Abstract
Aims
Leucocyte‐directed specialized pro‐resolving mediators (SPMs) are essential for cardiac repair, and their biosynthesis coincides with the expression of pro‐inflammatory mediators; however, the precise quantitation during an acute myocardial infarction (MI) event is poorly understood in race‐specific and sex‐specific manner. Coronary heart disease is the leading cause of death and disability in the USA. Although the prevalence of coronary heart disease is similar between Black and White patients, cardiovascular events (including MI), rehospitalization, and mortality are disproportionately higher in Black patients. Therefore, understanding differences in inflammation and resolution can enable the development of predictive, personalized, and precise treatment and attenuate sex/racial disparities. Thus, herein, we assess differences in bioactive lipids and SPMs, between Black and White patients experiencing an acute MI.
Methods and results
From the PRiME‐GGAT cohort, we collected plasma after MI within 24–48 h from 22 Black (15 male and 7 female) and 31 White (23 male and 8 female) subjects for a comparative race‐based and sex‐based analyses. MI was confirmed using a biochemical measurement of plasma troponin and ST elevation. Plasma levels of three essential polyunsaturated fatty acids [arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA)] and a set of 40 bioactive lipid mediators with major emphasis on SPMs were quantified by liquid chromatography–mass spectrometry. AA and DHA were higher in White male and female patients, and EPA was noted higher only in White male patients compared with White female and Black male and female patients. Lipoxygenase‐mediated AA‐derived 12‐hydroxyeicosatetraenoic acid (29–63%) and 15‐hydroxyeicosatetraenoic acid (3–9%) and DHA‐derived 17‐hydroxydocosahexaenoic acid (3–22%) and 14‐hydroxydocosahexaenoic acid (7–10%) were major bioactive lipid mediators in plasma. The SPM signature resolvin E1 was significantly lower in Black patients compared with White male and female patients, whereas protectin D1 was lower in White male patients compared with White female and Black male and female patients.
Conclusion
Our comparative analyses of fatty acids and respective cyclooxygenase‐derived and lipoxygenase‐derived SPM signatures capture the heterogeneity of disease pathology and elucidate potential mechanisms underlying sex‐based and race‐based differences following MI.
Keywords: Bioactive lipids, Heart failure, Inflammation, Racial disparity, Resolution of inflammation
Introduction
Coronary heart disease (CHD) is one of the leading causes of morbidity, mortality, and health‐care expenditures in the USA.1 Although the prevalence of coronary artery disease is similar between Black and White patients,2 outcomes following myocardial infarction (MI), or coronary intervention, that is, rehospitalization and mortality, are disproportionately higher in Black patients.3, 4, 5, 6, 7, 8 Although the disparities in heart disease and stroke risk are recognized, the pathophysiological mechanisms underlying this racial disparity are not clearly understood. This knowledge gap must be bridged to facilitate the development of possible treatments for precise and personalized therapy.3, 4, 5, 9, 10 Black men and women have higher rates of cardiovascular (CV) morbidity and mortality than White men and women.11 Further, there is also a racial disparity in the incidence of MI and the development of advanced heart failure (HF) post‐MI.12 MI is a leading contributor to the development of HF, which is substantially more common in Black than White patients.13, 14, 15 HF is the primary cause of hospitalization in the USA, accounting for 1–2% of our 3.3 trillion dollar health‐care expenditure. In total, CV disease is the number one cause of morbidity and mortality in the USA; approximately half of all adults have some type of CV disease like CHD, high blood pressure, obesity‐related cardiomyopathy, or HF.2
Lipid management is the primary focus of prevention and treatment of atherosclerotic CV disease.16, 17, 18 Fatty acid‐derived bioactive lipid mediators define cardiac healing, repair, and inflammation–resolution mechanisms.19, 20, 21, 22 Preclinical and clinical studies suggest that pro‐inflammatory lipid mediators (prostaglandins and leukotrienes/thromboxanes) and cytokines are markers of non‐resolving inflammation.23, 24, 25 In contrast, recent evidence indicates that immune responsive biosynthesis of resolving mediators [specialized pro‐resolving mediators (SPMs)] coincides with the expression of pro‐inflammatory mediators and that delineates leucocyte phenotypes in cardiac repair. This suggests that the acute inflammation directed resolution response is an active process of inflammation–resolution after injury, infection, or stress.19, 20, 26 In clinical practice, for diagnosis and prognosis, lipids are classified into triglycerides, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and very low density lipoprotein (cholesterol) quantified based on the density of protein–lipid complex.16 To better elucidate their pathological impact, a comprehensive assessment of bioactive lipid species at the peak of inflammation after an acute MI event is needed. In this report, we quantified lipid mediators of the cyclooxygenase and lipoxygenase (LOX) pathways using mass spectrometry (MS) to identify and quantify plasma SPMs in Black and White patients experiencing an acute MI event. Observational studies suggest that the incidence and prevalence of heart disease in Black individuals is higher due to lifestyle including the southern diet (fried and barbecue food), high dietary salt intake, and lack of physical activity.27 Our findings suggest that targeted estimation of bioactive lipids SPM signature of cyclooxygenase and LOX pathways provided race‐specific and sex‐specific difference that may be suitable for prevention, prognostic, precise, and personalized therapy of heart disease management.
Methods
Ethical compliance
The PRiME‐GGAT (Pharmacogenomic Resource to improve Medication Effectiveness Genotype Guided Antiplatelet Therapy) is a prospective cohort study conducted under the approval of the Institutional Review Board of the University of Alabama at Birmingham.
