Abstract
Aims
The aim of this study was to investigate the diagnostic and prognostic utility of the QRS‐T angle, an electrocardiogram (ECG) marker quantifying depolarization–repolarization heterogeneity, in patients with suspected acute decompensated heart failure (ADHF).
Methods and results
We prospectively enrolled unselected patients presenting to the emergency department with symptoms suggestive of ADHF. The QRS‐T angle was automatically derived from a standard 12‐lead ECG recorded at presentation. The primary diagnostic endpoint was a final adjudicated diagnosis of ADHF. The primary prognostic endpoint was all‐cause mortality during 2 years of follow‐up. Among the 1915 patients enrolled, those with higher QRS‐T angles were older, were more commonly male, and had a higher rate of co‐morbidities such as arterial hypertension, coronary artery disease, or chronic kidney disease. ADHF was the final adjudicated diagnosis in 1140 (60%) patients. The QRS‐T angle in patients with ADHF was significantly larger than in patients with non‐cardiac causes of dyspnoea {median 110° [inter‐quartile range (IQR) 46–156°] vs. median 33° [IQR 15–57°], P < 0.001}. The diagnostic accuracy of the QRS‐T angle as quantified by the area under the receiver operating characteristic curve (AUC) was 0.75 [95% confidence interval (CI) 0.73–0.77, P < 0.001], which was inferior to N‐terminal pro‐B‐type natriuretic peptide (AUC 0.93, 95% CI 0.92–0.94, P < 0.001), but similar to that of high‐sensitivity troponin T (AUC 0.78, 95% CI 0.76–0.80, P = 0.09). The AUC of the QRS‐T angle for discrimination between ADHF and non‐cardiac dyspnoea remained similarly high in subgroups of patients known to be diagnostically challenging, including patients older than 75 years [0.71 (95% CI 0.67–0.74)], renal failure [0.79 (95% CI 0.71–0.87)], and atrial fibrillation at presentation [0.68 (95% CI 0.60–0.76)]. Mortality rates according to QRS‐T angle tertiles were 4%, 6%, and 10% after 30 days (P < 0.001) and 24%, 31%, and 43% after 2 years (P < 0.001). After adjustment for clinical, laboratory, and ECG parameters, the QRS‐T angle remained an independent predictor for 2 year mortality with a 4% increase in mortality for every 20° increase in QRS‐T angle (P = 0.02).
Conclusions
The QRS‐T angle is a readily available and inexpensive marker that can assist in the discrimination between ADHF and non‐cardiac causes of acute dyspnoea and may aid in the risk stratification of these patients.
Keywords: Acute heart failure, Heart failure, Acute dyspnoea, QRS‐T angle, ECG
Introduction
Acute decompensated heart failure (ADHF) is the most common reason for hospitalizations in the elderly and is a major cause of morbidity and mortality in the western society. 1 The majority of patients initially present to the emergency department (ED) with the chief complaint of acute dyspnoea. Given the broad differential diagnosis of this common symptom—~7% of all non‐traumatic ED admissions present with respiratory distress—the identification of patients with ADHF remains challenging. 2
Besides history, physical examination, and blood tests, the 12‐lead electrocardiogram (ECG) plays a key role in the workup of patients with cardiorespiratory symptoms and is usually obtained within minutes in the ED. Its value in acute heart failure has, however, been limited predominantly to the identification of potentially precipitating causes of ADHF such as arrhythmias or myocardial infarction. Few studies evaluated its utility beyond this scope and, among others, identified prolongation of QRS and QTc duration to be associated with an increased risk for adverse events. 3
A readily available ECG parameter that has recently gained attention is the QRS‐T angle. It is the angle between the axis of the QRS complex and the axis of the T‐wave and represents a measure of cardiac depolarization–repolarization heterogeneity. It has recently been shown to improve the diagnostic accuracy in patients with suspected non‐ST‐elevation myocardial infarction, and large QRS‐T angles have been associated with increased risk for ventricular arrhythmias, hospitalizations, and mortality in various populations. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14
The utility of the QRS‐T angle in patients with suspected ADHF has not yet been assessed. The aim of this prospective multicentre study was therefore to evaluate the diagnostic and prognostic values of the QRS‐T angle in patients presenting to the ED with symptoms suggestive of ADHF.
Methods
Study design and population
Unselected patients presenting to the Emergency Departments of the University Hospital of Basel or the University Hospital of Zürich, Switzerland, with the leading symptom of acute dyspnoea were prospectively enrolled between May 2001 and December 2015. Exclusion criteria were age below 18 years, an obvious traumatic cause of dyspnoea, cardiogenic shock, and terminal kidney failure requiring dialysis. The study was approved by the local ethics committees and conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Routine clinical assessment
In all patients, routine clinical assessment including demographical factors, medical history, physical examination, 12‐lead ECG, continuous ECG monitoring, pulse oximetry, standard blood tests, and chest radiography were performed. Standard blood tests included high‐sensitivity troponin T (hs‐TnT; Roche Diagnostics, Rotkreuz, Switzerland) and the N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP; Roche Diagnostics, Rotkreuz, Switzerland). Additional diagnostic tests and therapeutic actions were left to the discretion of the attending physician.
Adjudication of final diagnoses
The final diagnosis was adjudicated by a single internal medicine specialist up to 2002 and by two independent cardiologists thereafter in the core laboratories of the University Hospitals Basel and Zürich after review of all available medical records, including the baseline evaluation, results of subsequent laboratory and radiologic tests, ECGs, echocardiographies, response to therapy, and autopsy data if available, from the time of ED presentation up to 90 days of follow‐up. In case of disagreement, a third cardiologist was consulted, and consensus was found upon joined review of available material. The diagnosis of ADHF was based on the applicable guidelines of the European Society of Cardiology for each period. 15 , 16 , 17 , 18
Digital electrocardiogram recording, automated calculation of the QRS‐T angle, and automated analysis of the QRS‐T confounder type
Ten‐second 12‐lead ECGs were acquired as part of the routine clinical assessment in the ED using an AT‐110 ECG device (Schiller AG, Baar, Switzerland) or a Page Writer TC30 ECG device (Philips Healthcare, Andover, MA, USA). The digital ECG raw data were recorded using a sampling rate of 500 Hz, a resolution of 5 μV/bit, and a diagnostic signal bandwidth of 0.05 to 150 Hz (fulfilling the requirements by current international ECG device standards).
The ETM V01.12.09.00 ECG analysis program (Schiller AG, Baar, Switzerland) was used to automatically measure ECG features, including amplitudes, depolarization and repolarization timings, and frontal plane QRS and T‐wave axis (summation vector of frontal plane QRS, respectively, T‐wave). To minimize random noise and to improve the signal to noise ratio, median beats were used for all quantitative measures as described by Kligfield et al. 19 To construct the median heartbeat, a high‐pass filter was applied to the ECG signal to remove baseline wandering. A QRS detector determined the position of all heartbeats in the 10 s 12‐lead ECGs. Beats were then classified by cross‐correlation of the QRS complexes. The QRS class with the most QRS complexes and the shortest QRS duration was defined as the predominant normal heartbeat. Those predominant normal heartbeats were then temporally aligned by maximization of the cross‐correlation of the QRS complexes. From the aligned heartbeats, which are time series, a robust median was calculated for each time point (sample) in the time series. The QRS‐T angle was subsequently calculated as the absolute difference between the QRS and T‐wave axis. If the difference exceeded 180°, the value was subtracted from 360° (Figure S1). Automated analysis of the ECG confounder types [intermediate: isolated right bundle branch block (RBBB) and left ventricular hypertrophy; remarkable: left bundle branch block, non‐specific bundle branch block, left anterior fascicular block (LAFB), and RBBB + LAFB] was classified based on automated diagnostic statement codes (ETM V01.12.09.00, Schiller AG, Baar, Switzerland), QRS duration, and QRS axis as previously reported by Strauss et al. 20
Follow‐up
Patients were contacted 1 and 2 years after hospital discharge by phone or in written form by trained researchers. In case of missing information, the national registry on mortality, the hospital's diagnosis registry, and the family physician's records were assessed.
