Abstract
Aims
The aim of this study was to compare the outcomes of surgical mitral valve repair or replacement (sMVR) and percutaneous edge‐to‐edge repair using MitraClip (pMVR) in patients with severe left ventricular dysfunction affected by functional mitral regurgitation (FMR).
Methods and results
We retrospectively identified 132 patients with left ventricular ejection fraction (LVEF) ≦ 30% submitted to sMVR (n = 47) or pMVR (n = 85) for FMR at our centre from January 2013 to December 2017. To adjust for baseline imbalances, we used a propensity score matching by age, logistic EuroSCORE, and left ventricular end‐systolic volume. After being matched, MitraClip therapy showed lower perioperative mortality and rate of complications yet increased residual mitral regurgitation (MR) grade than did surgery (0.2 ± 0.50 in sMVR vs. 1.3 ± 0.88 in pMVR, P < 0.0001). According to stratified multivariate Cox model analysis, residual MR severity was an independent risk factor for cardiac death [hazard ratio (HR), 2.81; 95% confidence interval [CI], 1.44–5.48, P = 0.0025] and re‐hospitalization for heart failure (HR, 3.07; 95% CI, 1.50–6.29, P = 0.0022) at 1 year follow‐up. Stratified multivariable Cox regression analysis at 3 years identified pMVR as risk factor for cardiac death (HR, 0.19; 95% CI, 0.040–0.86, P = 0.031) and re‐hospitalization for heart failure (HR, 0.28; 95% CI, 0.077–0.99, P = 0.048).
Conclusions
In patients with FMR and LVEF ≤ 30%, MitraClip therapy resulted in lower perioperative complications and mortality than sMVR. However, surgically treated patients who survived the perioperative stage had less residual MR and experienced lower rates of re‐hospitalization for heart failure at 1 year and lower cardiac mortality at 1 and 3 years of follow‐up than did patients undergoing pMVR.
Keywords: Heart failure, Left ventricular dysfunction, Functional mitral regurgitation, MitraClip, Mitral valve surgery
Introduction
Functional mitral regurgitation (FMR) is frequently observed in patients with ischaemic and non‐ischaemic cardiomyopathy and is associated with poor clinical outcome in patients with heart failure (HF) with reduced ejection fraction (HFrEF) due to dilative left ventricular remodelling. 1 , 2 While FMR is regarded by many as a marker rather than a driver of poor outcome in these patients, non‐pharmacological treatment of FMR is frequently considered by the treating physicians of these patients. 3 Although surgical mitral valve repair or replacement (sMVR) is regarded as the gold standard for therapy, in clinical practice, about 50% of patients with severe FMR are not referred for surgery owing to perceived high surgical risk. 4 , 5 , 6 Frequently, these are elderly patients (age > 80 years) with relevant co‐morbidities and severely reduced left ventricular ejection function (LVEF < 30%). 7 , 8 Since 2013, percutaneous edge‐to‐edge transcatheter mitral valve repair (pMVR) with the MitraClip system (Abbott Vascular, Menlo Park, CA) for FMR in patients with high risk of perioperative mortality and co‐morbidities is available and has become increasingly favoured over sMVR, representing a less invasive beating‐heart interventional technique. 9 , 10 , 11 , 12 , 13 In addition, while the MITRA‐FR (Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation) study did not find a significant benefit of sMVR over optical medical therapy, another recent randomized trial [‘Cardiovascular outcomes assessment of the MitraClip percutaneous therapy for heart failure patients with functional mitral regurgitation' (COAPT) trial] suggested that pMVR is superior to medical therapy with regard to survival and recurrent hospitalization for HF. 14 In the eyes of many readers, these results further support the use of pMVR with MitraClip in this population, despite the fact that both American and European guidelines only see a low level of evidence (class IIb) to support any procedure for correcting mitral regurgitation in patients with functional, not primary mitral regurgitation. 15 , 16
FMR in patients with HFrEF is often associated with functional tricuspid regurgitation, atrial fibrillation, or ischaemic disease. In sMVR, concomitant procedures can be performed to address these conditions including tricuspid valve surgery, maze procedures, and coronary artery bypass grafting (CABG). Meanwhile, pMVR—as a concept of minimal invasive therapy—consists of an isolated intervention on the mitral valve regardless of concomitant abnormalities. In the absence of conclusive evidence from randomized controlled trials, these two strategies have naturally been the subject of a contentious debate to determine which modality is superior in symptomatic patients with HFrEF and FMR. 17 In our study, we sought to compare the clinical outcomes (freedom from re‐hospitalization and cardiac death) after sMVR vs. pMVR in symptomatic patients with FMR and severe left ventricular dysfunction (LVEF ≦ 30%).
Methods
Study design and follow‐up
This single‐centre study was approved by the Institutional Review Board of Sana Heart Center in Cottbus (Germany). Hospital records were screened to retrospectively identify patients with moderate to severe FMR and severely reduced LVEF (≤30%) who were treated in our hospital between January 2013 and December 2017 with pMVR or sMVR. Treatment was performed electively (with elective admission) or urgently (during a non‐elective admission for HF). Patients with acute emergency treatment, endocarditis, mitral valve stenosis, sMVR after MitraClip implantation, or redo‐sMVR were excluded. The clinical course of all patients was retrospectively reviewed based on patients' charts as assessed in clinical routine preoperatively, post‐operatively in the intensive care unit, and at discharge. Follow‐up data of clinical status and transthoracic echocardiography were obtained from the patients' general practitioners or private cardiologists by telephone and fax communication and were complete in 91% of patients. The clinical follow‐up was closed on 31 December 2018, when the last enrolled patient had completed 1 year of follow‐up. Endpoints of the study were first re‐hospitalization for HF or cardiovascular death. Re‐hospitalization for HF was defined as new‐onset or worsening signs and symptoms of HF that require urgent therapy and result in hospitalization.