Study design and patient inclusion criteria
Patients (≥18 years old) admitted for acute coronary syndrome (ACS; Figure 1 (A)) who underwent percutaneous coronary intervention (PCI) were enrolled at the time of the ACS admission. Plasma was obtained within 24 h of the PCI. ACS was defined as a spectrum of clinical presentations ranging from occlusion of the coronary artery causing ST‐segment elevation MI (STEMI) to a significant compromise of blood flow presenting as non‐ST‐segment elevation MI or unstable angina. A structured case report form was used to record patients' demographics (age, sex, and race), lifestyle (e.g. smoking), socio‐economic status (SES; annual income, educational attainment, and health insurance), CV risk factors, and co‐morbidities. Serum creatinine at admission was used to calculate the estimated glomerular filtration rate using the Modification of Diet in Renal Disease formula.28 Other laboratory parameters included troponin I (TnI), haematocrit, haemoglobin (Hb), platelets, leucocytes (including neutrophils, lymphocytes, monocytes, eosinophils, and basophils), alkaline phosphatase, alanine transaminase, aspartate transaminase, glycated haemoglobin (HbA1C), total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides. Vital signs (e.g. blood pressure and heart rate) at time of PCI, PCI status (urgent or elective), indication for PCI, access site, coronary artery lesion location, peri‐PCI antiplatelet use, contrast volume, number of coronary arteries with ≥70% stenosis, and number and type of stents implanted were documented. Plasma samples from 53 patients with STEMI were used to comprehensively assess the lipidomic profile (Figure 1 (A) and (B)). To establish signatures of inflammation and non‐resolving lipid metabolites, parent essential polyunsaturated fatty acids, such as arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA), were measured.
Figure 1.
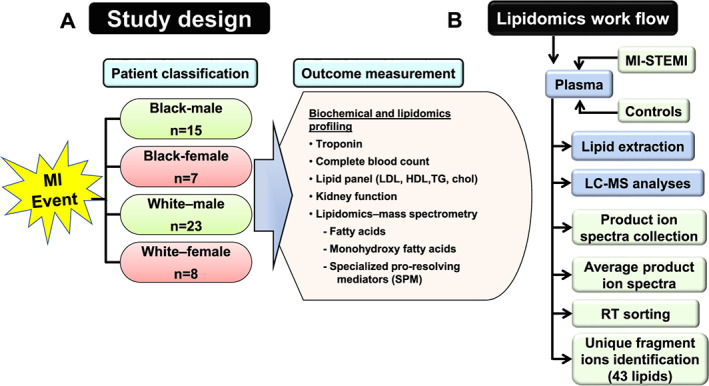
(A) Study design for biochemical and lipidomics profiling of 53 ST‐segment elevation myocardial infarction (STEMI) patients: Black (n = 15 men and 7 women) and White (n = 23 men and 8 women) patients with outcome parameters that are collected as a routine procedure of an acute myocardial infarction (MI) event. (B) Lipidomics workflow illustrates plasma lipid mediators extraction and unique identification of bioactive lipid mediators based on mass by the charge to ratio using mass spectrometry. Black, African American; LC‐MS, liquid chromatography–mass spectrometry; TG, triglycerides; White, European American.
Analytical standard and plasma sample preparation using solid phase extraction
All chemicals and lipid standards were analytical and MS grade. Aliquots of 50 μL human plasma were mixed with 5 μL deuterated internal standard mixture before loading onto 96‐well solid phase extraction cartridges (30 mg, HLB, Waters, Milford, MA, USA), which had been preconditioned with 1 mL MeOH and 1 mL water sequentially; 1.5 mL of 5% methanol was used to wash out the unbounded interferences. Elution was carried out with 1.2 mL of methanol, and eluents were dried under nitrogen and reconstituted with 50 μL of 50% methanol as described previously.29
Liquid chromatography–mass spectrometry analyses
A Vanquish UHPLC coupled with a Quantiva triple quadrupole mass spectrometer (Thermo Fisher Scientific, Haverhill, MA, USA) was used for liquid chromatography multiple reaction monitoring mass spectrometry analysis. A HSS T3 column(100 × 2.1 mm, 1.8 μm, Waters) with T3 VanGuard precolumn (5 × 2.1 mm, 1.8 μm, Waters) was employed for separation of analytes. The column was thermostated at 40°C. The mobile phase was composed of Solvent A [0.1% formic acid (FA) in H2O] and Solvent B (0.1% FA in acetonitrile). Gradient elution was used for 12 min at a flow rate of 0.3 mL/min. Detailed gradient conditions were as reported previously.29 A 10 μL aliquot of each sample was injected onto column for analysis. The effluent was monitored with the Quantiva mass spectrometer in negative ion mode. The detailed operation parameters for the mass spectrometer and the transition list for the scheduled multiple reaction monitoring were described previously.29
Multiple reaction monitoring data processing
Liquid chromatography multiple reaction monitoring datasets were processed with TraceFinder 4.1, and the auto‐integrated peaks were inspected manually. The calibration curves of each analyte were constructed by normalizing to the selected ISTD followed by linear regression with 1/x weighting. Absolute concentration of analyte in each sample was calculated from the calibration curves.29
Statistical analyses
Data are expressed as mean and standard error of the mean, and analyses of variance were used to assess differences at a bidirectional P‐value of ≤0.05. Statistical analyses were performed using Microsoft Excel for pie charts and GraphPad Prism 8 for the graphs.
Results
Demographics of patients with myocardial infarction without signs of sex‐based and race‐based differences between Black and White patients
Baseline characteristics for Black and White patients with confirmed acute MI who underwent PCI are shown in Table 1. There was no statistical difference between Black and White patients with regard to age, body mass index, and laboratory parameters including estimated glomerular filtration rate, alkaline phosphatase, alanine transaminase, aspartate transaminase, and HbA1C. Furthermore, there was no statistical difference between Black and White patients in maximum TnI pre‐PCI and post‐PCI (P = 0.12 and P = 0.83, respectively; Table 1), suggesting a similar extent of cardiac injury. Among Black patients, women had a higher pre‐PCI (P = 0.043) TnI but also had the longest time to the collection, which may explain the higher numbers. Plasma cholesterol carrier lipids showed that there was no statistical difference in total cholesterol, HDL, LDL, and triglycerides at baseline between Black and White female and male patients. Furthermore, there were no differences in leucocytes such as monocytes, eosinophils, basophils, lymphocytes, and neutrophils between race and sex subgroups (Figure 2 (A) and (B)), suggesting that current clinical laboratory assessments are inadequate to explain race disparities and sex‐specific differences.
Table 1.