Outcomes and statistical analysis
Data are presented as median [inter‐quartile range (IQR)] in case of continuous variables and as numbers and percentages in case of categorical variables. Comparison between groups was performed using Pearson's χ 2 test, Mann–Whitney U test, and Kruskal–Wallis test as appropriate. The primary diagnostic endpoint was an adjudicated final diagnosis of ADHF. Pre‐specified subgroup analyses for the diagnostic endpoint were planned in patients with advanced age (>75 years), atrial fibrillation (AF) at presentation, and renal failure [estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2]. Diagnostic accuracy was assessed by receiver operating characteristic (ROC) analysis with calculation of the area under the ROC curve (AUC) and comparison of ROC AUCs using the non‐parametric method as recommended by DeLong et al. 21 Additionally, univariable and multivariable binary logistic regression models were computed to quantify the association between selected baseline variables and the diagnosis of ADHF as odds ratios (ORs) with 95% confidence intervals (CIs). Variables were selected based on clinical importance and tested for their association with the diagnosis of ADHF in univariable analyses first. Parameters with a P‐value < 0.1 in univariate tests were entered into the multivariable model.
The primary prognostic endpoint was all‐cause mortality during 2 years of follow‐up. The secondary prognostic endpoint was all‐cause re‐hospitalizations during 2 years of follow‐up. To evaluate time to event outcomes, we constructed Kaplan–Meier curves and used the log‐rank test to assess statistical significance. Univariable and multivariable Cox proportional hazard analyses with calculation of hazard ratios (HRs) with 95% CI were performed to quantify 2 year mortality risk. As for logistic regression, we selected variables by clinical reasoning and entered those with a P‐value < 0.1 in univariable analysis into the multivariable model. For all tests, a two‐tailed P‐value < 0.05 was considered to be statistically significant. All analyses were performed using SPSS Statistics version 25 (IBM, Chicago, Illinois, USA) and STATA Statistical Software version 15.1 (StataCorp, College Station, Texas, USA).
Results
Baseline characteristics
A total of 3116 unselected patients presenting to the ED with the chief complaint of acute dyspnoea were enrolled between 2001 and 2015. After exclusion of patients without digital ECG records (n = 1158), with unclear final diagnoses (n = 13) or with an adjudicated final diagnosis of ST‐elevation myocardial infarction or ventricular arrhythmia (where no further diagnostics are required, n = 30), 1915 patients were available for analysis ( Figure S2 ).
Baseline characteristics are presented in Table 1. ADHF was the adjudicated final diagnosis in 60% of patients (n = 1140). Non‐cardiac causes of dyspnoea were responsible for the remaining 40% (n = 775) and included asthma or chronic obstructive pulmonary disease (COPD) exacerbations, bacterial or viral infections, pulmonary embolisms, and other causes.
Table 1.
Baseline characteristics
| Variable | All patients (n = 1915) | Acute decompensated heart failure (n = 1140) | Non‐cardiac dyspnoea (n = 775) | P‐value a |
|---|---|---|---|---|
| Age (years), median [IQR] | 76 [64–83] | 79 [70–85] | 69 [56–78] | <0.001 |
| Male sex, n (%) | 1071 (56) | 646 (43) | 425 (45) | 0.43 |
| BMI (kg/m2), median [IQR] | 26 [23–30] | 26 [23–30] | 26 [22–30] | 0.03 |
| Current smoking, n (%) | 396 (21) | 196 (18) | 200 (26) | <0.001 |
| Co‐morbidities, n (%) | ||||
| Art. hypertension | 1340 (71) | 893 (80) | 447 (58) | <0.001 |
| Dyslipidaemia | 841 (45) | 578 (51) | 263 (34) | <0.001 |
| Diabetes mellitus | 454 (24) | 323 (29) | 131 (17) | <0.001 |
| CAD | 708 (37) | 556 (50) | 152 (20) | <0.001 |
| Prior MI | 391 (21) | 314 (29) | 77 (10) | <0.001 |
| PM, ICD, or CRT | 147 (8) | 140 (12) | 7 (1) | <0.001 |
| Atrial fibrillation | 547 (30) | 472 (43) | 75 (10) | <0.001 |
| COPD | 610 (32) | 284 (25) | 326 (42) | <0.001 |
| CKD | 606 (32) | 502 (45) | 103 (13) | <0.001 |
| Dyspnoea, n (%) | <0.001 | |||
| NYHA II | 197 (10) | 74 (6.5) | 123 (16) | |
| NYHA III | 832 (43) | 522 (46) | 310 (40) | |
| NYHA IV | 805 (42) | 496 (44) | 309 (40) | |
| Vital signs, median [IQR] | ||||
| sBP (mmHg) | 138 [122–156] | 137 [20–156] | 139 [125–156] | 0.06 |
| dBP (mmHg) | 80 [68–91] | 80 [68–93] | 80 [69–90] | 0.72 |
| Heart rate (b.p.m.) | 86 [72–101] | 87 [71–105] | 85 [73–99] | 0.08 |
| ECG findings, n (%) or median [IQR] | ||||
| Rhythm | ||||
| Sinus | 1436 (75) | 712 (62) | 724 (93) | <0.001 |
| Atrial fibrillation | 479 (25) | 428 (38) | 51 (7) | <0.001 |
| Ventricular pacing | 72 (4) | 65 (6) | 7 (1) | <0.001 |
| QRS duration (ms) | 96 [86–118] | 104 [90–132] | 92 [84–100] | <0.001 |
| QTc time (ms) | 454 [430–481] | 468 [444–494] | 439 [420–458] | <0.001 |
| QRS‐T angle (°) | 66 [25–139] | 110 [46–156] | 33 [15–67] | <0.001 |
| QRS‐T confounders | <0.001 | |||
| None | 1134 (59) | 530 (47) | 604 (78) | <0.001 |
| Intermediate | 416 (22) | 298 (26) | 118 (15) | <0.001 |
| Remarkable | 365 (19) | 312 (27) | 53 (6.8) | <0.001 |
| Laboratory tests, median [IQR] | ||||
| Haemoglobin (g/L) | 132 [117–145] | 127 [113–141] | 139 [136–150] | <0.001 |
| CRP (mg/L) | 12 [3.7–38] | 11 [4.1–31] | 13 [3.0–58] | 0.07 |
| eGFR (mL/min/m2) | 65 [42–86] | 53 [35–74] | 83 [62–99] | <0.001 |
| NT‐proBNP (pg/mL) b | 2032 [354–6453] | 5083 [2237–9921] | 252 [83–750] | <0.001 |
| hs‐TnT (ng/mL) c | 0.03 [0.01–0.05] | 0.04 [0.02–0.07] | 0.01 [0.01–0.03] | <0.001 |
Abbreviations: Art, arterial; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CRT, cardiac resynchronization therapy; dBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; hs‐TnT, high‐sensitivity troponin T; ICD, implantable cardioverter‐defibrillator; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PM, pacemaker; sBP, systolic blood pressure.