Surgical procedures
All procedures were performed by experienced board‐certified cardiovascular surgeons via median sternotomy or right thoracotomy. sMVR with concomitant procedures including aortic valve replacement, tricuspid valve repair (TVR), CABG, pulmonary vein ablation and other procedures [atrial septal defect (ASD) closure and resection of left atrial appendage] were performed by full sternotomy, if possible. With sternotomy, cardiopulmonary bypass (CPB) was established through direct cannulation of ascending aorta and right atrium vein, or superior vena cava/inferior vena cava in cases requiring TVR and ASD closure. In the right‐thoracotomy approach, skin incision in the fourth intercostal space was performed after establishing CPB through femoral artery and vein cannulation. Access to mitral valve was gained either via a direct left atrial or transseptal incision depending on the need for TVR and ASD closure. Standard MV replacement and MV annuloplasty with semi‐rigid Colvin–Galloway ring were performed on the arrested heart under normal temperature (36°C). Intraoperative transesophageal echocardiography (TEE) was performed to verify that there was no mitral regurgitation (MR) in MV replacement and residual MR grade 0–1 in MV repair. All patients were treated with warfarin as anticoagulation therapy for three post‐operative months.
Interventional procedures
All interventional procedures with MitraClips were by one experienced interventional cardiologist. All clips (arm length 9 mm) were implanted according to standard practices under general anaesthesia with TEE and fluoroscopic guidance. Maximum residual MR grade 2 at a mean blood pressure of ≥ 60 mmHg was regarded as an acceptable procedural result; additional clips were placed until this requirement was met.
Choice of treatment strategy in clinical routine
Patients were regarded as potential candidates for pMVR if they met basic criteria for intervention from the German Cardiac Society. 18 The local heart team (consisting of a cardiologist, a cardiac surgeon, a perfusionist, and a cardio‐anaesthetist) discussed the individual therapeutic approach based on age, surgical risk as estimated by logistic EuroSCORE, cardiac and extra‐cardiac co‐morbidities, and mitral valve morphology as assessed by TEE.
Statistical analysis
Results are expressed as mean ± standard deviation (SD) or as median + 25th to 75th percentile interquartile range for continuous variables, and frequency and percentage for categorical variables. Univariable comparisons were performed with Student's unpaired t‐test for continuous normally distributed data. The Mann–Whitney U test was used for comparisons of non‐parametric continuous data and Fisher's exact test for categorical data. Survival and freedom from cardiac events were derived using the Kaplan–Meier method; comparisons were made using the log‐rank test. Patient characteristics of the pMVR and sMVR groups were compared, and propensity score (PS) analysis was performed to adjust for the three major aspects where significant differences were detected. Patients undergoing MitraClip therapy were matched on a one‐on‐one basis to patients undergoing surgical treatment on the basis of PSs by use of nearest‐neighbour matching without replacement with a matching tolerance (calliper) of 0.25 and an absolute standardized difference of ≦10%. Rates of freedom from re‐hospitalization for HF and cardiac death in the matched cohort were generated using the Kaplan–Meier method, and comparisons were made using the stratified log‐rank test. To estimate the independent effects for re‐hospitalization for HF and cardiac death, multivariable Cox proportional‐hazards regression analysis, also stratified on the matched pairs, was subsequently applied to the matched population to identify any independent predictor of mortality. Covariates were included via stepwise regression analysis using a probability for stepwise entry of 0.05. Candidate covariates were chosen based on previous medical knowledge. P‐value < 0.05 was considered statistically significant, and all reported P‐values are two‐sided. Statistical analysis was performed using SPSS for windows version 22.0 (IBM Japan, Tokyo, Japan).
Results
Baseline characteristics and perioperative outcomes in the full cohort
A total of 132 patients with moderate to severe FMR and preoperative LVEF ≦ 30% were retrospectively identified and included in the study. Of 132 patients, 47 patients (36%) underwent sMVR (n = 28/47, 60% ischemic; n = 19/47, 40% non‐ischemic) and 85 patients (64%) underwent pMVR (n = 41/85, 48% ischemic; n = 44/85, 52% non‐ischaemic) in our centre. All patients underwent coronary angiography prior to MVR to assess coronary artery state. Demographic and clinical features are shown in Table 1 .