Race‐based and sex‐based differences in haematological and biochemical parameters in Black and White patients after myocardial infarction
| Variable | Black | White | Black vs. White P‐value | ||||
|---|---|---|---|---|---|---|---|
| Female (n = 7) | Male (n = 15) | P‐value | Female (n = 8) | Male (n = 23) | P‐value | ||
| Age, years | 56.71 ± 7.43 | 56.60 ± 9.46 | 0.98 | 51.13 ± 13.13 | 60.78 ± 11.03 | 0.0306 | 0.83 |
| BMI, kg/m2 | 30.71 ± 6.40 | 28.20 ± 5.13 | 0.29 | 26.88 ± 3.14 | 29.61 ± 5.15 | 0.20 | 0.44 |
| eGFR, mL/min/1.73 m2 | 79.14 ± 25.68 | 77.30 ± 37.93 | 0.89 | 89.08 ± 37.49 | 71.83 ± 17.69 | 0.15 | 0.80 |
| HCT, % | 40.14 ± 3.08 | 43.93 ± 5.62 | 0.10 | 41.00 ± 3.12 | 45.30 ± 5.46 | 0.0410 | 0.47 |
| Hb, g/dL | 13.09 ± 1.01 | 14.42 ± 1.88 | 0.08 | 13.88 ± 1.23 | 15.17 ± 1.69 | 0.057 | 0.13 |
| Platelets, 109/L | 264.86 ± 57.09 | 260.13 ± 69.90 | 0.87 | 272.13 ± 71.69 | 220.83 ± 58.04 | 0.055 | 0.42 |
| Total chol, mg/dL | 174.00 ± 31.84 | 194.91 ± 47.42 | 0.42 | 186.57 ± 54.04 | 192.00 ± 55.05 | 0.80 | 0.77 |
| Triglycerides, mg/dL | 94.33 ± 16.61 | 94.64 ± 77.25 | 0.99 | 152.25 ± 109.58 | 125.89 ± 96.51 | 0.49 | 0.14 |
| HDL, mg/dL | 39.17 ± 5.81 | 44.73 ± 8.65 | 0.28 | 42.75 ± 14.24 | 38.56 ± 9.69 | 0.33 | 0.70 |
| LDL, mg/dL | 123.83 ± 33.84 | 122.36 ± 37.52 | 0.95 | 123.25 ± 59.26 | 129.00 ± 43.54 | 0.76 | 0.84 |
| Alk_Phos, U/L | 84.00 ± 22.05 | 75.71 ± 15.98 | 0.55 | 83.33 ± 13.29 | 72.29 ± 30.22 | 0.35 | 0.82 |
| ALT, U/L | 31.00 ± 18.55 | 24.29 ± 24.64 | 0.86 | 109.40 ± 160.96 | 46.59 ± 44.36 | 0.08 | 0.0561 |
| AST, U/L | 79.00 ± 64.63 | 59.86 ± 99.62 | 0.83 | 186.17 ± 299.59 | 82.71 ± 124.42 | 0.17 | 0.26 |
| Hb_A1C, % | 6.66 ± 1.61 | 6.48 ± 2.18 | 0.81 | 5.74 ± 0.99 | 6.51 ± 1.32 | 0.24 | 0.38 |
| max_TnI_pre_PCI, units/L | 13.64 ± 21.01 | 3.76 ± 7.88 | 0.0433 | 2.88 ± 5.61 | 4.48 ± 7.97 | 0.71 | 0.12 |
| max_TnI_post_PCI, units/L | 63.47 ± 50.39 | 108.73 176.60 | 0.54 | 41.33 ± 48.60 | 152.35 ± 187.25 | 0.094 | 0.83 |
| Neu_base, 109/L | 64.00 ± 15.94 | 68.50 ± 12.96 | 0.47 | 66.83 ± 9.35 | 64.19 ± 12.46 | 0.66 | 0.86 |
| Abs Neu, 109/L | 9.88 ± 5.95 | 7.83 ± 2.69 | 0.19 | 7.70 ± 2.40 | 6.66 ± 2.39 | 0.49 | 0.13 |
| Lymph pre, 109/L | 28.00 ± 14.05 | 22.33 ± 10.48 | 0.28 | 19.33 ± 10.09 | 25.00 ± 10.41 | 0.27 | 0.42 |
| Monocytes, 109/L | 6.57 ± 2.15 | 7.67 ± 2.67 | 0.40 | 7.83 ± 3.19 | 8.52 ± 2.71 | 0.58 | 0.24 |
| Eosinophils, 109/L | 1.00 ± 1.15 | 1.00 ± 0.85 | 0.99 | 1.83 ± 1.47 | 1.62 ± 1.32 | 0.70 | 0.078 |
| Basophils, 109/L | 0.14 ± 0.38 | 0.75 ± 0.62 | 0.0361 | 0.83 ± 0.41 | 0.67 ± 0.66 | 0.54 | 0.13 |
| Neu_post_PCI, 109/L | 69.57 ± 15.59 | 67.50 ± 12.57 | 0.69 | 73.38 ± 9.40 | 74.68 ± 8.63 | 0.77 | 0.11 |
| AbsNeu_post_PCI, 109/L | 10.23 ± 5.67 | 6.54 ± 2.84 | 0.077 | 7.72 ± 2.42 | 10.01 ± 5.26 | 0.22 | 0.73 |
| Lymph_post_PCI, 109/L | 21.71 ± 12.85 | 20.21 ± 8.09 | 0.70 | 17.75 ± 5.75 | 14.95 ± 7.35 | 0.42 | 0.078 |
| Time to collection, in h post‐MI | 37.73 ± 10.34 | 21.52 ± 8.20 | 0.0008 | 21.45 ± 5.805 | 23.531 ± 10.76 | 0.60 | 0.0209 |
| Socio‐economic status | |||||||
| Income | 0.99 | 0.34 | 0.09 | ||||
| ≥50,000 | 1 (16.7%) | 2 (18.2%) | 1 (20.0%) | 8 (50.0%) | |||
| <50,000 | 5 (83.3%) | 9 (81.8%) | 4 (80.0%) | 8 (50.0%) | |||
| Insurance | 0.65 | 0.58 | 0.04 | ||||
| Medicaid/no insurance | 2 (28.6%) | 7 (46.7%) | 2 (25.0%) | 3 (13.0%) | |||
| Private/Medicare | 5 (71.4%) | 8 (53.3%) | 6 (75.0%) | 20 (87.0%) | |||
| Education | 0.99 | 0.09 | 0.45 | ||||
| ≥High school | 7 (100%) | 13 (86.7%) | 5 (62.5%) | 21 (91.3%) | |||
| <High school | 0 | 2 (13.3%) | 3 (37.5%) | 2 (8.7%) | |||
Alk_Phos, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; chol, cholesterol; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; Hb_A1C, haemoglobin A1c; HCT, haematocrit; HDL, high density lipoprotein; LDL, low density lipoprotein; Lymph, lymphocytes; Neu, neutrophils; PCI, percutaneous coronary intervention; post‐MI, post‐myocardial infarction; TnI, troponin I; U, units.