For comparison between acute decompensated heart failure and non‐cardiac dyspnoea. Between‐group comparisons were performed using Pearson's χ 2 test in case of nominal and Mann–Whitney U test in case of ordinal or continuous variables.
Available for 1848 patients.
Available for 1791 patients.
Levels of the QRS‐T angle and QRS‐T confounders
The median QRS‐T angle in the overall population was 66° (IQR 25–139°). Levels of the QRS‐T angle were associated with several baseline characteristics, as shown in Table 2. Patients with higher QRS‐T angles were older, were more commonly male, and had a higher rate of co‐morbidities such as arterial hypertension, coronary artery disease, or chronic kidney disease (CKD).
Table 2.
Patient characteristics stratified on QRS‐T angle
| Variable | QRS‐T angle < 35° (n = 641) | QRS‐T angle 35–114° (n = 635) | QRS‐T angle > 114° (n = 639) | P‐value |
|---|---|---|---|---|
| Age (years), median [IQR] | 72 [59–82] | 76 [64–83] | 78 [69–84] | <0.001 |
| Male sex, n (%) | 321 (50) | 360 (57) | 390 (61) | <0.001 |
| BMI (kg/m2), median [IQR] | 26 [22–30] | 26 [23–30] | 26 [23–29] | 0.72 |
| Current smoking, n (%) | 142 (23) | 129 (21) | 124 (20) | 0.46 |
| Co‐morbidities, n (%) | ||||
| Art. hypertension | 413 (65) | 442 (70) | 485 (78) | <0.001 |
| Dyslipidaemia | 248 (39) | 258 (42) | 335 (55) | <0.001 |
| Diabetes mellitus | 120 (19) | 153 (24) | 181 (29) | <0.001 |
| CAD | 171 (27) | 207 (33) | 330 (53) | <0.001 |
| Prior MI | 83 (13) | 118 (19) | 190 (31) | <0.001 |
| PM, ICD, or CRT | 21 (3) | 37 (6) | 89 (14) | <0.001 |
| Atrial fibrillation | 113 (18) | 183 (30) | 251 (41) | <0.001 |
| COPD | 232 (36) | 213 (34) | 165 (26) | 0.003 |
| CKD | 140 (22) | 175 (28) | 291 (47) | <0.001 |
| Dyspnoea, n (%) | <0.001 | |||
| NYHA II | 84 (13) | 77 (12) | 36 (5.6) | |
| NYHA III | 288 (45) | 255 (40) | 289 (45) | |
| NYHA IV | 244 (38) | 271 (43) | 290 (45) | |
| Vital signs, median [IQR] | ||||
| sBP (mmHg) | 138 [124–157] | 140 [125–158] | 133 [117–152] | <0.001 |
| dBP (mmHg) | 80 [68–91] | 81 [68–93] | 79 [67–91] | 0.29 |
| Heart rate (b.p.m.) | 83 [70–97] | 87.3 [74–103] | 88.6 [73–106] | <0.001 |
| ECG findings, n (%) or median [IQR] | ||||
| Rhythm | ||||
| Sinus | 548 (86) | 478 (75) | 410 (64) | <0.001 |
| Atrial fibrillation | 93 (15) | 157 (25) | 229 (36) | <0.001 |
| Ventricular pacing | 8 (1) | 13 (2) | 51 (8) | <0.001 |
| QRS duration (ms) | 90 [84–98] | 96.0 [86–110] | 116.0 [96–144] | <0.001 |
| QTc time (ms) | 442 [423–465] | 453.0 [431–473] | 473.0 [446–501] | <0.001 |
| QRS‐T confounders, n (%) | ||||
| None | 535 (84) | 403 (64) | 196 (31) | <0.001 |
| Intermediate | 88 (14) | 156 (25) | 172 (27) | <0.001 |
| Remarkable | 18 (2.8) | 76 (12) | 271 (42) | <0.001 |
| Laboratory tests, median [IQR] | ||||
| Haemoglobin (g/L) | 133 [119–146] | 133 [120–146] | 130 [115–142] | 0.001 |
| CRP (mg/L) | 11 [3–47] | 14 [4–42] | 11 [4–30] | 0.03 |
| eGFR (mL/min/m2) | 75 [52–93] | 65 [44–88] | 52 [36–75] | <0.001 |
| NT‐proBNP (pg/mL) a | 508 [118–2536] | 1831 [312–5240] | 5556 [2066–11 663] | <0.001 |
| hs‐TnT (ng/mL) b | 0.02 [0.01, 0.04] | 0.02 [0.01–0.05] | 0.04 [0.02–0.07] | <0.001 |
| Discharge diagnosis, n (%) | <0.001 | |||
| ADHF | 232 (36) | 365 (58) | 543 (85) | |
| Non‐cardiac cause | 409 (64) | 270 (43) | 96 (15) |
Abbreviations: ADHF, acute decompensated heart failure; Art, arterial; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CRP, C‐reactive protein; CRT, cardiac resynchronization therapy; dBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; hs‐TnT, high‐sensitivity troponin T; ICD, implantable cardioverter‐defibrillator; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PM, pacemaker; sBP, systolic blood pressure.
Between‐group comparisons were performed using Pearson's χ 2 test in case of nominal and Kruskal–Wallis test in case of ordinal or continuous variables.
Available for 1848 patients.
Available for 1791 patients.
Presence and type of ECG confounders of cardiac depolarization and repolarization resulted in significant changes in the QRS‐T angle. The ECG was free of confounders in 59% of patients. In those, the median QRS‐T angle was 39° (IQR 17–88°). It increased to 99° (IQR 41–149°) in patients with intermediate ECG confounders (22% of patients overall) and to 154° (IQR 108–170°) in those with remarkable ECG confounders (19% of patients overall).
Diagnostic value of the QRS‐T angle for the discrimination between acute decompensated heart failure and non‐cardiac causes of dyspnoea
The QRS‐T angle was significantly greater in patients with ADHF compared with patients with non‐cardiac causes of dyspnoea [110° (IQR 46–156°) vs. 33° (IQR 15–57°), P < 0.001, Figure 1A]. The diagnostic accuracy of the QRS‐T angle at presentation for discrimination between ADHF or non‐cardiac causes of dyspnoea as quantified by the AUC in the overall cohort was 0.75 (95% CI 0.73–0.77, P < 0.001). Choosing a cut‐off value of 63° (based on Youden's index), 70% of patients were correctly classified (sensitivity 69%, specificity 73%, positive predictive value 79%, and negative predictive value 61%; Table S1).
Figure 1.
