Abstract
Aims
Anaemia and iron deficiency (ID) are frequently found in patients with chronic heart failure (CHF) and associated with adverse outcome. However, it is unclear whether absolute [transferrin saturation (TSAT) <20%, ferritin <100 μg/L] or inflammation‐driven functional ID (TSAT <20%, ferritin >100 μg/L) with and without anaemia had similar or different consequences for such patients.
Methods and results
Within this retrospective cohort study, 2223 patients (1601 men and 622 women) with CHF, referred to our department, between 2000 and 2018, were followed for a median time of 84 months. Anaemia was found in 393 patients and was an independent predictor for an adverse outcome [HR 2.164 (95% CI 1.865–2.512), P < 0.001]. In 674 patients with available parameters of iron metabolism, ID was present in 228 patients and was associated with an unfavourable outcome [HR 1.499 (95% CI 1.158–1.940), P = 0.002]. ID was best predicting an adverse outcome in men ≤59 years, with heart failure with reduced ejection fraction, preserved kidney function, no inflammation, and a body mass index (BMI) ≥25.5 kg/m2. Functional ID in women and absolute ID in men were associated with poor prognosis. Of note, TSAT <20% but not low ferritin levels were predictive for an adverse outcome. Anaemic patients with high ferritin levels, advanced inflammation, older age, low BMI, male gender, and reduced glomerular filtration rate had the worst prognosis.
Conclusions
Anaemia and low tissue iron availability as reflected by TSAT <20% are negative predictors of outcome in patients with CHF. Systemic inflammation, renal function, BMI, age, and gender are important contributors for the clinical course.
Keywords: Anaemia, Gender, Heart failure, Iron deficiency, Outcome
Introduction
Anaemia and iron deficiency (ID) are frequently encountered in patients with chronic heart failure (CHF). 1 , 2 , 3 They affect the quality of life and reduce the exercise capacity of patients by limiting their physical efficiency. 4 , 5 The presence of anaemia and/or ID is associated with a poor outcome in patients with CHF 6 , 7 , 8 , 9 , 10 , 11 and linked to an increased mortality. 12 , 13 ID can be caused by insufficient iron absorption or chronic blood losses resulting in low iron storage, termed as absolute or true ID being reflected by low circulating concentrations of iron and of the iron storage protein ferritin. ID can also originate from inflammation‐driven alterations of iron homeostasis leading to defective iron utilization and transport, termed as functional ID being reflected by low circulating iron but normal or increased ferritin concentrations. In both cases, iron availability for erythropoiesis is limited resulting in the development of anaemia over time. 14 , 15 The aetiology of anaemia in CHF is often unclear, but inflammation might be a central underlying component because immune activation and increased circulating cytokine levels are observed in patients with advanced CHF. 12 , 16 , 17 Inflammation mediated induction of cytokines and of the master regulatory of iron homeostasis, hepcidin, block dietary iron uptake, and iron release of macrophages. 18 , 19 Hepcidin controls body iron homeostasis by interacting with the cellular iron exporter ferroportin‐1, resulting in its internalization, thereby impairing duodenal iron absorption and iron egress from macrophages. 20 Consequently, in the setting of inflammation, iron is retained in the mononuclear phagocyte system, resulting in low circulating iron levels, but expansion of mononuclear phagocyte system iron stores as reflected by increased ferritin concentrations. 21 Originally, these alterations of iron homeostasis developed as a defence mechanism of the innate immune system to restrict the availability of the microbial nutrient iron for extracellular pathogens and to regulate cytokine activities during inflammation. 19 , 21 , 22
Ongoing immune activation leads to the development of anaemia of chronic disease (ACD), which is a common clinical picture seen in patients with chronic inflammation. In contrast, true or absolute ID and subsequently ID anaemia develop when dietary iron absorption cannot compensate for blood loss or for an increased iron demand. In addition, patients with ACD may also suffer from absolute ID, termed as ACD + ID, mostly as a consequence of chronic gastro‐intestinal bleeding, urogenital bleeding, phlebotomy, or reduced iron absorption that occurs as a consequence of inflammation. However, no good laboratory markers have been established to correctly identify absolute ID in the setting of inflammation. 23 , 24 Of note, ID without anaemia has been shown also to impact on functional parameters in CHF such as oxygen consumption or myocardial contractility. 11 , 25 This may be related in part to impaired mitochondrial function as a consequence of ID given that iron stimulates Krebs cycles activity and oxidative phosphorylation. 26 , 27
The objective of this study was to investigate the impact of absolute versus functional ID with and without anaemia on disease severity and outcome in patients with CHF and how this relates to inflammation.