Values are mean ± standard error of the mean; n indicates sample size/group. Income information was missing in 15 patients.
Figure 2.
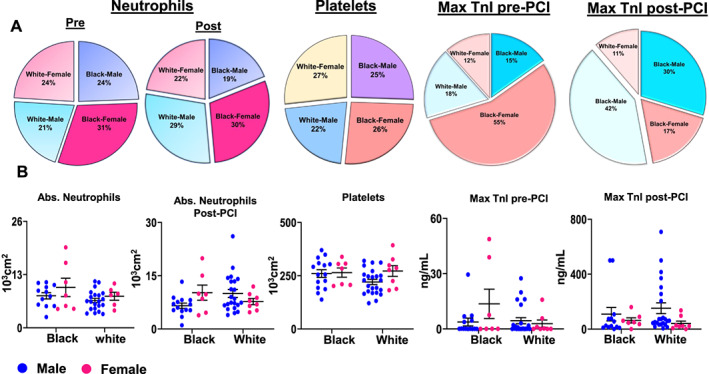
(A) Distribution of neutrophils, platelets, and troponin in Black and White male and female patients during acute myocardial infarction event (24–48 h; mean 37.73 h post‐myocardial infarction). (B) An absolute number of neutrophils, platelets, and troponin concentration by race/ethnicity and sex. Black, African American; PCI, percutaneous coronary intervention; White, European American.
White patients showed higher n‐6 and n‐3 fatty acid concentrations compared with Black patients after an acute myocardial infarction event
Circulating fatty acids exclusively depend on personnel dietary fat intake and are critically important for the incident of CV disease and mortality.30, 31 IMPROVE pan‐European cohort and JELIS trial established that systemic levels of omega‐6 and omega‐3 fatty acids are associated with reduced future CV events.17, 32 Thus, to determine the racial and sex‐specific difference between fatty acids in White and Black patients with STEMI, we measured 3 fatty acids (substrate) and 40 related lipid metabolites (mediators and products) along with the parent fatty acids (Supporting Information, Table S1 and Supporting Information, Figure S1 ).
Comparing the overall distribution of the three major fatty acids, AA was highest in concentration followed by DHA and EPA, demonstrating the dominance of omega‐6 fatty acids among White and Black patients with no significant differences by race and sex observed in AA (Figure 3 (A); P = 0.098). The pie chart (Figure 3 (A)) shows that the highest levels of AA were present in White female patients (85%), while White male patients showed 82% AA, followed by Black male and female patients having 81%, respectively. The MS data revealed that the levels of species of AA (upper limit of 6027 ng/mL vs. 4643 ng/mL) were higher than DHA (1761 ng/mL vs. 799 ng/mL) and EPA (210 ng/mL vs. 82 ng/mL) in White male patients compared with Black male patients. No differences were observed among female patients (Figure 3 (B)). MS spectra indicated the peaks of AA, DHA, and EPA in White (male and female) and Black (male and female) population (Figure 3 (C)). Quantitative analyses confirmed that White male patients had a higher relative trend of circulating levels of AA, DHA, and EPA compared with White female, Black male, and Black female patients.
Figure 3.
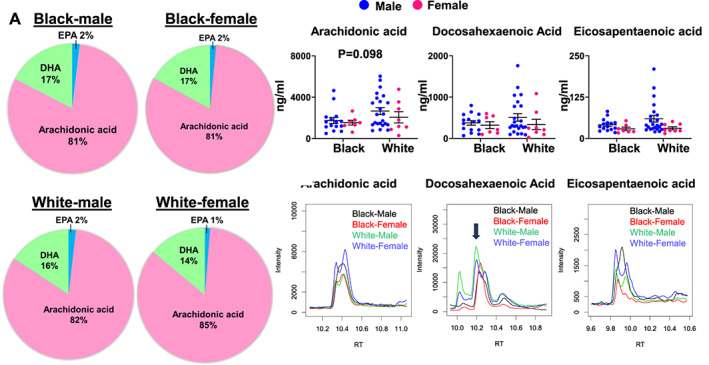
Plasma polyunsaturated fatty acids vary between Black and White patients after an acute myocardial infarction (MI) event. (A) Distribution of percentage of essential polyunsaturated fatty acids such as arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) in Black and White quantified by targeted lipidomics analyses post‐MI (24–48 h). (B) Quantitative levels of AA, DHA, and EPA in Black and White participants post‐MI (24–48 h; mean: 37.73 post‐MI). (C) Representative mass spectrometry spectra display the peaks of AA, DHA, and EPA in Black (male and female) and White (male and female) participants. The black arrow indicates the precise peak of fatty acid. Black, African American; White, European American.
White male patients experience marked activation of 12‐hydroxyeicosatetraenoic acid levels during an acute myocardial infarction event
In response to immune activation events like MI, particularly, 12 LOX metabolizes fatty acids AA, DHA, and EPA to differential bioactive lipid metabolites.20, 33 We therefore tested racial and sex‐associated differences in lipid signatures with respect to essential fatty acids AA, DHA, and EPA. First, we classified the distribution pattern of hydroxyeicosatetraenoic acids (HETEs), hydroxydocosahexaenoic acids (HDHAs), and hydroxyeicosapentaenoic acids (HEPEs). Second, we categorized HETEs, HDHAs, and HEPEs to describe the respective metabolites from AA, DHA, and EPA, respectively. Third, we classified single bioactive lipid mediators for AA (pink‐red), DHA (light‐dark green), and EPA (light‐dark blue) and decoded in the pie chart (Figure 4 (A)). The 12‐HETE level was the highest among HETEs, HDHAs, and HEPEs. Both White men (63%) and women (47%) had a higher proportion of 12‐HETE compared with Black men (29%) and women (33%). In contrast, 17‐HDHA distribution was lower in White individuals compared with Black individuals (Figure 4 (A)). The graphs and MS spectra showed that the upper limit for 12‐HETE in White male patients was 1365 ng/mL vs. 72 ng/mL compared with Black male patients with no major differences in the concentrations of pro‐inflammatory mediators (leukotriene B4 and prostaglandins), and pro‐resolving mediators 17‐HDHA and 14‐HDHA (Figure 4 (B) and (C); Supporting Information, Figures S2 and S3 ). Thus, targeted and quantitative measurement of 12 LOX‐derived monohydroxy lipid mediators of AA, DHA, and EPA allowed us to define a differential lipidomic milieu in Black and White patients in an acute MI event.