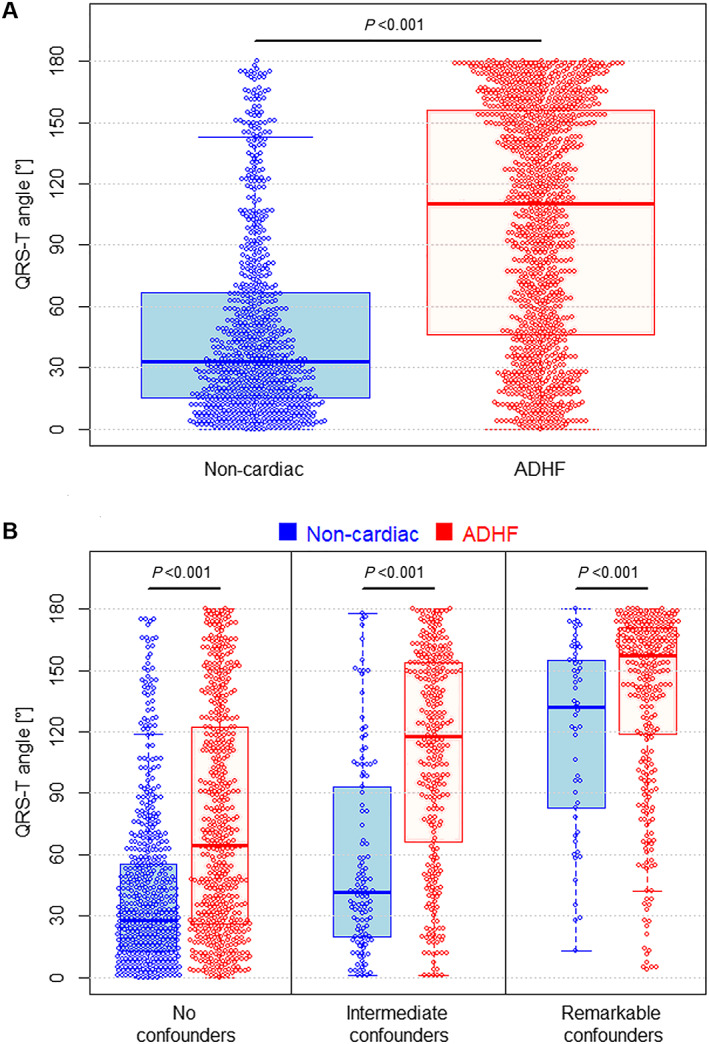
QRS‐T angle in patients with acute decompensated heart failure versus other causes of acute dyspnoea. QRS‐T angle in patients with ADHF (displayed in red) and non‐cardiac causes of acute dyspnoea (displayed in blue) in the overall population (A), and stratified according to the presence of no, intermediate (RBBB, LVH), and remarkable (LAFB, RBBB+LAFB, LBBB, and NBBB) ECG confounders (B). Mann–Whitney U test used for comparison. Abbreviations: ADHF, acute decompensated heart failure; RBBB, right bundle branch block; LVH, left ventricular hypertrophy; LAFB, left anterior fascicular block; LBBB, left bundle branch block; NBBB, non‐specific bundle branch block.
QRS‐T angles were significantly higher in patients with ADHF as compared with patients with non‐cardiac causes of dyspnoea irrespective of the presence and type of QRS‐T confounders (Figure 1B, all P < 0.001). Accordingly, the AUC was not different for patients with no ECG confounders (AUC 0.69, 95% CI 0.65–72), intermediate ECG confounders (AUC 0.76, 95% CI 0.71–0.81), and remarkable ECG confounders (AUC 0.65, 95% CI 0.58–0.72).
The AUC of the QRS‐T angle for discrimination between ADHF and non‐cardiac dyspnoea remained similarly high in subgroups of patients known to be diagnostically challenging, including patients older than 75 years [0.71 (95% CI 0.67–0.74)], with renal failure [0.79 (95% CI 0.71–0.87)], and with AF at presentation [0.68 (95% CI 0.60–0.76)]. For comparison, the diagnostic accuracy of hs‐TnT decreased from an AUC of 0.78 (95% CI 0.76–0.80) in the overall population and 0.68 (95% CI 0.65–0.72) in patients > 75 years, to 0.60 (95% CI 0.50–0.70) in patients with renal failure, and to 0.52 (95% CI 0.43–0.61) in patients presenting with AF. The AUCs of the QRS‐T angle and hs‐TnT were statistically not different in the overall population and in patients > 75 years, while in patients with renal failure and AF, the QRS‐T angle was superior to hs‐TnT (P < 0.001 and P = 0.002). Compared with those of NT‐proBNP (the biomarker assisting in gold‐standard adjudication in this study), the AUCs of both the QRS‐T angle and hs‐TnT were lower in the overall population as well as in all subgroups (Table S1). Cut‐offs with respective sensitivities, specificities, and positive and negative predictive values are summarized in Table S2 .
Without adjustment for other factors, the odds for ADHF increased by 42% (OR 1.42, 95% CI 1.37–1.48, P < 0.001) for every 20° increase of the QRS‐T angle ( Table S3 ). In a multivariable model including patient age, body mass index (BMI), medical history (arterial hypertension, dyslipidaemia, coronary artery disease, AF, and COPD), eGFR, NT‐proBNP, hs‐TnT, and QRS and QTc duration, the QRS‐T angle remained predictive for ADHF with an OR of 1.17 (95% CI 1.10–1.24) per 20° increase (P < 0.001; Table S3).
Prognostic value of the QRS‐T angle
A total of 793 patients (42%) died during a median follow‐up time of 28 months (IQR 27–39). Those who died had significantly higher QRS‐T angles than had survivors [96° (IQR 35–153°) vs. 51° (IQR 20–121°), P < 0.001]. Survival according to QRS‐T angle tertiles was 96%, 94%, and 90% after 30 days (P < 0.001) and 76%, 69%, and 57% after 2 years (P < 0.001, Figure 2A). The QRS‐T angle was associated with survival both in patients with ADHF (Figure 2B) and in patients with non‐cardiac causes of dyspnoea (Figure 2C). Similarly, the QRS‐T angle predicted freedom from re‐hospitalizations during 2 years of follow‐up in the overall cohort (Figure 3A), as well as in the subgroups of patients with ADHF (Figure 3B) and non‐cardiac causes of dyspnoea (Figure 3C).
Figure 2.
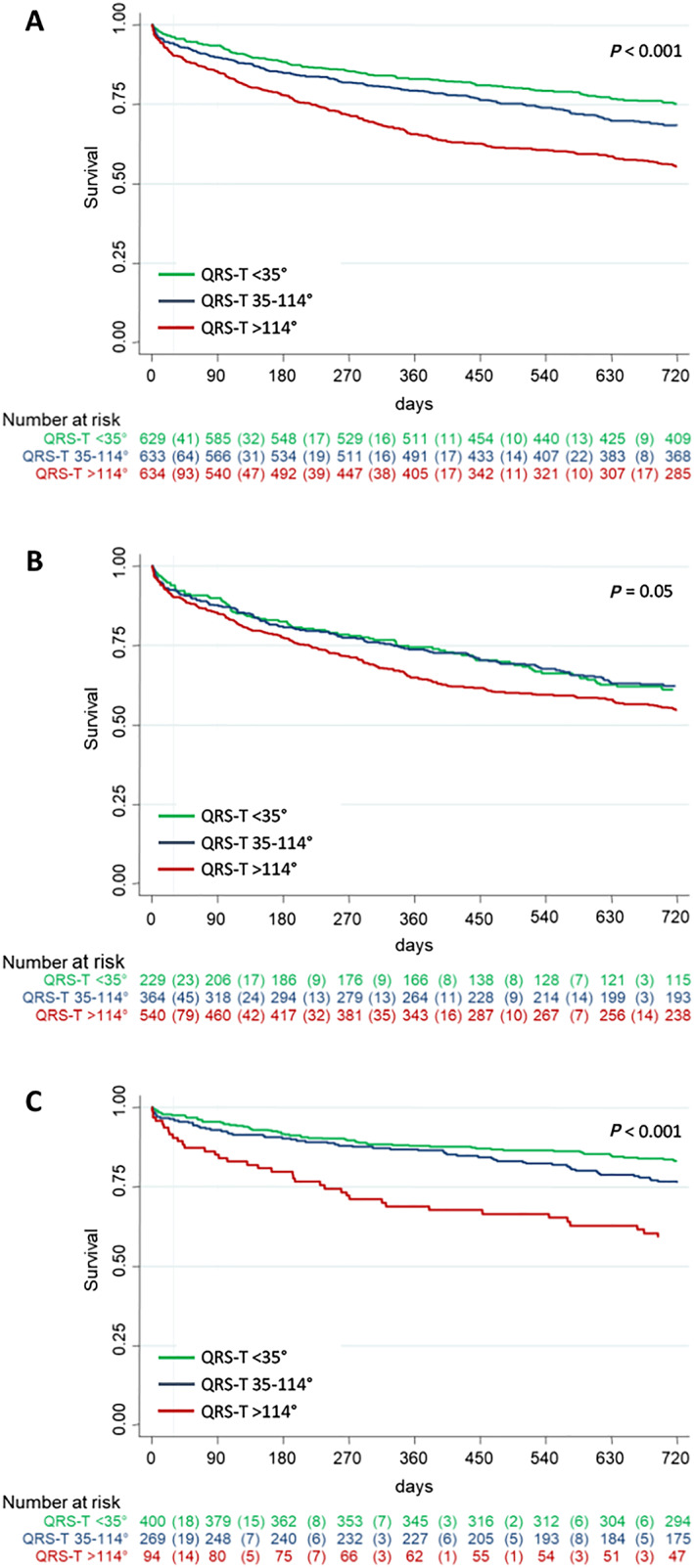
Prognostic value of the QRS‐T angle with respect to all‐cause mortality. Kaplan–Meier survival estimates according to QRS‐T angle tertiles in the overall population (A), in patients with acute decompensated heart failure (B), and in patients with non‐cardiac cause of acute dyspnoea (C).