Table 1.
Baseline characteristics of the full cohort and PS‐matched cohort; n (%) if not otherwise specified
| Full cohort | Total | sMVR | pMVR | P‐value |
|---|---|---|---|---|
| n = 132 | n = 47 | n = 85 | ||
| Age, mean ± SD (years) | 70 ± 9.0 | 68 ± 9.6 | 72 ± 8.5 | 0.0429 |
| Age ≧ 80 years old | 17 (13%) | 3 (6%) | 14 (16%) | 0.112 |
| Male gender | 91 (69%) | 28 (60%) | 63 (74%) | 0.116 |
| Body mass index, mean ± SD (kg/m2) | 26 ± 4.9 | 27 ± 5.5 | 26 ± 4.6 | 0.655 |
| COLD | 22 (17%) | 7 (15%) | 15 (18%) | 0.809 |
| Arterial hypertension | 118 (89%) | 41 (87%) | 77 (91%) | 0.566 |
| Chronic renal disease | 48 (36%) | 12 (26%) | 36 (42%) | 0.0611 |
| Diabetes mellitus | 51 (39%) | 14 (30%) | 37 (44%) | 0.138 |
| Logistic EuroSCORE, mean ± SD | 31 ± 21 | 25.0 ± 22 | 33.5 ± 20 | 0.0377 |
| EuroSCORE, mean ± SD | 16 ± 13 | 15 ± 13 | 16 ± 14 | 0.853 |
| Ischaemic cardiomyopathy | 69 (52%) | 28 (60%) | 41(48%) | 0.275 |
| Dilated cardiomyopathy | 63 (48%) | 19 (40%) | 44 (52%) | 0.275 |
| Atrial fibrillation | 77 (58%) | 26 (55%) | 51 (60%) | 0.713 |
| Previous CRT | 43 (33%) | 6 (13%) | 37 (44%) | <0.001 |
| Previous ICD | 46 (35%) | 3 (6%) | 43 (51%) | <0.001 |
| Previous cardiac surgery | 31 (23%) | 5 (11%) | 26 (31%) | 0.0102 |
| Previous percutaneous coronary intervention | 45 (34%) | 9 (19%) | 36 (42%) | 0.0121 |
| NYHA functional class, mean ± SD | 3.2 ± 0.46 | 3.2 ± 0.46 | 3.2 ± 0.46 | 0.683 |
| NYHA II | 2 (2%) | 1 (2%) | 1 (1%) | |
| NYHA III | 97 (73%) | 35 (74%) | 62 (73%) | |
| NYHA IV | 33 (25%) | 11 (23%) | 22 (26%) | |
| Medication | ||||
| ACE inhibitor/ARB | 94 (71%) | 31 (66%) | 63 (74%) | 0.856 |
| Beta‐blocker | 113 (86%) | 40 (85%) | 73 (86%) | 1 |
| Mineralocorticoid receptor antagonist | 81 (61%) | 17 (36%) | 64 (75%) | <0.01 |
| Loop diuretics | 118 (89%) | 37 (79%) | 81(95%) | 0.00592 |
| Digitoxin | 27 (20%) | 6 (13%) | 21(25%) | 0.119 |
| PS‐matched cohort | Total | SMVR | PMVR | P‐value |
|---|---|---|---|---|
| n = 60 | n = 30 | n = 30 | ||
| Age, mean ± SD (years) | 71 ± 8.3 | 71 ± 8.5 | 71 ± 8.2 | 0.963 |
| Age ≧ 80 years old | 6 (10%) | 3 (10%) | 3 (10%) | 1 |
| Male gender | 38 (63%) | 17(61%) | 21(70%) | 0.422 |
| Body mass index, mean ± SD (kg/m2) | 27 ± 4.4 | 26 ± 3.7 | 28 ± 4.9 | 0.122 |
| COLD | 10 (17%) | 5 (17%) | 5 (17%) | 1 |
| Arterial hypertension | 56 (93%) | 27 (90%) | 29 (97%) | 0.612 |
| Chronic renal disease | 23 (38%) | 9 (30%) | 14 (47%) | 0.288 |
| Diabetes mellitus | 22 (37%) | 7 (23%) | 15 (50%) | 0.06 |
| Logistic EuroSCORE, mean ± SD | 30 ± 20 | 30 ± 24 | 29 ± 16 | 0.859 |
| EuroSCORE, mean ± SD | 14 ± 13 | 17 ± 15 | 11 ± 9.8 | 0.104 |
| Ischaemic cardiomyopathy | 31 (52%) | 16 (53%) | 15 (50%) | 1 |
| Dilated cardiomyopathy | 29 (48%) | 14 (47%) | 15 (50%) | 1 |
| Atrial fibrillation | 33 (55%) | 20 (67%) | 13 (43%) | 0.119 |
| Previous CRT | 16 (27%) | 6 (20%) | 10 (33%) | 0.382 |
| Previous ICD | 17 (28%) | 1 (3.3%) | 16 (53%) | <0.001 |
| Previous cardiac surgery | 9 (15%) | 4 (13%) | 5 (17%) | 1 |
| Previous percutaneous coronary intervention | 16 (27%) | 6 (20%) | 10 (3.3%) | 0.0292 |
| NYHA functional class, mean ± SD | 3.2 ± 0.46 | 3.2 ± 0.48 | 3.1 ± 0.43 | 0.577 |
| NYHA II | 2 (3%) | 1 (3.3%) | 1 (3.3%) | |
| NYHA III | 46 (77%) | 22 (73%) | 24 (80%) | |
| NYHA IV | 12 (20%) | 7 (23%) | 5 (17%) | |
| Medication | ||||
| ACE inhibitor/ARB | 47 (78%) | 19 (63%) | 28 (93%) | 0.0102 |
| Beta‐blocker | 53 (88%) | 26 (87%) | 27 (90%) | 1 |
| Mineralocorticoid receptor antagonist | 30 (50%) | 10 (33%) | 20 (66%) | 0.0194 |
| Loop diuretics | 51 (85%) | 24 (80%) | 27 (90%) | 0.472 |
| Digitoxin | 10 (17%) | 5 (17%) | 5 (17%) | 1 |
ARB, angiotensin receptor blocker; COLD, chronic obstructive lung disease; CRT, cardiac resynchronization therapy; ICD, implanted cardioverter defibrillator.
Patients in the pMVR group were older (P = 0.043) and had a higher predicted surgical risk by logistic EuroSCORE (P = 0.038) than are sMVR patients. The prevalence of implanted cardioverter defibrillator (ICD) or cardiac resynchronization therapy device therapy, previous cardiac surgery, percutaneous coronary artery intervention, and use of spironolactone were significantly higher in the pMVR than in the sMVR group (Table 1 ). Preoperative echocardiography revealed lower LVEF (P < 0.001) and higher LV volumes [P < 0.001 for LV end‐diastolic volume and P < 0.001 for left ventricular end‐systolic volume LV end‐systolic volume (LVESV)] and reduced right ventricular function (P = 0.012 for tricuspid annular plane systolic excursion) in the pMVR group. Table 2 shows preoperative echocardiography data of both groups in more detail.
Table 2.