Figure 4.
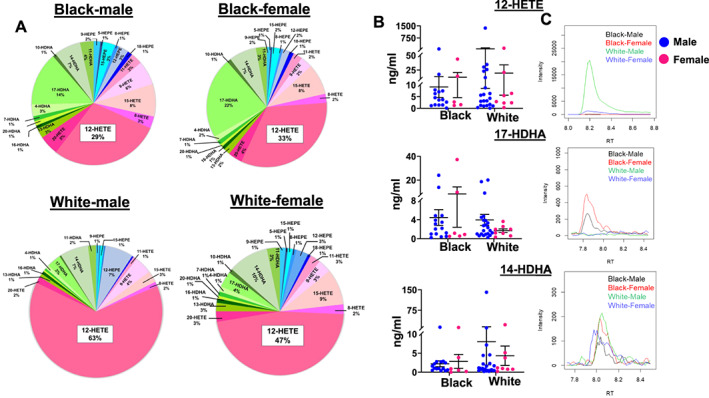
Arachidonic acid‐derived 12‐HETE is a highly responsive bioactive lipid signature in White participants after a myocardial infarction (MI) event (24–48 h; mean: 37.73 post‐MI). (A) Distribution of bioactive lipid mediators for arachidonic acid (HETEs: pink‐red), docosahexaenoic acid (HDHAs: light‐dark green), eicosapentaenoic acid (HEPEs: light–dark blue) between Black and White subjects after an MI event within 24–48 h quantified by liquid chromatography–mass spectrometry. (B) Quantified levels in ng/mL of 12‐HETE, 17‐HDHA, and 14‐HDHA in Black and White patients within 24–48 h post‐MI. (C) Representative mass spectrometry spectra displays the peaks of 12‐HETE, 17‐HDHA, and 14‐HDHA in Black (male and female) and White (male and female) participants. HEPE, hydroxyeicosapentaenoic acids; HETE, hydroxyeicosatetraenoic acid; HDHA, hydroxydocosahexaenoic acid. Black, African American; White, European American.
White and Black patients have distinct specialized pro‐resolving mediator signatures during an acute myocardial infarction event
After MI, the immune system is known to be activated, but the precise pattern of bioactive lipid mediators in the circulation mostly produced by cells of the immune system remains poorly characterized. Thus, we next characterized the liquid chromatography–mass spectrometry profile of fatty acids (AA, DHA, and EPA) targeted lipid mediators endogenously produced by immune cells in our STEMI patients. Biosynthesis of SPMs occurs within 24 h of an acute MI. We determined whether there are racial differences in the fatty acids‐derived bioactive SPM signature. Liquid chromatography with tandem mass spectrometry data revealed that the White population has significantly higher levels of resolvin E1 (RvE1; 34% in men and 36% in women) compared with the Black population (21% in men and 9% in women). In contrast, Black patients had higher levels of protectin D1 (37% in men and 33% in women) compared with White patients (14% in men and 16% in women; Figure 5 (A)), indicating that the White and Black patients have distinct SPM signatures following MI. The upper limit of plasma RvE1 was 0.099 ng/mL in White male patients compared with 0.058 ng/mL in Black male patients. Similarly, in White female patients, the upper limit of RvE1 was 0.095 ng/mL compared with 0.026 ng/mL in Black female patients. Plasma protectin D1 was higher in Black male patients than in White male patients (upper limit 0.391 ng/mL vs. 0.131 ng/mL) with no major differences in female patients irrespective of race (Figure 5 (B)). The black arrow in the MS spectra showed peaks of RvE1 and protectin D1, respectively (Figure 5 (C) ). Thus, the quantitative measurement of SPMs in Black and White patients provided personalized and precise distinct signatures of inflammation and resolution after an acute MI event.
Figure 5.
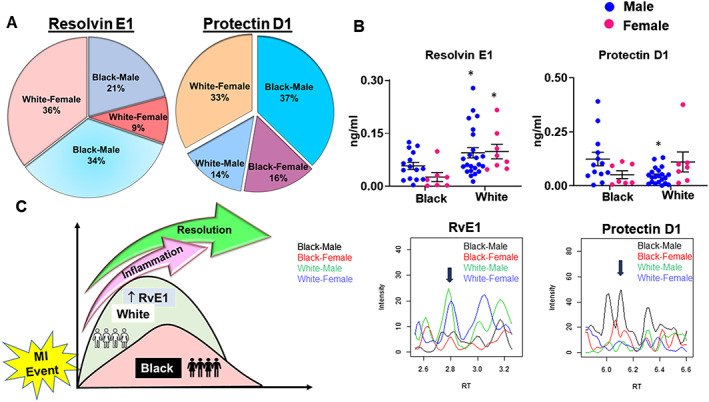
Plasma specialized pro‐resolving mediator signatures are distinct in Black and White patients during acute myocardial infarction (MI) events. (A) Distribution of resolvin E1 (RvE1) and protectin D1 among Black and White male and female participants within 24–48 h (mean: 37.73 h) post‐MI determined by mass spectrometry. (B) Plasma quantitative levels and representative mass spectrometry spectra of RvE1 and protectin D1 in Black and White male and female patients. The black arrows indicate the precise peak of a specialized pro‐resolving mediator signature. (C) Summary figure illustrating overall changes in bioactive lipids signature in Black and White patients. Black, African American; White, European American.