Figure 3.
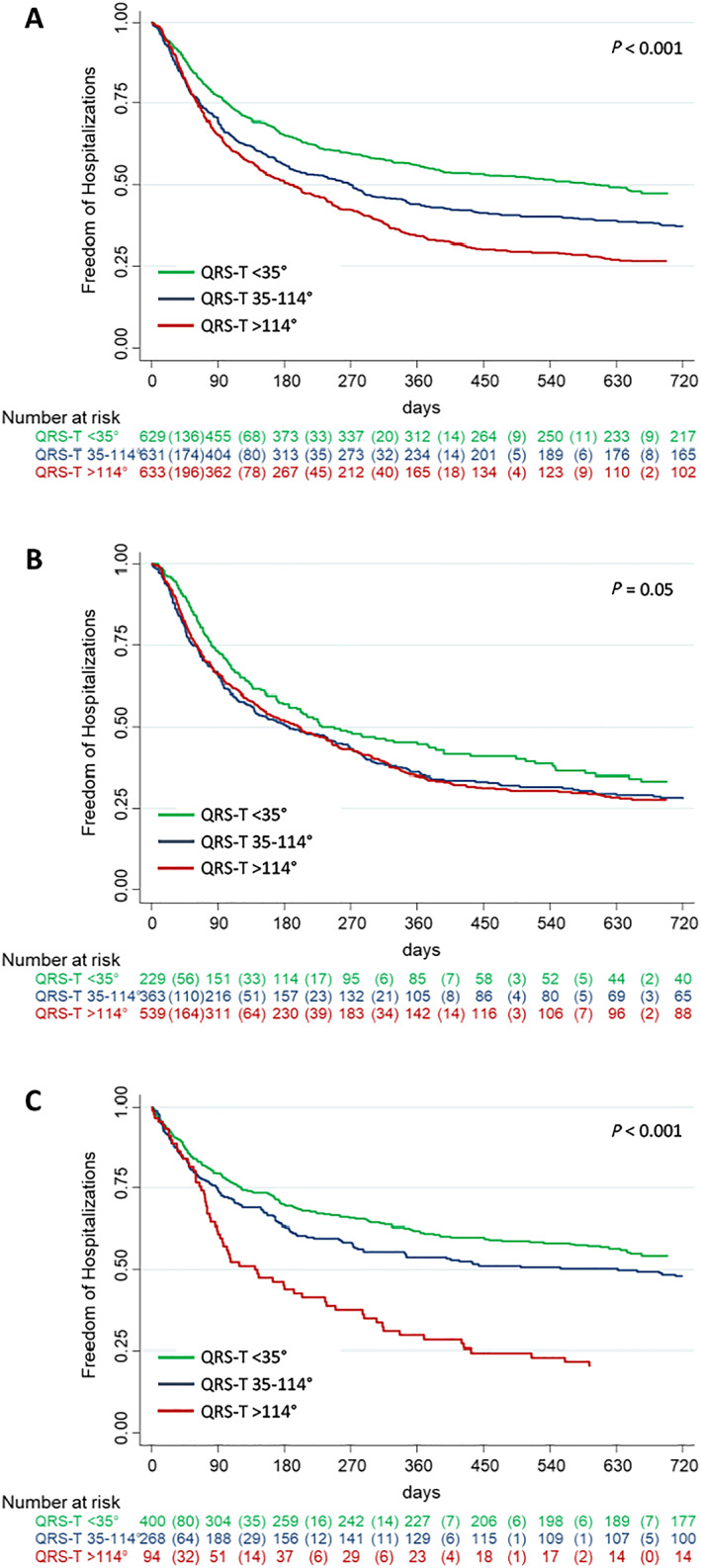
Prognostic value of the QRS‐T angle with regard to all‐cause re‐hospitalizations. Cumulative re‐hospitalization risk according to QRS‐T angle tertiles in the overall population (A), in patients with acute decompensated heart failure (B), and in patients with non‐cardiac cause of acute dyspnoea (C).
In univariable Cox proportional hazard analysis, the QRS‐T angle predicted mortality after 2 years in the whole population with an HR of 1.12 (95% CI 1.09–1.14, P < 0.001) for every 20° increment (Table 3). It remained predictive of 2 year mortality even after adjustment for age, sex, BMI, co‐morbidities (arterial hypertension, dyslipidaemia, coronary artery disease, AF, and COPD), systolic blood pressure, eGFR, NT‐proBNP, hs‐TnT, and QRS and QTc duration (HR 1.04, 95% CI 1.01–1.07, P = 0.02; Table 3). Similar results were observed also for re‐hospitalizations (HR 1.42, 95% CI 1.37–1.48, P < 0.001 in univariable analysis, HR 1.17, 95% CI 1.10–1.24, P < 0.001 in multivariable analysis adjusting for the factors mentioned above, per 20° increase of the QRS‐T angle), as shown in Table S3.
Table 3.