Baseline results of transthoracic echocardiography in the full cohort and the PS‐matched cohort; n (%) if not otherwise specified
| Full cohort | sMVR | pMVR | P‐value |
|---|---|---|---|
| n = 47 | n = 85 | ||
| LVEF mean ± SD (%) | 26 ± 5.2 | 22 ± 5.3 | <0.001 |
| MR grade, mean ± SD | 3 ± 0.44 | 3 ± 0.35 | 0.76 |
| MR grade 2 | 5 (11%) | 5 (6%) | |
| MR grade 3 | 38 (81%) | 75 (88%) | |
| MR grade 4 | 4 (8%) | 5 (6%) | |
| TR grade, mean ± SD | 1.6 ± 0.93 | 1.7 ± 0.75 | 0.478 |
| TR grade 0 | 5 (11%) | 3 (3.5%) | |
| TR grade 1 | 20 (42%) | 34 (40%) | |
| TR grade 2 | 13 (28%) | 37 (43.5%) | |
| TR grade 3 | 9 (19%) | 11 (13%) | |
| RVESP, mean ± SD (mmHg) | 49 ± 3.1 | 54 ± 15 | 0.0731 |
| LVDd, mean ± SD (mm) | 70 ± 7.3 | 73 ± 6.4 | 0.0674 |
| LVDs, mean ± SD (mm) | 63 ± 8.4 | 66 ± 7.2 | 0.0974 |
| RVDd, mean ± SD (mm) | 40 ± 6.0 | 39 ± 6.3 | 0.876 |
| RVDs, mean ± SD (mm) | 32 ± 5.7 | 34 ± 6.2 | 0.275 |
| LA, mean ± SD (mm) | 53 ± 6.5 | 53 ± 8.4 | 0.797 |
| TAPSE, mean ± SD (mm) | 17 ± 4.2 | 14 ± 4.6 | 0.00206 |
| LVEDV, mean ± SD (mL) | 197 ± 71 | 243 ± 68 | 0.00056 |
| LVESV, mean ± SD (mL) | 133 ± 58 | 182 ± 64 | 0.00004 |
| LVSV, mean ± SD (mL) | 64 ± 30 | 61 ± 28 | 0.604 |
| PS‐matched cohort | sMVR | pMVR | P‐value |
|---|---|---|---|
| n = 30 | n = 30 | ||
| LVEF mean ± SD (%) | 25 ± 5.8 | 22 ± 5.2 | 0.05 |
| MR grade, mean ± SD | 3.0 ± 0.32 | 3.0 ± 0.32 | 1 |
| MR grade 2 | 1 (3.3%) | 1 (3.3%) | |
| MR grade 3 | 27 (90%) | 27 (90%) | |
| MR grade 4 | 2 (6.7%) | 2 (6.7%) | |
| TR grade, mean ± SD | 1.6 ± 1.0 | 1.5 ± 0.73 | 0.549 |
| TR grade 0 | 3 (10%) | 2 (6.7%) | |
| TR grade 1 | 13 (43%) | 14 (47%) | |
| TR grade 2 | 7 (23%) | 12 (40%) | |
| TR grade 3 | 7 (23%) | 2 (6.7%) | |
| RVESP, mean ± SD (mmHg) | 46 ± 12 | 48 ± 18 | 0.389 |
| LVDd, mean ± SD (mm) | 72 ± 8.0 | 71 ± 5.2 | 0.28 |
| LVDs, mean ± SD (mm) | 66 ± 9.0 | 63 ± 6.0 | 0.165 |
| RVDd, mean ± SD (mm) | 41 ± 6.2 | 39 ± 7.2 | 0.196 |
| RVDs, mean ± SD (mm) | 34 ± 5.6 | 33 ± 7.4 | 0.755 |
| LA, mean ± SD (mm) | 53 ± 7.4 | 50 ± 5.7 | 0.14 |
| TAPSE, mean ± SD (mm) | 16 ± 4.8 | 14 ± 4.2 | 0.0503 |
| LVEDV, mean ± SD (mL) | 217 ± 71 | 215 ± 58 | 0.923 |
| LVESV, mean ± SD (mL) | 149 ± 61 | 151 ± 50 | 0.882 |
| LVSV, mean ± SD (mL) | 68 ± 30 | 64 ± 27 | 0.611 |
EDV, end‐diastolic volume; ESV, end‐systolic volume; LA, left atrium; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; MR, mitral valve regurgitation; RVDd, right ventricular diastolic diameter; RVDs, right ventricular systolic diameter; RVESP, right ventricular end‐systolic pressure; SV, systolic volume; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid valve regurgitation.
In the sMVR group (35% urgent), post‐operative MR grade was ≦2 in all patients. In the pMVR group (1.1% urgent), 85 patients had an average of 2.1 ± 0.75 clips with post‐interventional MR grade ≦ 2 in 97.6%. Post‐operative MR grade at discharge was reduced to a mean ± SD of 0.17 ± 0.44 in sMVR as compared with 1.4 ± 0.69 in pMVR (P < 0.001). Intensive care unit (ICU) stay and hospital stay were shorter in the pMVR group (P < 0.001); 47% (n = 22/47) of sMVR patients were discharged to home as compared with 86% (73/85) in pMVR (P < 0.001). There was a trend towards higher in‐hospital mortality (P = 0.0966) and 30 day mortality (P = 0.132) in sMVR that did not reach statistical significance.
Propensity score matching
To minimize potential effects of selection bias and to decrease variability of both groups, a second series of analyses were performed on selected pMVR and sMVR patients with corresponding clinical and echocardiography characteristics on the basis of PS matching.
Baseline characteristics and perioperative outcomes in the propensity score‐matched cohort
Based on the results of the logistic regression analysis, significant differences in characteristics of sMVR and pMVR groups were found for age, logistic EuroSCORE, and LVESV. 19 Accordingly, PS matching was performed for age, logistic EuroSCORE, and LVESV (absolute standardized difference 1%, 5%, and 4%, respectively), resulting in 30 matching pairs of pMVR and sMVR subjects. In the PS‐matched cohort, only ICD implantation, history of PCI, systolic pulmonary hypertension, and preoperative use of renin–angiotensin–aldosterone system‐directed medication remained different (Table 1 ). Results of echocardiography in the matched cohort are shown in Table 2 . In sMVR, simple MV repair/replacement was performed in eight patients (27%), while 22 patients underwent additional procedures (CABG, n = 14; tricuspid valvuloplasty, n = 7; ablation, n = 5). In pMVR, a mean of 2.1 ± 0.73 clips was used with an acute success rate of 93%. Post‐operative MR grade at discharge was reduced to a mean of 0.20 ± 0.50 in sMVR, compared with a mean of 1.33 ± 0.88 in pMVR (P < 0.0001, Table 3 ). In the PS‐matched cohort, one pMVR patient with ischaemic cardiomyopathy who had entered the MitraClip procedure in prior cardiogenic shock subsequently died of low output syndrome. In contrast, four sMVR patients died post‐operatively (cardiogenic shock, n = 2; right HF, n = 1; septic shock, n = 1) at a mean of 6.5 ± 3.3 days despite the use of extracorporeal membrane oxygenation (ECMO) therapy in three (Table 4 ). In pMVR, 25 patients (83%) could be extubated in the hybrid operation hall, and no patients had major perioperative events such as stroke, cardiac tamponade, or clip‐related complications. ICU stay and hospital stay were shorter in the pMVR group (P < 0.001). The rate of discharge to home was higher in pMVR than in sMVR (P ≤ 0.001). Differences in in‐hospital mortality (P = 0.36) or 30 day mortality (P = 0.67) between the two groups did not reach statistical significance (Table S1 ).
Table 3.
