. Tolvaptan was considered as the independent variable related to the prevention of WRF (odds ratio, 0.19; 95% confidence interval, 0.05–0.69; P = 0.01; Table 2). A pre‐specified subgroup analysis indicated that a more favourable effect of tolvaptan was observed in patients with creatinine ≥1.1 mg/dL on admission and an ejection fraction <50% (Figure 4 ).
Figure 3.
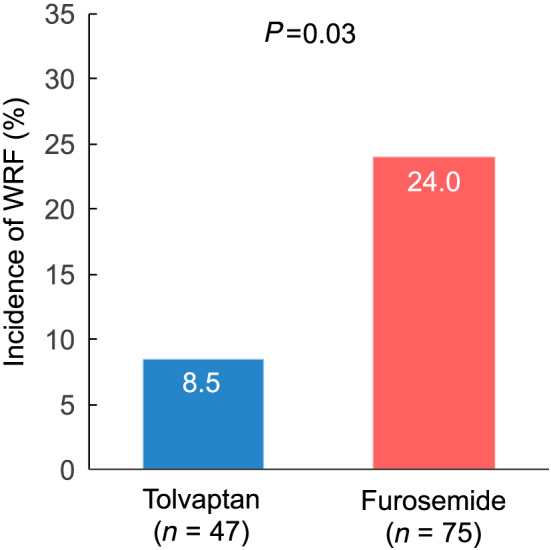
Incidence of worsening renal function.
Table 2.
Factors related to WRF by the multivariate analysis
| OR | 95% CI | P value | |
|---|---|---|---|
| Age, years | 1.00 | 0.96–1.05 | 0.90 |
| Male | 4.64 | 1.12–19.18 | 0.03 |
| Maximum daily dose of furosemide, mg/day | 1.00 | 0.96–1.04 | 0.90 |
| Creatinine at admission, mg/dL | 1.74 | 0.75–4.00 | 0.21 |
| BNP at admission, pg/mL | 1.00 | 0.99–1.01 | 0.93 |
| EF < 50% | 0.51 | 0.16–1.60 | 0.25 |
| Tolvaptan | 0.19 | 0.05–0.69 | 0.01 |
BNP, B‐type natriuretic peptide; CI, confidence interval; EF, ejection fraction; OR, odds ratio; WRF, worsening renal function.
Figure 4.
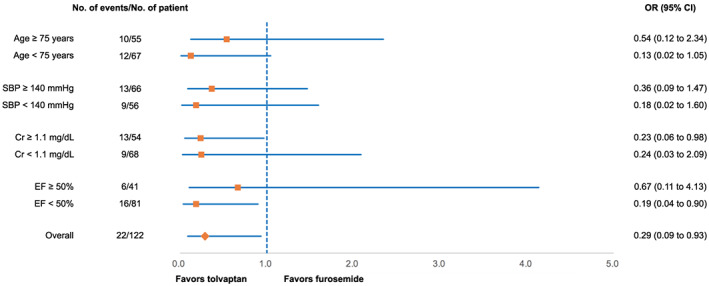
Forrest plot of odds ratios by pre‐specified patient subgroups for the incidence of worsening renal function. CI, confidence interval; Cr, creatinine; EF, ejection fraction; OR, odds ratio; SBP, systolic blood pressure.
Discussion
We investigated a possible renoprotective effect of tolvaptan in patients with new‐onset AHF. Study patients were initially treated with intravenous furosemide on admission. Subsequently, tolvaptan add‐on treatment was initiated in patients who did not meet the following criteria within 3 days after hospitalization: improvement of dyspnoea, decrease in pleural effusion by X‐ray, or decrease in body weight. The dose of tolvaptan and timing of initiation of drug therapy after hospitalization were dependent on the physician's decision. We found that tolvaptan add‐on therapy had lower incidence of WRF compared with those with furosemide alone group. Additionally, multivariate analysis revealed that tolvaptan add‐on therapy was an independent factor of preventing WRF. Our data suggest that tolvaptan add‐on therapy may be considered in individual treatment to protect renal function in selected cases in patients with new‐onset AHF.