Univariable and multivariable predictors for long‐term mortality in patients presenting to the emergency department with acute dyspnoea
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | LCI | UCI | P‐value | HR | LCI | UCI | P‐value | |
| Age (per year) | 1.05 | 1.04 | 1.06 | <0.001 | 1.04 | 1.03 | 1.05 | <0.001 |
| Male sex | 0.87 | 0.76 | 1.01 | 0.08 | 0.76 | 0.65 | 0.90 | 0.001 |
| BMI | 0.95 | 0.94 | 0.96 | <0.001 | 0.96 | 0.94 | 0.97 | <0.001 |
| Medical history | ||||||||
| Art. hypertension | 1.63 | 1.37 | 1.94 | <0.001 | 0.97 | 0.79 | 1.20 | 0.80 |
| Dyslipidaemia | 1.24 | 1.07 | 1.43 | 0.004 | 0.93 | 0.77 | 1.11 | 0.42 |
| CAD | 1.60 | 1.39 | 1.85 | <0.001 | 1.05 | 0.87 | 1.25 | 0.63 |
| Atrial fibrillation | 1.60 | 1.38 | 1.86 | <0.001 | 0.99 | 0.84 | 1.18 | 0.98 |
| COPD | 1.21 | 1.04 | 1.40 | 0.01 | 1.37 | 1.16 | 1.61 | <0.001 |
| Diabetes mellitus | 1.17 | 0.99 | 1.37 | 0.05 | 1.35 | 1.12 | 1.63 | 0.002 |
| Vital signs | ||||||||
| Heart rate (b.p.m.) | 1.00 | 1.00 | 1.00 | 0.74 | — | — | — | — |
| sBP (per 10 mmHg) | 0.92 | 0.90 | 0.95 | <0.001 | 0.94 | 0.91 | 0.97 | <0.001 |
| Laboratory tests | ||||||||
| eGFR > 60 mL/min/m2 | 0.40 | 0.35 | 0.47 | <0.001 | 0.64 | 0.54 | 0.77 | <0.001 |
| NT‐proBNP (per 100 pg/mL) | 1.001 | 1.001 | 1.001 | <0.001 | 1.000 | 1.000 | 1.001 | <0.001 |
| hs‐TnT (per ng/mL) | 1.61 | 1.28 | 2.03 | <0.001 | 1.18 | 0.87 | 1.56 | 0.29 |
| ECG parameters | ||||||||
| QRS‐T angle (per 20°) | 1.12 | 1.09 | 1.14 | <0.001 | 1.04 | 1.01 | 1.07 | 0.02 |
| QRS duration (per 10 ms) | 1.07 | 1.05 | 1.10 | <0.001 | 0.99 | 0.99 | 1.00 | 0.64 |
| QTc duration (per 10 ms) | 1.05 | 1.03 | 1.07 | <0.001 | 1.00 | 0.99 | 1.10 | 0.18 |
Abbreviations: Art, arterial; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; hs‐TnT, high‐sensitivity troponin T; LCI, lower confidence interval; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; sBP, systolic blood pressure; UCI, upper confidence interval.
Discussion
This large multicentre observational cohort study assessed the diagnostic and prognostic values of the QRS‐T angle, a simple and readily available, observer‐independent ECG marker quantifying depolarization–repolarization heterogeneity, in patients admitted to the ED with the chief complaint of acute dyspnoea suggestive of ADHF. We observed five major findings:
First, the QRS‐T angle was significantly higher—nearly three times as high—in patients with ADHF compared with non‐cardiac causes of acute dyspnoea. After adjustment for several clinical, laboratory, and ECG variables, the odds for ADHF increased by 17% for every 20° increase in the QRS‐T angle. Second, the diagnostic accuracy of the QRS‐T angle to detect patients with ADHF was similar to that of hs‐TnT and, in contrast to the latter, remained high in subgroups of patients known to be diagnostically challenging, such as those with renal failure and AF. Third, patients with higher QRS‐T angles had a higher risk for re‐hospitalizations and mortality. During 2 years of follow‐up, nearly 50% of patients in the highest QRS‐T angle tertile died, which was almost twice as many as those in the lowest tertile. Fourth, even after adjustment for multiple baseline characteristics, including but not limited to age, co‐morbidities, and importantly NT‐proBNP, the QRS‐T angle remained independently predictive of 2 year mortality with a risk increase of 4% for every 20°. Fifth, the QRS‐T angle predicted re‐hospitalizations and mortality both in patients with ADHF as well as those with non‐cardiac causes of dyspnoea independently, highlighting its prognostic value irrespective from the underlying condition.
These findings have several clinical implications. Despite advances in diagnostic abilities, rapid and reliable identification of patients with acute heart failure in the emergency setting remains challenging. Imaging modalities provide ample information, yet their limited availability and dependence on operator skills restrict their applicability to centres with on‐site expertise. Blood tests are invaluable in the assessment of suspected ADHF. However, the time it takes until results are obtained may delay diagnosis and treatment initiation, which has been associated with prolonged hospitalization durations and increased mortality. 22 , 23 A 12‐lead ECG is routinely obtained in any patient with potentially cardiac mediated symptoms and has the advantage of general availability and immediate interpretability. Its role in acute heart failure, however, has been primarily restricted to the identification of potentially precipitant causes for ADHF. 15 , 24 So far, no single ECG parameter has been found to predict the probability of ADHF even though it is recognized that patients with heart failure often have abnormal ECGs. 24 Accordingly, the QRS‐T angle reported in this study is the first readily available and easily interpretable ECG parameter that may aid in the diagnosis of patients with suspected ADHF. To put it in context, we compared its diagnostic value for a diagnosis of ADHF with that of hs‐TnT, which has increasingly gained attention owing to its potential diagnostic and prognostic impact, 25 , 26 , 27 as well as with that of NT‐proBNP, which was used as the biochemical gold standard for the adjudicated final diagnosis of ADHF. Not surprisingly, both the QRS‐T angle and hs‐TnT had a lower diagnostic performance than NT‐proBNP. On the other hand, the diagnostic accuracy of the QRS‐T angle in the overall population was similar to that of hs‐TnT and, in contrast to troponin, which lost its informative value in patients with renal failure and AF, remained high in these populations. Patients with co‐morbidities are known to be diagnostic challenging; nevertheless, they present a substantial proportion of patients presenting to the ED with respiratory distress—in our study, ~30% of patients had a history of AF or CKD, which is comparable with other cohorts in this setting. Including the easily available QRS‐T angle—together with any other available information—into the full picture may aid in the diagnostic process and enhance fast yet accurate triage and resource allocation in order to ensure optimal management of patients.
Triage and resource allocation are central in EDs, which are faced with ever‐rising patient volumes with increasingly complex disease manifestations. 28 Proper triage relies on adequate risk stratification and can be improved by incorporation of prognostic markers. 29 A number of previous investigations examined the prognostic value of the QRS‐T angle in various populations, including patients with arterial hypertension, diabetes, CKD, myocarditis, implanted cardiac devices, chronic heart failure, and suspected and confirmed myocardial ischaemia, as well as in the general population. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Across the spectrum, greater QRS‐T angles were associated with increased risk of adverse events, re‐hospitalizations, and mortality. These findings were corroborated in our study, which was the first to evaluate the prognostic value of the QRS‐T angle in patients with symptoms suggestive of ADHF. Also, in our population, larger QRS‐T angles were predictive of re‐hospitalizations and mortality, even after adjustment for multiple clinically relevant variables. Other ECG parameters that have been previously implied in a greater risk of adverse events, including death, in patients with ADHF are the QRS and QTc duration. 3 , 30 Also, in our cohort, both QRS and QTc duration were associated with mortality during follow‐up in univariable analysis but lost their significance upon adjustment for other baseline variables. Thus, among the ECG parameters readily available to the caretaker, the QRS‐T angle may provide the greatest prognostic information, as has been suggested also in earlier reports. 13 A reason for this observation may lie in the fact that the QRS‐T angle is likewise affected by abnormal depolarization as well as repolarization, which makes it more robust and less susceptible to noise than parameters measuring either of these factors alone. Moreover, the QRS‐T angle is a measure of electrical heterogeneity, which can occur at various stages in cardiac disease and is known to predispose for potentially lethal arrhythmias.