Discussion
Black patients are prone to a variety of CV diseases at an early age and develop long‐term chronic disease with disease manifesting at an earlier age, higher severity, and poorer long‐term outcomes. Despite these differences, medical management is generally agnostic of race. Perhaps understanding quantitative differences in prognostic and/or predictive biomarkers could facilitate the development of race‐specific personalize therapies.3, 4, 5, 34 In the present report, we quantify the essential fatty acids and define the possible immune responsive SPM signatures in patients undergoing PCI after an acute STEMI event. We also examined the bioactive lipid mediators because these serve as molecular messengers derived from AA, DHA, and EPA and act as necessary initiators of early inflammation and resolution pathways.26, 33, 35 We have three key findings: (i) heterogeneous activation of fatty acids; (ii) profound expansion of monohydroxyl fatty acids such as 12‐HETE, 14‐HDHA, and 17‐HDHA; and (iii) distinct increase of SPM signature in Black and White patients, particularly higher RvE1 in White male patients compared with Black male patients.
Inflammation is the hallmark of cardiac injury post‐MI. During this period, prior to troponin (clinical biomarker) release, biosynthesis of inflammatory‐resolving lipid mediators involved in the resolution (safe clearance of inflammation) that limits chronic inflammation.19, 36 Before troponin markers, leucocyte‐directed SPMs are immune responsive polyunsaturated fatty acids‐derived biomolecules generated within a narrow time window after the cardiac injury that initiate resolution of inflammation.20, 36 Deficiency of SPMs associated with monocyte/macrophage depletion or in obesogenic ageing leads to non‐resolving inflammation.20, 37 Ethyl EPA lowered the risk of MI events, stroke, and overall CV death by 25% in patients with hypertriglyceridaemia in the REDUCE‐IT trial and by 19% in the JELIS trial, suggesting preventive therapy.17, 18 Also, high levels of EPA reduced the risk of HF observed in the MESA trial, and acute administration of high doses of omega‐3 fatty acids increased the SPM signature in patients with evidence of peripheral artery disease in the OMEGA‐PAD I trial.38, 39, 40 Higher levels of EPA‐derived RvE1 in White men and women indicative of increased specific SPM signature after an MI event provide a rationale for personalized and precise medicine in CV therapy. SPMs RvD1 and RvE1 facilitate cardiac repair and clearance of neutrophils in the setting of ischaemic insult, suggesting the possibility of a precise and personalized treatment strategy if SPMs biosynthesis failed due to deficiency of substrate or LOXs activity. Early treatment with RvE1 or D1 supports cardiac healing by clearing inflammation in an ischaemic mouse model.41, 42 Thus, selective measurement of SPM signature supports the overlapping concept of inflammation and resolution and help to prognosticate the resolution and non‐resolving inflammation in CV medicine. Time‐dependent cardiac repair in pathogen and risk‐free rodents clearly suggests that the timing of the acute inflammatory phase coincides with the beginning of the resolving phase, to the same extent as MI patients biosynthesize resolution molecules within 2.5 h as the onset of initial inflammation post‐MI.20, 33, 36 Thus, beginning of acute inflammation‐resolution programmes the termination of inflammatory response after cardiac injury of acute inflammation programmes the beginning of inflammatory resolution after cardiac injury.43 A previous report indicates that SPMs levels in atheroma define the stable and unstable milieu, providing the rationale for precise and personalized therapy for atherosclerotic patients.44
Lipid profiling is commonly used for diagnosis and in such prevention in CV medicine; however, patient stratification based on LDL and HDL levels has the limited biological significance, as it does not account for lipid and protein carrier physiology after injury. Our report defines the specific immune responsive species such as AA, DHA, and EPA in the setting of STEMI. Recently published temporal analyses of SPMs at the onset of and at Day 1 and Day 8 post‐MI define the differential signatures of 5, 12, and 15 LOX‐derived mediators post‐MI.36 The docosapentaenoic acid metabolome is one of the major fatty acids in the plasma lipidome that facilitates biosynthesis of T‐series resolvins and reduces the risk of CV disease.45 Amplified pro‐inflammatory markers are signs of an acute inflammatory response, despite detectable levels of HETEs in all samples, limited pro‐inflammatory lipid species that noted despite race‐based and sex‐based differences. Thus, SPMs are unique diagnostic and prognostic signatures that allowed us to define the initiation of resolution independent of pro‐inflammatory lipid mediators.
Study strengths
This report quantified individual fatty acids and respective lipid mediators within a 24–48 h window after an MI event. Within the heterogeneity of risk factors and severity of MI events, race‐based and sex‐based SPM signatures can potentially provide additional stratification.
Study limitations
Our study has limitations; our data are from a single academic centre and a single time point with a limited sample size with heterogeneous CV risk profiles and severity of the myocardial damage from the infarction. Although the inclusion of healthy controls could have allowed us to assess SPM signatures in patients with stable CHD, based on current evidence, we expect these to be below the level of detection or low abundance in the absence of infarction. Measured SPM signatures vary based on dietary fatty acids, marine oils‐based supplements, co‐medications (e.g. aspirin), the magnitude of infarction (minor, major, or massive), or ageing. We did not have data on diet or over‐the‐counter medications and recognize that these factors need to be considered in interpreting levels of lipid mediators in plasma of the individual patient.37, 46, 47, 48 The influence of SES on CV disease is widely recognized. Although we collected information on education, income, and health insurance, we could not stratify by SES due to the small sample size. The differences in SPM signatures need to be replicated in independent patient cohorts.
In conclusion, this study identified the race‐based and sex‐based SPM signatures following an acute MI event. These data show a distinct signature of SPMs species in Black and White post‐MI patients highlighting differences in pathophysiology. Understanding these differences can facilitate tailoring of HF medical management and provide precision medicine. This study in humans confirms data from rodent studies demonstrating the overlapping timeline of inflammation and resolution following cardiac injury and highlights the importance of these processes for cardiac repair after an MI event. Assessment of SPM signatures may support the development of prognostic and diagnostic tools enabling quantification of resolution potential.
Conflict of interest
The authors declare no competing financial interests.
Author contributions
G.V.H. and N.A.L. conceived, designed, co‐ordinated plasma samples, and executed the project. Plasma samples were analysed in double‐blinded manner. G.V.H., N.A.L., V.K., T.D., and S.O. performed the analyses, interpreted the results, and drafted the manuscript. G.V.H., N.A.L., V.K., S.O., and T.D. revised the manuscript and approved for the submission.