Procedural characteristics of PS‐matched cohort; n (%) if not otherwise specified
| sMVR | pMVR | P‐value | |
|---|---|---|---|
| n = 30 | n = 30 | ||
| Urgent | 12 (40%) | 1 (3%) | 0.00105 |
| Elective | 18 (60%) | 29 (97%) | 0.00105 |
| Isolated MV replacement or repair | 8 (27%) | — | — |
| MV replacement + additional procedures | 22 (73%) | — | — |
| MV repair | 8 (27%) | — | — |
| Redo‐surgery | 4 (13%) | — | — |
| Sternotomy | 24 (80%) | — | — |
| RT approach | 6 (20%) | — | — |
| ACC, mean ± SD (min) | 62 ± 23 | — | — |
| ECC, mean ± SD (min) | 107 ± 36 | — | — |
| MitraClip, mean ± SD | — | 2.1 ± 0.78 | — |
| Procedural success rate (MR ≦ 2 grade) | 30 (100%) | 28 (93%) | 0.492 |
| Concomitant procedures | |||
| AVR | 4 (13%) | — | — |
| TVR | 7 (23%) | — | — |
| CABG | 13 (43%) | — | — |
| LA ablation | 5 (17%) | — | — |
| LAA closure | 4 (13%) | — | — |
ACC, aortic cross clamping; AVR, aortic valve replacement; CABG, cardiopulmonary bypass grafting; ECC, extracorporeal circulation; LAA, left atrial appendage; MV, mitral valve; RT, right thoracotomy; TVR, tricuspid valve repair.
Table 4.
Cases of perioperative death in PS‐matched cohort (pMVR n = 1, sMVR n = 4)
| Nr. | Age (years)/gender | DCM/ICM | LVEF | Technical approach | Mode of death | Survival (days) | log EuroSCORE | |
|---|---|---|---|---|---|---|---|---|
| pMVR | 1 | 64/male | ICM | 10% | 1 clip | Low output syndrome, cardiogenic shock | 8 | 43.6 |
| sMVR | 1 | 74/male | ICM | 23% |
redo‐MVR + CABGx3 IABP + ECMO |
Low output syndrome, cardiogenic shock | 4 | 37.2 |
| 2 | 78/male | DCM | 20% | MVR + TVR | Septic shock | 11 | 43.5 | |
| 3 | 66/male | DCM | 10% | MVR + TVR + AVR IABP + ECMO | Low output syndrome, cardiogenic shock | 4 | 33.0 | |
| 4 | 78/male | ICM | 21% | MVR + TVR ECMO | Right heart failure | 7 | 84.5 |
AVR, aortic valve replacement; CABG, coronary artery bypass grafting; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pumping; ICM, ischaemic cardiomyopathy; MVR, mitral valve replacement; TVR, tricuspid valve repair.
Comparison of early and midterm outcomes for re‐hospitalization and cardiac death
For the full cohort, median follow‐up was 24 months for sMVR [inter‐quartile range (IQR) 13–42 months, range 0–65 months] and 23 months for pMVR (IQR 8.4–34 months, range 0.17–70 months). In sMVR, there were no cases of endocarditis, early degeneration of the implanted valve prosthesis, or severe valvular dysfunction requiring redo‐surgery during the follow‐up period. In pMVR, three patients required surgical revision due to recurrent severe MR with partial clip detachment, and four patients needed second‐intervention clip implantations for recurrent severe MR. On Kaplan–Meier analysis of the un‐matched cohort, the rates of freedom from re‐hospitalization for HF and cardiac death were significantly higher for sMVR at 3 years of follow‐up (P = 0.0013 and P = 0.0037, respectively) (Figure 1 ).
Figure 1.
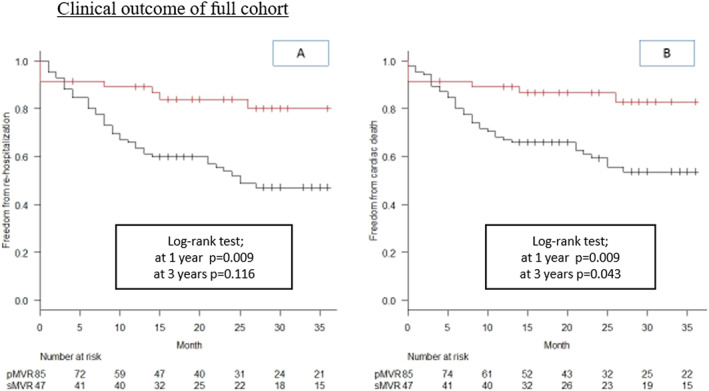
Clinical outcome of full cohort. Kaplan–Meier curves for freedom from re‐hospitalization (A) and freedom from cardiac death (B) for sMVR (red line) vs. pMVR (black line), showing better outcome for sMVR at 3 years (log‐rank P = 0.0013 and P = 0.0037, respectively). sMVR, surgical mitral valve repair or replacement; pMVR, percutaneous edge‐to‐edge repair using MitraClip.
In the PS‐matched cohort, re‐hospitalization for HF and cardiac death was not significantly different in sMVR vs. pMVR groups (stratified log‐rank test: P = 0.28 and P = 0.15, respectively) (Figure 2 ). The rates of freedom from re‐hospitalization for HF and cardiac death were the same at 4 months. Within 4 months after pMVR, three patients died of HF, of which one patient was associated with residual MR grade 4 and the other two had MR grade ≤ 2. At 1 year, the rates of freedom from re‐hospitalization for HF in sMVR and pMVR were 90% (95% CI, 0.79–1.00) and 73.3% (95% CI, 0.58–0.89%), respectively (P = 0.19); and the rates of cardiac death in sMVR and pMVR were 90% (95% CI, 0.79–1.00) and 73.3% (95% CI, 0.58–0.89), respectively (P = 0.094). When comparing only the patients who survived the perioperative phase in the matched cohort (n = 26 in sMVR vs. n = 26 in pMVR), the rates of freedom from re‐hospitalization and cardiac death were significantly higher in the sMVR group than in the pMVR group (P = 0.009 and P = 0.009, respectively) at 1 year follow‐up. This advantage of the sMVR group remained statistically significant at 3 years of follow‐up for freedom from cardiac death (P = 0.043), but not for freedom from re‐hospitalization for HF (stratified log‐rank test; P = 0.12) (Figure S1 ). New York Heart Association grade in survivors at 1 year follow‐up was not significantly different with 2.2 ± 0.59 in sMVR vs. 2.2 ± 0.79 in pMVR patients (P = 0.89).