The findings of this study have to be interpreted in consideration of following limitations: First, patients with terminal renal failure requiring dialysis were excluded, wherefore the findings do not apply to this population. Second, the effect estimates of our multivariable analyses may be considered small. However, as the QRS‐T angle is by no means meant to replace other parameters but shall rather be seen in conjuncture with them, also small contributions may sum up to a common whole and should therefore not be neglected—especially when they are free of additional effort and cost. Third, we calculated only the frontal QRS‐T angle, while some authors have suggested that the spatial QRS‐T angle may have higher diagnostic and prognostic accuracy. 31 Fourth, we did not discriminate between newly diagnosed acute heart failure and decompensation of previously known chronic heart failure. Further investigations are needed to examine possible differences between these populations. Fifth, no detailed information was available on pacing modes in patients with cardiac devices. We were able to report the amount of patients with ventricular pacing but lack the data on atrial pacing. However, from a pathophysiological point of view, we do not think that atrial pacing should have had a relevant impact on the QRS‐T angle. And finally, the analyses are based on QRS‐T angles derived at a single point in time (i.e. at ED admission). While previous studies suggested that the QRS‐T angle was relatively stable over time, it is possible that it may be acutely affected in the setting of decompensated heart failure. 4 , 9 In the context of our current study, only admission ECGs were recorded. This precludes us from analysing the QRS‐T angle at discharge of the index hospitalization, and accordingly of the temporal QRS‐T angle variability. The topic however should be addressed in future studies and could even find a potential value of the QRS‐T angle as an indicator of treatment response in patients with ADHF.
Conclusions
The QRS‐T angle, a quickly obtainable and readily interpretable ECG parameter, may aid in the diagnosis of patients with suspected ADHF and independently predicts mortality during 2 years of follow‐up. Being usually available within minutes after patients contact, it can assist in the early management and decision process until results of more advanced tests become available.
Conflict of Interest
T.B. has received research grants from the Swiss National Science Foundation (PASMP3‐134362), the Department of Internal Medicine, University Hospital Basel, Abbott, and Roche as well as speaker honoraria from Roche. C.M. has received research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the KTI, the University of Basel, Abbott, Alere, AstraZeneca, Beckman Coulter, BG Medicine, Biomerieux, BRAHMS, Critical Diagnostics, Roche, Siemens, Singulex, Sphingotec, and 8sense as well as speaker/consulting honoraria from Abbott, Alere, AstraZeneca, Biomerieux, BMS, Boehringer Ingelheim, BRAHMS, Cardiorentis, Eli Lilly, Novartis, Roche, Sanofi, Siemens, and Singulex. T.R. has received research grants from the Goldschmidt‐Jacobson Foundation, the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union (Eurostars 9799—ALVALE), the Professor Max Cloëtta Foundation, the Cardiovascular Research Foundation Basel, the University of Basel, and the University Hospital Basel, all for work outside the submitted study. He has received speaker/consulting honoraria or travel support from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense‐Webster, Biotronik, Boston‐Scientific, Daiichi Sankyo, Medtronic, Pfizer‐BMS, and Roche, all for work outside the submitted study. He has received support for his institution's fellowship programme from Abbott/SJM, Biosense‐Webster, Biotronik, Boston‐Scientific, and Medtronic for work outside the submitted study. All other authors declare no conflict of interest.
Funding
This work was supported by research grants from the European Union, the Swiss National Science Foundation, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the University of Basel, the University Hospital Basel, Critical Diagnostics, Abbott, Alere, BRAHMS, Roche, and Singulex.
Supporting information
Figure S1. Calculation of the QRS‐T Angle.
Figure S2. Patient flow chart.
Table S1. Diagnostic accuracy of the QRS‐T angle to discriminate between acute decompensated heart failure and non‐cardiac causes of dyspnea compared to hs‐TnT and the gold‐standard NT‐proBNP.
Table S2. Cut‐off QRS‐T angles to discriminate between acute decompensated heart failure and non‐cardiac causes of dyspnea.
Table S3. Prediction of acute decompensated heart failure versus non‐cardiac cause of dyspnea by clinically available information at emergency department presentation: univariable and multivariable logistic regression.
Acknowledgements
We thank the patients who participated in the study, the staff of the participating emergency departments, the research coordinators, and the laboratory technicians (particularly Michael Freese, Caroline Kulangara, Claudia Stelzig, Kathrin Meissner, Christine Kruse, Irina Klimmeck, Janine Voegele, Beate Hartmann, Ina Ferel, Natascha Herr, and Fausta Chiaverio) for their most valuable efforts. R.S., Z.S., C.M., and T.R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sweda, R. , Sabti, Z. , Strebel, I. , Kozhuharov, N. , Wussler, D. , Shrestha, S. , Flores, D. , Badertscher, P. , Lopez‐Ayala, P. , Zimmermann, T. , Michou, E. , Gualandro, D. M. , Häberlin, A. , Tanner, H. , Keller, D. I. , Nowak, A. , Pfister, O. , Breidthardt, T. , Mueller, C. , and Reichlin, T. (2020) Diagnostic and prognostic values of the QRS‐T angle in patients with suspected acute decompensated heart failure. ESC Heart Failure, 7: 1817–1829. 10.1002/ehf2.12746.
Romy Sweda and Zaid Sabti have contributed equally to this analysis and should be considered co‐first authors.
Participating Institutions: University Hospital Bern, Switzerland; University Hospital Basel, Switzerland; University Hospital Zurich, Switzerland
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Pang PS, Collins SP, Miró Ò, Bueno H, Diercks DB, Di Somma S, Gray A, Harjola V‐P, Hollander JE, Lambrinou E, Levy PD, Papa A, Möckel M. Editor's choice—the role of the emergency department in the management of acute heart failure: an international perspective on education and research. Eur Heart J Acute Cardiovasc Care 2017; 6: 421–429. [DOI] [PubMed] [Google Scholar]
- 3. Gouda P, Brown P, Rowe BH, McAlister FA, Ezekowitz JA. Insights into the importance of the electrocardiogram in patients with acute heart failure. Eur J Heart Fail 2016; 18: 1032–1040. [DOI] [PubMed] [Google Scholar]
- 4. Pavri BB, Hillis MB, Subačius H, Brumberg GE, Schaechter A, Levine JH, Kadish A. Prognostic value and temporal behavior of the planar QRS‐T angle in patients with nonischemic cardiomyopathy. Circulation 2008; 117: 3181–3186. [DOI] [PubMed] [Google Scholar]
- 5. Borleffs CJW, Scherptong RWC, Man S‐C, van Welsenes GH, Bax JJ, van Erven L, Swenne CA, Schalij MJ. Predicting ventricular arrhythmias in patients with ischemic heart disease. Circ Arrhythmia Electrophysiol. 2009; 2: 548–554. [DOI] [PubMed] [Google Scholar]
- 6. Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS‐T angle predicts cardiac death in a clinical population. Heart Rhythm 2005; 2: 73–78. [DOI] [PubMed] [Google Scholar]