Funding
Authors acknowledge the funding support from National Institutes of Health (HL132989 and HL144788) to G.V.H. and National Institutes of Health (R01HL092173 and K24HL133373) and Clinical and Translational Science Award (UL1TR000165) to N.A.L.
Supporting information
Figure S1. Overlaid total ion chromatograms for selected samples
Figure S2. Quantitative levels of 12‐HEPE and representative MS Spectra peaks in black and white patients after an MI event.
Figure S3. Quantified levels in ng/ml of proinflammatory lipid mediators (A) 5‐iso‐PGF2α VI (B) dhk‐PGF2α (C) 6 k‐PGE1 (D) TxB3 (E) TxB2 (F) LTB4 in black and white patients within 24–48 h post‐MI.
Table S1. List of liquid chromatography retention time and multiple reaction monitoring transitions for 3 fatty acids and differential 40 lipid mediators.
Halade, G. V. , Kain, V. , Dillion, C. , Beasley, M. , Dudenbostel, T. , Oparil, S. , and Limdi, N. A. (2020) Race‐based and sex‐based differences in bioactive lipid mediators after myocardial infarction. ESC Heart Failure, 7: 1700–1710. 10.1002/ehf2.12730.
References
- 1. Khazanie P, Krumholz HM, Kiefe CI, Kressin NR, Wells B, Wang TY, Peterson ED. Priorities for cardiovascular outcomes research: a report of the National Heart, Lung, and Blood Institute's Centers for Cardiovascular Outcomes Research Working Group. Circ Cardiovasc Qual Outcomes 2017; 10: pii: e001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS. Heart Disease and Stroke Statistics–2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi T, Glorioso TJ, Armstrong EJ, Maddox TM, Plomondon ME, Grunwald GK, Bradley SM, Tsai TT, Waldo SW, Rao SV, Banerjee S, Nallamothu BK, Bhatt DL, Rene AG, Wilensky RL, Groeneveld PW, Giri J. Comparative outcomes after percutaneous coronary intervention among Black and White patients treated at US Veterans Affairs Hospitals. JAMA Cardiol 2017; 2: 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hess CN, Kaltenbach LA, Doll JA, Cohen DJ, Peterson ED, Wang TY. Race and sex differences in post‐myocardial infarction angina frequency and risk of 1‐year unplanned rehospitalization. Circulation 2017; 135: 532–543. [DOI] [PubMed] [Google Scholar]
- 5. Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G. Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012; 308: 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li S, Fonarow GC, Mukamal KJ, Liang L, Schulte PJ, Smith EE, DeVore A, Hernandez AF, Peterson ED, Bhatt DL. Sex and race/ethnicity‐related disparities in care and outcomes after hospitalization for coronary artery disease among older adults. Circ Cardiovasc Qual Outcomes 2016; 9: S36–S44. [DOI] [PubMed] [Google Scholar]
- 7. Pradhan J, Schreiber TL, Niraj A, Veeranna V, Ramesh K, Saigh L, Afonso L. Comparison of five‐year outcome in African Americans versus Caucasians following percutaneous coronary intervention. Catheter Cardiovasc Interv 2008; 72: 36–44. [DOI] [PubMed] [Google Scholar]
- 8. Iantorno M, Torguson R, Kolm P, Gajanana D, Suddath WO, Rogers T, Bernardo NL, Ben‐Dor I, Gai J, Satler LF, Garcia‐Garcia HM, Weintraub WS, Waksman R. Relation of sex and race to outcomes in patients undergoing percutaneous intervention with drug‐eluting stents. Am J Cardiol 2019; 123: 913–918. [DOI] [PubMed] [Google Scholar]
- 9. Mensah GA. Black–White disparities: More than just race. J Am Heart Assoc 2019; 8: e014272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai A, Dillon C, Hillegass WB, Beasley M, Brott BC, Bittner VA, Perry GJ, Halade GV, Prabhu SD, Limdi NA. Risk of major adverse cardiovascular events and major hemorrhage among White and Black patients undergoing percutaneous coronary intervention. J Am Heart Assoc 2019; 8: e012874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 12. Howard G, Safford MM, Moy CS, Howard VJ, Kleindorfer DO, Unverzagt FW, Soliman EZ, Flaherty ML, McClure LA, Lackland DT, Wadley VG, Pulley L, Cushman M. Racial differences in the incidence of cardiovascular risk factors in older Black and White adults. J Am Geriatr Soc 2017; 65: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Failure 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 14. Pullen AB, Jadapalli JK, Rhourri‐Frih B, Halade GV. Re‐evaluating the causes and consequences of non‐resolving inflammation in chronic cardiovascular disease. Heart Fail Rev 202025: 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360: 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med 2019; 381: 1557–1567. [DOI] [PubMed] [Google Scholar]
- 17. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet 2007; 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 18. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11–22. [DOI] [PubMed] [Google Scholar]
- 19. Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol 2014; 109: 444. [DOI] [PubMed] [Google Scholar]
- 20. Halade GV, Norris PC, Kain V, Serhan CN, Ingle KA. Splenic leukocytes define the resolution of inflammation in heart failure. Sci Signal 2018; 11: pii: eaao1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halade GV, Dorbane A, Ingle KA, Kain V, Schmitter JM, Rhourri‐Frih B. Comprehensive targeted and non‐targeted lipidomics analyses in failing and non‐failing heart. Anal Bioanal Chem 2018; 410: 1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Back M, Yurdagul A Jr, Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 2019; 16: 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peters‐Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med 2007; 357: 1841–1854. [DOI] [PubMed] [Google Scholar]