In a meta‐analysis investigating the association between WRF and prognosis in heart failure, WRF was observed in 26% of AHF patients during hospitalization.1 In patients with AHF, WRF during hospitalization is associated with an increased risk of adverse cardiovascular outcomes.3, 20, 21 Furosemide, which is a loop diuretic, has been used for AHF, but it leads to high prevalence of WRF. Additionally, insufficient volume reduction due to WRF during hospitalization for AHF was associated with increased 1‐year mortality or urgent heart transplantation.22 Therefore, a renoprotective strategy in AHF is important to avoid renal impairment and improve subsequent long‐term prognosis. Furosemide contributes to a volume reduction with poor fluid refilling from the extravascular space, which leads to a decrease in mean arterial blood pressure and cardiac output and a subsequent decrease in renal blood flow. A decrease in renal blood flow causes the activation of the renin–angiotensin–aldosterone system and the progression of WRF.23, 24 In contrast, tolvaptan causes free‐water diuresis, which increases the serum osmolality and maintains intravascular fluid due to the shifting of fluid from the extravascular space, preserving the mean arterial blood pressure and renal blood flow without activating the renin–angiotensin–aldosterone system.10, 25 Although effective renoprotective strategies in the early stage of AHF have not been fully determined, several prospective studies have shown that the aquaresis effect of tolvaptan, which is a vasopressin type 2 receptor antagonist with electrolyte‐free water diuretic properties, inhibits intravascular hypovolaemia and subsequent incidence of WRF.5, 7, 8, 26 In patients with AHF, Shirakabe et al. reported that tolvaptan significantly reduced the incidence of WRF and had a better mid‐term cardiovascular outcome than furosemide.5 However, these studies highlighted the effect of tolvaptan in patients with an advanced stage of AHF who were already receiving a high dose of furosemide (over 40 mg/day furosemide) before admission. In our study, the patients with new‐onset AHF receiving less than 40 mg/day of furosemide on admission were evaluated, and tolvaptan add‐on therapy was found to be associated with a significantly lower incidence of WRF than conventional furosemide therapy.
Only two small clinical studies elucidated the effect of tolvaptan in preventing WRF in AHF based on left ventricular systolic function.8, 26 Although a favourable effect of tolvaptan in preventing WRF in patients with preserved ejection fraction has been reported, the effect of tolvaptan in preventing WRF in patients with reduced left ventricular systolic function has not been established. In our patients with new‐onset AHF, tolvaptan add‐on therapy demonstrated a more favourable effect in preventing WRF in patients with left ventricular systolic dysfunction and renal impairment compared with furosemide therapy on admission. The predominant cause of AHF with preserved left ventricular systolic function is related to a central shift of volume distribution rather than volume overload, whereas volume overload is the main cause of AHF in patients with reduced left ventricular systolic function.27
Dupont et al. reported that mean arterial blood pressure, cardiac output, and central venous pressure are key factors in the prevention of WRF in AHF patients.28 Considering the fact that the diuretic effect of tolvaptan is associated with the preservation of intravascular fluid, our data indicate that the favourable effect of tolvaptan in preventing WRF was due to the proper maintenance of arterial blood pressure, specifically in patients with left ventricular systolic dysfunction and renal impairment on admission.
Limitations
This study has the following limitations. First, this study had a small size. Hence, a double‐blinded study with a larger sample size is required to evaluate the long‐term effect of tolvaptan on patient prognosis. Second, we did not evaluate the highly sensitive markers of kidney injury such as heart‐type fatty acid‐binding protein, neutrophil gelatinase‐associated lipocalin, and kidney injury molecule‐1. Although the marker used to evaluate kidney injury is serum creatinine in clinical practice, this is the first study to report a renoprotective effect of tolvaptan in patients with new‐onset AHF.
Conclusions
A lower incidence of WRF was observed in patients with new‐onset AHF who were treated with the tolvaptan add‐on therapy, specifically those with left ventricular systolic dysfunction and renal impairment on admission.
Conflict of interest
None declared.
Funding
None.
Supporting information
Figure S1. Body weight on admission and at discharge.
Table S1. Medication at discharge
Kin, H. , Matsumura, K. , Yamamoto, Y. , Fujii, K. , Otagaki, M. , Takahashi, H. , Park, H. , Yoshioka, K. , Yokoi, M. , Sugiura, T. , and Shiojima, I. (2020) Renoprotective effect of tolvaptan in patients with new‐onset acute heart failure. ESC Heart Failure, 7: 1764–1770. 10.1002/ehf2.12738.