- 7. May O, Graversen CB, Johansen MØ, Arildsen H. A large frontal QRS‐T angle is a strong predictor of the long‐term risk of myocardial infarction and all‐cause mortality in the diabetic population. J Diabetes Complications 2017; 31: 551–555. [DOI] [PubMed] [Google Scholar]
- 8. Chen S, Hoss S, Zeniou V, Shauer A, Admon D, Zwas DR, Lotan C, Keren A, Gotsman I. Electrocardiographic predictors of morbidity and mortality in patients with acute myocarditis: the importance of QRS‐T angle. J Card Fail 2018; 24: 3–8. [DOI] [PubMed] [Google Scholar]
- 9. Gotsman I, Shauer A, Elizur Y, Zwas DR, Lotan C, Keren A. Temporal changes in electrocardiographic frontal QRS‐T angle and survival in patients with heart failure. PLoS One. 2018;13:e0194520. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Zhu Q, Zhu L, Jiang H, Xie J, Huang W, Xu B. Spatial/frontal QRS‐T angle predicts all‐cause mortality and cardiac mortality: a meta‐analysis. PLoS One. 2015;10:e0136174. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Z, Prineas RJ, Case D, Soliman EZ, Rautaharju PM, ARIC Research Group . Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the Atherosclerosis Risk In Communities Study). Am J Cardiol 2007; 100: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Bie MK, Koopman MG, Gaasbeek A, Dekker FW, Maan AC, Swenne CA, Scherptong RW, Van Dessel PF, Wilde AA, Schalij MJ, Rabelink TJ, Wouter JJ. Incremental prognostic value of an abnormal baseline spatial QRS‐T angle in chronic dialysis patients. Europace 2013; 15: 290–296. [DOI] [PubMed] [Google Scholar]
- 13. Kardys I, Kors JA, Van der Meer IM, Hofman A, Van der Kuip DAM, Witteman JCM. Spatial QRS‐T angle predicts cardiac death in a general population. Eur Heart J 2003; 24: 1357–1364. [DOI] [PubMed] [Google Scholar]
- 14. Strebel I, Twerenbold R, Wussler D, Boeddinghaus J, Nestelberger T, du Fay de Lavallaz J, Abächerli R, Maechler P, Mannhart D, Kozhuharov N, Rubini Giménez M, Wildi K, Sazgary L, Sabti Z, Puelacher C, Badertscher P, Keller DI, Miró Ò, Fuenzalida C, Calderón S, Martin‐Sanchez FJ, Iglesias SL, Osswald S, Mueller C, Reichlin T. Incremental diagnostic and prognostic value of the QRS‐T angle, a 12‐lead ECG marker quantifying heterogeneity of depolarization and repolarization, in patients with suspected non‐ST‐elevation myocardial infarction. Int J Cardiol 2019; 277: 8–15. [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016; 37: 2129–2200m. [DOI] [PubMed] [Google Scholar]
- 16. Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez‐Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: The Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 26: 384–416. [DOI] [PubMed] [Google Scholar]
- 17. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole‐Wilson PA, Strömberg A, Van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Auricchio A, Bax J, Böhm M, Corra U, Della Bella P, Elliott PM, Follath F, Gheorghiade M, Hasin Y, Hernborg A, Jaarsma T, Komajda M, Kornowski R, Piepoli M, Prendergast B, Tavazzi L, Vachiery JL, Verheugt FWA, Zannad F. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 18. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 19. Kligfield P, Badilini F, Denjoy I, Babaeizadeh S, Clark E, De Bie J, Devine B, Extramiana F, Generali G, Gregg R, Helfenbein E, Kors J, Leber R, Macfarlane P, Maison‐Blanche P, Rowlandson I, Schmid R, Vaglio M, van Herpen G, Xue J, Young B, Green CL. Comparison of automated interval measurements by widely used algorithms in digital electrocardiographs. Am Heart J 2018; 200: 1–10. [DOI] [PubMed] [Google Scholar]
- 20. Strauss DG, Mewton N, Verrier RL, Nearing BD, Marchlinski FE, Killian T, Moxley J, Tereshchenko LG, Wu KC, Winslow R, Cox C, Spooner PM, Lima JAC. Screening entire health system ECG databases to identify patients at increased risk of death. Circ Arrhythmia Electrophysiol 2013; 6: 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 22. Maisel AS, Peacock WF, McMullin N, Jessie R, Fonarow GC, Wynne J, Mills RM. Timing of immunoreactive B‐type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol 2008; 52: 534–540. [DOI] [PubMed] [Google Scholar]
- 23. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Matsukawa R, Kato K, Suzuki S, Naruke T, Yoshioka K, Miyoshi T, Baba Y, Yamamoto M, Murai K, Mizutani K, Yoshida K, Kitai T. Time‐to‐furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 3042–3051. [DOI] [PubMed] [Google Scholar]
- 24. Khan NK, Goode KM, Cleland JGF, Rigby AS, Freemantle N, Eastaugh J, Clark AL, de Silva R, Calvert MJ, Swedberg K, Komajda M, Mareev V, Follath F. Prevalence of ECG abnormalities in an international survey of patients with suspected or confirmed heart failure at death or discharge. Eur J Heart Fail 2007; 9: 491–501. [DOI] [PubMed] [Google Scholar]
- 25. Pang PS, Teerlink JR, Voors AA, Ponikowski P, Greenberg BH, Filippatos G, Felker GM, Davison BA, Cotter G, Kriger J, Prescott MF, Hua TA, Severin T, Metra M. Use of high‐sensitivity troponin T to identify patients with acute heart failure at lower risk for adverse outcomes. an exploratory analysis from the RELAX‐AHF trial. JACC Heart Fail 2016; 4: 591–599. [DOI] [PubMed] [Google Scholar]
- 26. Felker GM, Mentz RJ, Teerlink JR, Voors AA, Pang PS, Ponikowski P, Greenberg BH, Filippatos G, Davison BA, Cotter G, Prescott MF, Hua TA, Lopez‐Pintado S, Severin T, Metra M. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX‐AHF study. Eur J Heart Fail 2015; 17: 1262–1270. [DOI] [PubMed] [Google Scholar]
- 27. O'Connor CM, Fiuzat M, Lombardi C, Fujita K, Jia G, Davison BA, Cleland J, Bloomfield D, Dittrich HC, DeLucca P, Givertz MM, Mansoor G, Ponikowski P, Teerlink JR, Voors AA, Massie BM, Cotter G, Metra M. Impact of serial troponin release on outcomes in patients with acute heart failure: analysis from the PROTECT pilot study. Circ Hear Fail 2011; 4: 724–732. [DOI] [PubMed] [Google Scholar]
- 28. Hinson JS, Martinez DA, Cabral S, George K, Whalen M, Hansoti B, Levin S. Triage performance in emergency medicine: a systematic review. Ann Emerg Med 2019; 74: 140–152. [DOI] [PubMed] [Google Scholar]
- 29. Kutz A, Hausfater P, Amin D, Amin A, Canavaggio P, Sauvin G, Bernard M, Conca A, Haubitz S, Struja T, Huber A, Mueller B, Schuetz P. TRIAGE study group for the T study. The TRIAGE‐ProADM Score for an Early Risk Stratification of Medical Patients in the Emergency Department—development based on a multi‐national, prospective, observational study. PLoS One 2016; 11: e0168076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC, Grinfeld L, Swedberg K, Udelson JE, Cook T, Traver B, Zimmer C, Orlandi C, Gheorghiade M. Investigators for the E of VA in HFOSWT (EVEREST). Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA 2008; 299: 2656. [DOI] [PubMed] [Google Scholar]
- 31. Oehler A, Feldman T, Henrikson CA, Tereshchenko LG. QRS‐T angle: a review. Ann Noninvasive Electrocardiol 2014; 19: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Calculation of the QRS‐T Angle.
Figure S2. Patient flow chart.
Table S1. Diagnostic accuracy of the QRS‐T angle to discriminate between acute decompensated heart failure and non‐cardiac causes of dyspnea compared to hs‐TnT and the gold‐standard NT‐proBNP.
Table S2. Cut‐off QRS‐T angles to discriminate between acute decompensated heart failure and non‐cardiac causes of dyspnea.
Table S3. Prediction of acute decompensated heart failure versus non‐cardiac cause of dyspnea by clinically available information at emergency department presentation: univariable and multivariable logistic regression.