Figure 2.
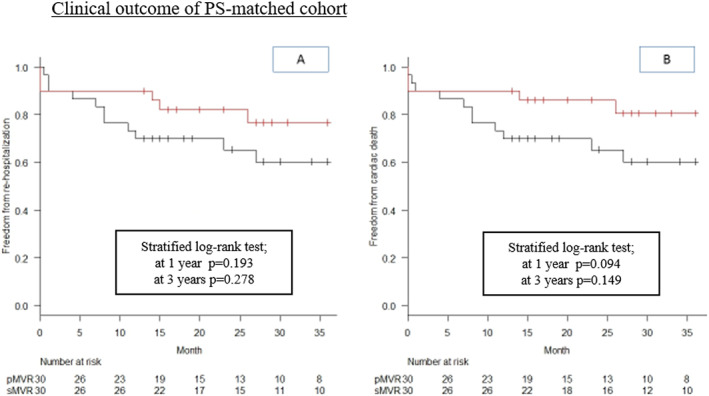
Clinical outcome of PS‐matched cohort. Kaplan–Meier curves for freedom from re‐hospitalization (A) and freedom from cardiac death (B) for sMVR (red line) vs. pMVR (black line), showing better outcome regarding both events for sMVR at 1 year (stratified log‐rank test P = 0.193 and P = 0.094, respectively) and 3 years (stratified log‐rank test P = 0.278 and P = 0.149, respectively). pMVR, percutaneous edge‐to‐edge repair using MitraClip; PS, propensity score; sMVR, surgical mitral valve repair or replacement.
At stratified multivariable Cox regression analysis at 1 year follow‐up, post‐operative MR severity represented an independent risk factor for re‐hospitalization [hazard ratio (HR), 3.07; 95% CI, 1.5–6.3, P = 0.0022] and cardiac death (HR, 2.8; 95% CI, 1.4–5.5, P = 0.0025) across all matched patients (Table S2 ). Stratified multivariable Cox regression analysis at 3 years identified pMVR (vs. sMVR) as risk factor for cardiac death (HR, 0.19; 95% CI, 0.040–0.86, P = 0.048) and for re‐hospitalization for HF (HR, 0.28; 95% CI, 0.077–0.99, P = 0.048). Also, an elevated grade of residual tricuspid valve regurgitation (TR) acted as a risk factor for cardiac death (HR, 2.69; 95% CI, 1.14–0.99, P = 0.048) (Table S3 ).
Discussion
In this study, we compared the clinical outcomes of two different therapeutic approaches in symptomatic patients with severely reduced ejection fraction and functional MR: sMVR, which allows for full repair/replacement and concomitant surgical procedures yet requires cardiac arrest, and pMVR using edge‐to‐edge repair, a less comprehensive approach performed on a beating heart.
There are two main findings of the current study. First, in a cohort of HFrEF patients with treated FMR, perioperative mortality is higher in patients with sMVR than pMVR, yet when surviving the perioperative stage, sMVR is significantly associated with longer freedom from re‐hospitalization (P = 0.048) and cardiac death (P = 0.030) during 3 years of follow‐up as compared with pMVR. Second, residual MR is associated with cardiac death and re‐hospitalization due to HF at 1 year follow‐up (P < 0.01); in pMVR, residual MR grade was an independent risk factor for re‐hospitalization for HF and cardiac death.
Outcome in surgical mitral valve replacement or repair and percutaneous edge‐to‐edge transcatheter mitral valve repair
As expected, pMVR was found to be safer than surgery regarding peri‐procedural risk. With an acute success rate of 97.6% (defined as post‐operative MR grade ≦ 2), the functional results of pMVR in our cohort were better than those recently reported in the MITRA‐FR and COAPT trials. 14 , 20 Perioperative mortality in the PS‐matched pMVR group was only 3.3%, based on one death in a patient presenting with preoperative cardiogenic shock. More than 90% of pMVR patients were stable on medical HF therapy and could be discharged home, even if LVEF was poor. In contrast to sMVR, there were no such complications as respiratory failure with need for re‐intubation, bleeding requiring blood transfusion, or stroke. On the other hand, several patients in the sMVR group needed short‐term circulatory support including ECMO and intra‐aortic balloon pump. Other sMVR patients had to undergo redo‐thoracotomy owing to severe perioperative bleeding, which was associated with prolonged post‐operative ICU stay. In the PS‐matched analysis, perioperative mortality in the sMVR group reached 13% as opposed to 3.3% in the pMVR group, even if the difference was not statistically significant (P = 0.36).
The picture changed once the perioperative stage was over. Four months after the procedure, the same number of patients had died from cardiac death in both groups (13%). At 1 year of follow‐up, mortality and rate of re‐hospitalization for HF were significantly higher in the MitraClip group than in the surgical group, with the difference in mortality remaining significant until 3 years of follow‐up despite the relatively low number of cases available for analysis at this time point. Thus, for those patients who survive the perioperative stage, a surgical approach to treating FMR in HFrEF seems to be superior to percutaneous edge‐to‐edge repair.
Our results indicate that the reason for the favourable midterm/long_x2010;term outcome of short‐term sMVR survivors might be related to the more effective reduction in mitral valve regurgitation in sMVR. There were only four sMVR patients with residual MR > grade 0 (vs. 26 pMVR), and none of these four were free from re‐hospitalization and cardiac death. In pMVR, residual MR grade was an independent risk factor for cardiac death and re‐hospitalization for HF at 1 year follow‐up. According to Notomi et al., post‐operative LVEF is reduced early (1 to 6 months) after correction for mitral regurgitation in severely reduced LVEF, when the MR volume is fully eliminated or at least significantly reduced. 21 In our study, the severity of residual MR was lower in the sMVR group (P < 0.001), and sMVR patients exhibited more reduced post‐operative LV function than pMVR patients. Accordingly, more patients in the PS‐matched cohort died of cardiogenic shock in the days after sMVR than after pMVR. During 1 year of follow‐up, only one patient undergoing pMVR needed mitral valve surgery owing to recurrence of severe MR with mitral ring dilatation, indicating that the durability of the procedural result might be comparable in pMVR and sMVR. However, residual MR (as assessed immediately after the procedure) had a strong impact on cardiac death and re‐hospitalization for HF. Owing to the nature of the MitraClip procedure, pMVR rarely achieved full resolution of MR, while sMVR frequently did. 17 Accordingly, the HRs for cardiac death (HR, 0.33; 95% CI, 0.089–1.2, P = 0.095) and re‐hospitalization for HF (HR, 0.33; 95% CI, 0.090–1.2, P = 0.10) at 1 year follow‐up were lower after sMVR compared with pMVR. Thus, HFrEF patients with FMR whose left ventricles were able to tolerate the immediate effects of full resolution of MR on the long run seemed to benefit more from sMVR than from pMVR.