References
- 1. Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta‐analysis. J Card Fail 2007; 13: 599–608. [DOI] [PubMed] [Google Scholar]
- 2. Kociol RD, Greiner MA, Hammill BG, Phatak H, Fonarow GC, Curtis LH, Hernandez AF. Long‐term outcomes of medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am J Cardiol 2010; 105: 1786–1793. [DOI] [PubMed] [Google Scholar]
- 3. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 4. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shirakabe A, Hata N, Yamamoto M, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Shimizu W. Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid‐term prognosis of patients with severely decompensated acute heart failure. Circ J 2014; 78: 911–921. [DOI] [PubMed] [Google Scholar]
- 6. Inomata T, Ikeda Y, Kida K, Shibagaki Y, Sato N, Kumagai Y, Shinagawa H, Ako J, Izumi T, Kanagawa Aquaresis I . Effects of additive tolvaptan vs. increased furosemide on heart failure with diuretic resistance and renal impairment—results from the K‐STAR study. Circ J 2017; 82: 159–167. [DOI] [PubMed] [Google Scholar]
- 7. Kimura K, Momose T, Hasegawa T, Morita T, Misawa T, Motoki H, Izawa A, Ikeda U. Early administration of tolvaptan preserves renal function in elderly patients with acute decompensated heart failure. J Cardiol 2016; 67: 399–405. [DOI] [PubMed] [Google Scholar]
- 8. Tamaki S, Sato Y, Yamada T, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kondo T, Ozaki T, Seo M, Ikeda I, Fukuhara E, Abe M, Nakamura J, Fukunami M. Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure and preserved left ventricular ejection fraction—prospective randomized controlled study. Circ J 2017; 81: 740–747. [DOI] [PubMed] [Google Scholar]
- 9. Matsumura K, Morishita S, Taniguchi N, Takehana K, Takahashi H, Otagaki M, Yoshioka K, Yamamoto Y, Takagi M, Shiojima I. Prognostic factors for long‐term outcomes in acute decompensated heart failure patients under tolvaptan treatment. Heart Vessels 2019; 34: 607–615. [DOI] [PubMed] [Google Scholar]
- 10. Goldsmith SR, Bart BA, Burnett J. Decongestive therapy and renal function in acute heart failure: time for a new approach? Circ Heart Fail 2014; 7: 531–535. [DOI] [PubMed] [Google Scholar]
- 11. Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993; 22: A6–A13. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury N . Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 15. Matsumura K, Sugiura T. Effect of sodium glucose cotransporter 2 inhibitors on cardiac function and cardiovascular outcome: a systematic review. Cardiovasc Ultrasound 2019; 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox H, Hemmann K, Lehmann R. Comparison of transthoracic and transesophageal echocardiography for transcatheter aortic valve replacement sizing in high‐risk patients. J Echocardiogr 2020; 18: 47–56. [DOI] [PubMed] [Google Scholar]
- 17. Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J 2014; 35: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 18. Masuyama T, Tsujino T, Origasa H, Yamamoto K, Akasaka T, Hirano Y, Ohte N, Daimon T, Nakatani S, Ito H. Superiority of long‐acting to short‐acting loop diuretics in the treatment of congestive heart failure. Circ J 2012; 76: 833–842. [DOI] [PubMed] [Google Scholar]
- 19. Rickham PP. Human experimentation. Code of Ethics of the World Medical Association Declaration of Helsinki. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Logeart D, Tabet JY, Hittinger L, Thabut G, Jourdain P, Maison P, Tartiere JM, Solal AC. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol 2008; 127: 228–232. [DOI] [PubMed] [Google Scholar]
- 21. Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K. Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J 2013; 77: 687–696. [DOI] [PubMed] [Google Scholar]
- 22. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? the role of congestion and its interaction with renal function. Circ Heart Fail 2012; 5: 54–62. [DOI] [PubMed] [Google Scholar]
- 23. Sarraf M, Schrier RW. Cardiorenal syndrome in acute heart failure syndromes. Int J Nephrol 2011; 2011 293938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol 2012; 60: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 25. Costello‐Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C, Burnett JC Jr. Vasopressin‐2‐receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol 2006; 290: F273–F278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jujo K, Saito K, Ishida I, Furuki Y, Kim A, Suzuki Y, Sekiguchi H, Yamaguchi J, Ogawa H, Hagiwara N. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail 2016; 3: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takei M, Kohsaka S, Shiraishi Y, Goda A, Izumi Y, Yagawa M, Mizuno A, Sawano M, Inohara T, Kohno T, Fukuda K, Yoshikawa T, West Tokyo Heart Failure Registry I. Effect of estimated plasma volume reduction on renal function for acute heart failure differs between patients with preserved and reduced ejection fraction. Circ Heart Fail 2015; 8: 527–532. [DOI] [PubMed] [Google Scholar]
- 28. Dupont M, Mullens W, Finucan M, Taylor DO, Starling RC, Tang WH. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013; 15: 433–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Body weight on admission and at discharge.
Table S1. Medication at discharge