Methods
Study population
In this retrospective analysis, we utilized data of 2223 Caucasian patients with diagnosed heart failure (HF) based on the presence of current or previous symptoms or characteristic clinical signs and evidence of left ventricular dysfunction. Patients included in this study were in stable cardiac conditions admitted to the Department of Cardiology at the Innsbruck Medical University for further diagnostic evaluation between 2000 and 2018. Exclusion criteria were acute HF, coronary artery disease on coronary angiography, or vitamin D or calcium supplementation within the last 6 months. Patients were treated according to CHF guidelines. The final study population comprised only patients with available haemoglobin concentrations (n = 2223). Among those, we investigated a subgroup of 674 patients in detail, of whom parameters of iron homeostasis were available.
This study agrees with the principles outlined in the Declaration of Helsinki and was approved by the local Ethics Committee of the Innsbruck Medical University (ID of the Ethical vote: UN4280, session number 298/4.11). All patients participating in this study gave written informed consent.
Follow‐up analysis
For the outcome analysis, patients were followed up until August 2018. The event‐free survival was defined as the period of time between the first hospitalization and the combined endpoint, which was either patients' death, heart transplantation (HTx), or ventricular assist device (VAD) implantation. Information about those events were received from the clinical information system, from the local mortality registry, from the patients' relatives, or from the patients themselves.
Measurements
Clinical evaluation, laboratory measurements, and non‐invasive and invasive were performed at first contact. Blood samples were stored at −80°C, and laboratory measurements were performed at the central laboratory of the Innsbruck University Hospital, which has to undergo internal and external quality assurances regularly. All parameters were determined by fully automated tests. Specifically, C‐reactive protein (CRP) was measured by an immunoturbidimetry test (Roche, Mannheim, Germany). Creatinine and NTpro‐BNP were determined by standardized automated tests. For measuring serum iron levels, the FerroZine™ method without deproteinization was used (Merck KGaA, Darmstadt, Germany). Ferritin levels were detected with an immunoturbidimetry test containing anti‐ferritin antibodies from rabbits (Roche, Mannheim, Germany). Transferrin was determined by an immunoturbidimetry test containing specific antibodies from rabbits (Roche, Mannheim, Germany). The transferrin saturation (TSAT) was calculated as followed: iron/transferrin × 70.9. Haemoglobin levels were analysed photometrically at 555 nm (XE‐5000, Sysmex GmbH, Vienna, Austria). We used the IDMS‐traceable MDRD study equation [estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) = 175 × (serum creatinine) − 1.154 × age − 0.203 (× 0.742 if female)] to estimate the eGFR.
Left ventricular ejection fraction was measured by echocardiography. Mean pulmonary artery pressure and pulmonary capillary wedge pressure were measured during right heart catheterization. Cardiac output was calculated by the Fick's formula.
Classifications
According to criteria of the World Health Organization, anaemia was defined as Hb < 130 g/L in men and Hb < 120 g/L in women. We classified ID into absolute (true) ID and functional ID. Absolute ID indicating low iron storage (LIS) was defined as serum ferritin <100 μg/L plus transferrin saturation (TSAT) <20% and functional ID indicating defective iron utilization and transport (DIU) was defined as serum ferritin between 100 and 300 μg/L plus TSAT of <20% in patients with HF. 2 , 13 , 28 , 29 , 30 , 31 Accordingly, anaemia with absolute ID/LIS would be categorized as ID anaemia, whereas functional ID/DIU and anaemia would suggest the diagnosis of ACD.
Heart failure with reduced ejection fraction (HFrEF), heart failure with mid‐range ejection fraction (HFmrEF), and heart failure with preserved ejection fraction (HFpEF) were diagnosed by echocardiographic, clinical, and biochemical parameters according to the ESC 2016 guidelines for the diagnosis and treatment of acute and CHF. 32
Statistical analysis
To test for Gaussian distribution, we used the Shapiro–Wilk test. Parameters are depicted as means ± SD when normally distributed, medians (25th and 75th percentile) when not normally distributed or n (%). To test for significant differences between groups, the two‐sample t‐test, ANOVA test, Mann–Whitney U test, Kruskal–Wallis test, or Pearson χ 2 test was used. Spearman rank correlation tests for correlation analysis. Parameters were investigated by univariate and multivariate proportional hazard regression analyses for their predictive power for an adverse outcome. Univariate and multivariate proportional hazard regression analyses including ID were performed in the subpopulation with available iron metabolism parameters. All parameters showing a skewed distribution were logarithmically transformed before regression analysis.