Interestingly, residual TR was also an independent risk factor for cardiac death in our study (HR, 2.69; 95% CI, 1.1–6.4, P = 0.024), pointing at the important role of the right ventricle in advanced stages of HF. According to previous studies, up to 19% of patients with severe FMR have moderate to severe TR associated with a poor clinical course of HF. 22 , 23 At the moment, interventional strategies for the treatment of FMR address the mitral valve only, whereas sMVR is frequently combined with TVR when TR is present. While TR is seen by many as a consequence rather than a cause of poor RV function, its correction might still be worthwhile in a situation where an optimization of LV haemodynamics is attempted by MVR. Our results support the hypothesis that combined interventions for MR and TR or early interventions for residual TR should be performed in cases where TR is moderate or severe. 24
According to a recent article analysing the results of COAPT and MITRA‐FR, the degree of LV dilatation may indicate if patients will benefit from pMVR or not. 25 Our Transthoracic echocardiography (TTE) parameters representing LV dilatation (LVEF, LV end‐diastolic diameter, and LVESV) in sMVR were comparable with those in MITRA‐FR, but survival at 3 years of follow‐up in our sMVR patients (81%) was superior to MitraClip patients in both COAPT and MITRA‐FR. Our results may support that sMVR is superior to pMVR in patients with HFrEF and dilated left ventricle. 25
Study limitations
In our study, there are various limitations. This is a non‐randomized retrospective, single‐centre observational study with a limited number of patients. Owing to this design, the higher risk patients would be selected naturally for non‐open surgery; therefore, the intrinsic risk of patients receiving pMVR would potentially be higher. In addition, differences in frailty could be a factor rejecting patients to open surgery, but it might not be reflected in the PS‐matched analysis, which in turn might potentially affect the outcome of patients after pMVR. Moreover, our TTE data were evaluated by experienced cardiologists, but not adjudicated by an external core lab. Thus, conclusions from our study should be taken with caution until confirmed by prospective and randomized clinical trials such as the currently ongoing MATTERHORN trial (NCT 02371512).
Conclusions
In a single‐centre retrospective analysis of patients with FMR and severely reduced LVEF, MitraClip therapy resulted in lower perioperative complications and mortality than surgical therapy but yielded less reduction in FMR. In contrast, surgically treated patients who survived the perioperative stage had less residual MR and experienced lower rates of re‐hospitalization for HF at 1 year as well as lower cardiac mortality at 1 and 3 years of follow‐up than patients undergoing pMVR.
Conflict of interest
None declared.
Supporting information
Figure S1. Outcome of perioperative survivors (excluding cases with peri‐operative death) of PS‐matched cohort.
Table S1. Perioperative course of PS‐matched cohort; n (%) if not otherwise specified. There were no cases of myocardial infarction or AV blockage. ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; AV, atrioventricular; ICU, intensive care unit; LVEF, left ventricular ejection fraction; MR, mitral valve regurgitation; TR, tricuspid valve regurgitation.
Table S2. Cox regression model analysis at 1‐year follow‐up. DCM, dilated cardiomyopathy.
Table S3. Cox regression model analysis in 3 years follow‐up. DCM, dilated cardiomyopathy.
Acknowledgement
None.
Gyoten, T. , Schenk, S. , Rochor, K. , Herwig, V. , Harnath, A. , Grimmig, O. , Just, S. , Fritzsche, D. , and Messroghli, D. (2020) Outcome comparison of mitral valve surgery and MitraClip therapy in patients with severely reduced left ventricular dysfunction. ESC Heart Failure, 7: 1781–1790. 10.1002/ehf2.12741.
References
- 1. Goliasch G, Bartko PE, Pavo N, Neuhold S, Wurm R, Mascherbauer J, Lang IM, Strunk G, Hülsmann M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018; 39: 39–46. [DOI] [PubMed] [Google Scholar]
- 2. Yiu SF, Enriquez‐sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: a quantitative clinical study. Circulation 2000; 102: 1400–1406. [DOI] [PubMed] [Google Scholar]
- 3. Giannini C, D'ascenzo F, Fiorelli F, Spontoni P, Swaans MJ, Velazquez EJ, Armeni P, Adamo M, De CM, Petronio AS. A meta‐analysis of MitraClip combined with medical therapy vs. medical therapy alone for treatment of mitral regurgitation in heart failure patients. ESC Hear Fail 2018; 5: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mirabel M, Iung B, Baron G, Messika‐Zeitoun D, Détaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 5. Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of The Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67: 943–951. [DOI] [PubMed] [Google Scholar]
- 6. Bach DS, Awais M, Gurm HS, Kohnstamm S. Failure of guideline adherence for intervention in patients with severe mitral regurgitation. J Am Coll Cardiol American College of Cardiology Foundation 2009; 54: 860–865. [DOI] [PubMed] [Google Scholar]
- 7. Tamburino C, Ussia GP, Maisano F, Capodanno D, La CG, Scandura S, Colombo A, Giacomini A, Michev I, Mangiafico S, Cammalleri V, Barbanti M, Alfieri O. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J 2010; 31: 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goel SS, Bajaj N, Aggarwal B, Gupta S, Poddar KL, Ige M, Bdair H, Anabtawi A, Rahim S, Whitlow PL, Tuzcu EM, Griffin BP, Stewart WJ, Gillinov M, Blackstone EH, Smedira NG, Oliveira GH, Barzilai B, Menon V, Kapadia SR. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63: 185–186. [DOI] [PubMed] [Google Scholar]