- 24. Halade GV, Kain V. Obesity and cardiometabolic defects in heart failure pathology. Compr Physiol 2017; 7: 1463–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kasikara C, Doran AC, Cai B, Tabas I. The role of non‐resolving inflammation in atherosclerosis. J Clin Invest 2018; 128: 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature 2014; 510: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, Manly JJ, Flaherty ML, Judd SE, Wadley VG, Long DL, Howard VJ. Association of clinical and social factors with excess hypertension risk in Black compared with White US adults. JAMA 2018; 320: 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, Johnson CA, Kausz A, Kimmel PL, Kusek J, Levin A. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 29. Chen GY, Zhang Q. Comprehensive analysis of oxylipins in human plasma using reversed‐phase liquid chromatography‐triple quadrupole mass spectrometry with heatmap‐assisted selection of transitions. Anal Bioanal Chem 2019; 411: 367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, Mozaffarian D. Circulating omega‐6 polyunsaturated fatty acids and total and cause‐specific mortality: the Cardiovascular Health Study. Circulation 2014; 130: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marklund M, Wu JHY, Imamura F, del Gobbo LC, Fretts A, de Goede J, Shi P, Tintle N, Wennberg M, Aslibekyan S, Chen TA, de Oliveira Otto MC, Hirakawa Y, Eriksen HH, Kroger J, Laguzzi F, Lankinen M, Murphy RA, Prem K, Samieri C, Virtanen J, Wood AC, Wong K, Yang WS, Zhou X, Baylin A, Boer JMA, Brouwer IA, Campos H, Chaves PHM, Chien KL, de Faire U, Djousse L, Eiriksdottir G, El‐Abbadi N, Forouhi NG, Michael Gaziano J, Geleijnse JM, Gigante B, Giles G, Guallar E, Gudnason V, Harris T, Harris WS, Helmer C, Hellenius ML, Hodge A, Hu FB, Jacques PF, Jansson JH, Kalsbeek A, Khaw KT, Koh WP, Laakso M, Leander K, Lin HJ, Lind L, Luben R, Luo J, McKnight B, Mursu J, Ninomiya T, Overvad K, Psaty BM, Rimm E, Schulze MB, Siscovick D, Skjelbo Nielsen M, Smith AV, Steffen BT, Steffen L, Sun Q, Sundstrom J, Tsai MY, Tunstall‐Pedoe H, Uusitupa MIJ, van Dam RM, Veenstra J, Monique Verschuren WM, Wareham N, Willett W, Woodward M, Yuan JM, Micha R, Lemaitre RN, Mozaffarian D, Riserus U. Biomarkers of dietary omega‐6 fatty acids and incident cardiovascular disease and mortality. Circulation 2019; 139: 2422–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamleh MA, McLeod O, Checa A, Baldassarre D, Veglia F, Gertow K, Humphries SE, Rauramaa R, de Faire U, Smit AJ, Giral P, Kurl S, Mannarino E, Tremoli E, Silveira A, Orvik J, Hamsten A, Wheelock CE. Increased levels of circulating fatty acids are associated with protective effects against future cardiovascular events in nondiabetics. J Proteome Res 2018; 17: 870–878. [DOI] [PubMed] [Google Scholar]
- 33. Halade GV, Black LM, Verma MK. Paradigm shift–metabolic transformation of docosahexaenoic and eicosapentaenoic acids to bioactives exemplify the promise of fatty acid drug discovery. Biotechnol Adv 2018; 36: 935–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018; 243: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tourki B, Kain V, Pullen AB, Norris PC, Patel N, Arora P, Leroy X, Serhan CN, Halade GV. Lack of resolution sensor drives age‐related cardiometabolic and cardiorenal defects and impedes inflammation‐resolution in heart failure. Molecul Metabol 2020; 31: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fosshaug LE, Colas RA, Anstensrud AK, Gregersen I, Nymo S, Sagen EL, Michelsen A, Vinge LE, Oie E, Gullestad L, Halvorsen B, Hansen TV, Aukrust P, Dalli J, Yndestad A. Early increase of specialized pro‐resolving lipid mediators in patients with ST‐elevation myocardial infarction. EBioMedicine 2019; 46: 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D‐ and E‐series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 2016; 8: 2611–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, Investigators R‐I. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11–22. [DOI] [PubMed] [Google Scholar]
- 39. Block RC, Liu L, Herrington DM, Huang S, Tsai MY, O'Connell TD, Shearer GC. Predicting risk for incident heart failure with omega‐3 fatty acids: from MESA. JACC Heart Fail 2019; 7: 651–661. [DOI] [PubMed] [Google Scholar]
- 40. Grenon SM, Owens CD, Nosova EV, Hughes‐Fulford M, Alley HF, Chong K, Perez S, Yen PK, Boscardin J, Hellmann J, Spite M, Conte MS. Short‐term, high‐dose fish oil supplementation increases the production of omega‐3 fatty acid‐derived mediators in patients with peripheral artery disease (the OMEGA‐PAD I trial). J Am Heart Assoc 2015; 4: e002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu G, Liu Q, Shen Y, Kong D, Gong Y, Tao B, Chen G, Guo S, Li J, Zuo S, Yu Y, Yin H, Zhang L, Zhou B, Funk CD, Zhang J, Yu Y. Early treatment with resolvin E1 facilitates myocardial recovery from ischaemia in mice. Br J Pharmacol 2018; 175: 1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 2015; 84: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005; 6: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 44. Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro‐resolving lipid mediators and pro‐inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun 2016; 7: 12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Colas RA, Souza PR, Walker ME, Burton M, Zaslona Z, Curtis AM, Marques RM, Dalli J. Impaired production and diurnal regulation of vascular RvDn‐3 DPA increase systemic inflammation and cardiovascular disease. Circ Res 2018; 122: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Halade GV, Kain V, Wright GM, Jadapalli JK. Subacute treatment of carprofen facilitate splenocardiac resolution deficit in cardiac injury. J Leukoc Biol 2018; 104: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jadapalli JK, Wright GW, Kain V, Sherwani MA, Sonkar R, Yusuf N, Halade GV. Doxorubicin triggers splenic contraction and irreversible dysregulation of COX and LOX that alters the inflammation‐resolution program in the myocardium. Am J Physiol Heart Circ Physiol 2018; 315: H1091–h1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15‐epi‐lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A 2004; 101: 15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overlaid total ion chromatograms for selected samples
Figure S2. Quantitative levels of 12‐HEPE and representative MS Spectra peaks in black and white patients after an MI event.
Figure S3. Quantified levels in ng/ml of proinflammatory lipid mediators (A) 5‐iso‐PGF2α VI (B) dhk‐PGF2α (C) 6 k‐PGE1 (D) TxB3 (E) TxB2 (F) LTB4 in black and white patients within 24–48 h post‐MI.
Table S1. List of liquid chromatography retention time and multiple reaction monitoring transitions for 3 fatty acids and differential 40 lipid mediators.