- 9. Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DR, Stebbins A, Kar S, Thourani V, Ailawadi G. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT Registry Report. J Am Coll Cardiol Elsevier 2017; 70: 2315–2327. [DOI] [PubMed] [Google Scholar]
- 10. Sorajja P, Mack M, Vemulapalli S, Holmes DR Jr, Stebbins A, Kar S, Lim DS, Thourani V, McCarthy P, Kapadia S, Grayburn P, Pedersen WAAG. Initial experience with commercial transcatheter mitral valve repair in the United States. J Am Coll Cardiol Elsevier 2016; 67: 1129–1140. [DOI] [PubMed] [Google Scholar]
- 11. Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE, Jacobs JP, Hanzel G, Bavaria JE, Tuzcu EM, Peterson ED, Fitzgerald S, Kourtis M, Michaels J, Christensen B, Seward WF, Hewitt K, Holmes DR. 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol 2017; 69: 1215–1230. [DOI] [PubMed] [Google Scholar]
- 12. Maisano F, Franzen O, Baldus S, Schäfer U, Hausleiter J, Butter C, Ussia GP, Sievert H, Richardt G, Widder JD, Moccetti T, Schillinger W. Percutaneous mitral valve interventions in the real world: early and 1‐year results from the ACCESS‐EU, A prospective, multicenter, nonrandomized post‐approval study of the MitraClip therapy in Europe. J Am Coll Cardiol Elsevier Inc 2013; 62: 1052–1061. [DOI] [PubMed] [Google Scholar]
- 13. Baldus S, Schillinger W, Franzen O, Bekeredjian R, Sievert H, Schofer J, Kuck KH, Konorza T, Möllmann H, Hehrlein C, Ouarrak T, Senges J, Meinertz T. MitraClip therapy in daily clinical practice: initial results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Eur J Heart Fail 2012; 14: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 14. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, Investigators COAPT. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, Voors AA, Anker SD, Bueno H'c, Cleland JGF, Coats AJS, Falk V, Juanatey J'R 'nG 'l, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, (UK) , Van der JP. The N. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 16. Baumgartner H, Bax VFJJ, De Bonis M, Hamm C. Per Johan Holm, Bernard Iung, Patrizio Lancellotti, Emmanuel Lansac, Daniel Rodriguez Mu~noz, Raphael Rosenhek, Johan Sjo¨gren, Pilar Tornos Mas, Alec Vahanian, Thomas Walt JLZ. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 17. Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L. Randomized comparison of percutaneous repair and surgery for mitral regurgitation 5‐year results of EVEREST II. J Am Coll Cardiol 2015; 66: 2844–2854. [DOI] [PubMed] [Google Scholar]
- 18. Boekstegers P, Hausleiter J, Baldus S, Von BRS, Beucher H, Butter C, Franzen O, Hoffmann R, Ince H, Kuck KH, Rudolph V, Schäfer U, Schillinger W, Wunderlich N. Interventionelle Behandlung der Mitralklappeninsuffizienz mit dem MitraClip®‐Verfahren: Empfehlungen des Arbeitskreises Interventionelle Mitralklappentherapie der Arbeitsgemeinschaft Interventionelle Kardiologie (AGIK) der Deutschen Gesellschaft für Kardi. Kardiologe 2013; 7: 91–104. [Google Scholar]
- 19. Roques F, Michel P, Goldstone AR, Nashef SAM. The logistic EuroSCORE [3]. Eur Heart J 2003; 24: 881–882. [DOI] [PubMed] [Google Scholar]
- 20. Obadia J‐F, Messika‐Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint EC, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu J‐N, Cormier B, Armoiry X, Boutitie F, Maucort‐Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 21. Notomi Y. To meet the unmet needs. Eur Heart J Cardiovasc Imaging 2018; 81: 498–500. [DOI] [PubMed] [Google Scholar]
- 22. Ohno Y, Attizzani GF, Capodanno D, Cannata S, Dipasqua F, Immé S, Barbanti M, Ministeri M, Caggegi A, Pistritto AM, Chiarandà M, Ronsivalle G, Giaquinta S, Farruggio S, Mangiafico S, Scandura S, Tamburino C, Capranzano P, Grasso C. Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip System: 30‐day and 12‐month follow‐up from the GRASP Registry. Eur Heart J Cardiovasc Imaging 2014; 15: 1246–1255. [DOI] [PubMed] [Google Scholar]
- 23. Di MM, Bivona A, Iacò AL, Contini M, Gagliardi M, Varone E, Gallina S, Calafiore AM. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg 2009; 35: 635–640. [DOI] [PubMed] [Google Scholar]
- 24. Taramasso M, Alessandrini H, Latib A, Asami M, Attinger‐Toller A, Biasco L, Braun D, Brochet E, Connelly KA, Denti P, Deuschl F, Englmeier A, Fam N, Frerker C, Hausleiter J, Himbert D, Ho E, Juliard JM, Kaple R, Kreidel F, Kuck KH, Ancona M, Lauten A, Lurz P, Mehr M, Nazif T, Nickening G, Pedrazzini G, Pozzoli A, Praz F, Puri R, Rodés‐Cabau J, Schäfer U, Schofer J, Sievert H, Sievert K, Tang GHL, Tanner FC, Vahanian A, Webb JG, Windecker S, Yzeiray E, Zuber M, Maisano F, Leon MB, Hahn RT. Outcomes after current transcatheter tricuspid valve intervention: mid‐term results from the International TriValve Registry. JACC Cardiovasc Interv 2019; 12: 155–165. [DOI] [PubMed] [Google Scholar]
- 25. Bartko PE, Heitzinger G, Arfsten H, Pavo N, Spinka G, Andreas M, Mascherbauer J, Hengstenberg C, Huelsmann M, Goliasch G. Disproportionate functional mitral regurgitation: advancing a conceptual framework to clinical practice. JACC Cardiovasc Imaging 2019; 12: 2088–2090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Outcome of perioperative survivors (excluding cases with peri‐operative death) of PS‐matched cohort.
Table S1. Perioperative course of PS‐matched cohort; n (%) if not otherwise specified. There were no cases of myocardial infarction or AV blockage. ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; AV, atrioventricular; ICU, intensive care unit; LVEF, left ventricular ejection fraction; MR, mitral valve regurgitation; TR, tricuspid valve regurgitation.
Table S2. Cox regression model analysis at 1‐year follow‐up. DCM, dilated cardiomyopathy.
Table S3. Cox regression model analysis in 3 years follow‐up. DCM, dilated cardiomyopathy.