All tests were two tailed, and P‐values <0.05 were regarded to indicate statistical significance. Bonferroni correction was applied to address type I errors in univariate analyses. Statistical analysis was performed using SPSS Statistics Version 25.0 for Macintosh (IBM Corporation, Armonk, NY, USA).
Results
Clinical characteristics of patients and gender differences
The study population comprised 2223 patients with CHF: 1601 men (72.0%) and 622 women (28.0%). Baseline characteristics for the patient cohort and differentiated for patients with or without anaemia are listed in Table 1 .
Table 1.
Baseline characteristics
| Total | Anaemic | Non‐anaemic | Sig. | |
|---|---|---|---|---|
| n = 2223 | n = 393 | n = 1830 | P‐value | |
| Demographic and clinical characteristics | ||||
| Age (years) | 57.2 ± 14.7 | 61.3 ± 57.2 | 56.4 ± 14.6 | <0.001 |
| Sex (male/female) | 72.0% / 28.0% | 18.6% / 15.3% | 81.4% / 84.7% | 0.064 |
| BMI (kg/m2) | 26.0 ± 4.5 | 25.2 ± 4.4 | 26.2 ± 4.5 | <0.001 |
| Heart rate (b.p.m.) | 76 ± 17 | 77 ± 16 | 76 ± 17 | 0.206 |
| Syst. BP (mmHg) | 126 ± 22 | 122 ± 23 | 126 ± 22 | <0.001 |
| NYHA class | <0.001 | |||
| NYHA class I | 28.0% | 16.9% | 30.4% | |
| NYHA class II | 43.4% | 40.3% | 44.1% | |
| NYHA class III/IV | 28.6% | 42.9% | 25.6% | |
| Hypertension | 50.8% | 52.8% | 50.4% | 0.379 |
| Atrial fibrillation | 18.5% | 19.3% | 18.3% | 0.632 |
| Diabetes mellitus | 17.3% | 22.9% | 16.1% | 0.003 |
| Smoking | 10.8% (16.6% a ) | 8.5% (19.6% a ) | 11.3% (16.0% a ) | 0.419 |
| Alcohol abuse | 2.2% (2.6% a ) | 2.2% (1.9% a ) | 2.2% (2.7% a ) | 0.798 |
| Laboratory testing (serum) | ||||
| NT‐proBNP (ng/L) | 2812 ± 5431 | 5615 ± 8968 | 2245 ± 4164 | <0.001 |
| eGFR (mL/min/1.73 m2) | 81.21 ± 47.16 | 65.43 ± 52.49 | 84.36 ± 45.39 | <0.001 |
| CRP (mg/L) | 0.96 ± 2.41 | 2.11 ± 4.54 | 0.70 ± 1.47 | <0.001 |
| Ferritin (μg/L) | 231 ± 389 | 275 ± 430 | 220 ± 378 | 0.174 |
| Iron (μmol/L) | 16 ± 8 | 11 ± 9 | 17 ± 8 | 0.009 |
| TSAT (%) | 24 ± 14 | 17 ± 13 | 26 ± 13 | <0.001 |
| Haemoglobin (g/L) | 142 ± 18 | 115 ± 12 | 147 ± 13 | <0.001 |
| Haemodynamics | ||||
| LVEF (%) | 34 ± 15 | 35 ± 16 | 34 ± 15 | 0.160 |
| Mean PAP (mmHg) | 28 ± 11 | 27 ± 11 | 28 ± 11 | 0.347 |
| PCWP (mmHg) | 19 ± 9 | 18 ± 10 | 19 ± 9 | 0.730 |
| CO (L/min) | 4.4 ± 2.1 | 4.9 ± 5.1 | 4.3 ± 1.3 | 0.454 |
| Medications | ||||
| ACE inhibitor | 65.4% | 58.2% | 66.8% | 0.014 |
| ARB | 15.5% | 18.7% | 14.9% | 0.223 |
| Beta‐blocker | 64.7% | 63.9% | 64.8% | 0.862 |
| MRA | 27.1% | 29.8% | 26.6% | 0.446 |
| Diuretics | 65.6% | 76.3% | 63.6% | <0.001 |
| Statins | 35.9% | 41.5% | 34.8% | 0.099 |
Parameters from 2223 patients are listed as mean ± SD or n (%) for the whole cohort and separately for patients with or without anaemia. Two‐sample t‐test and the Pearson χ 2 test was used for comparisons between anaemic and non‐anaemic subgroups. After a Bonferroni correction for multiple comparisons, a P‐value <0.0018 was considered significant.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CO, cardiac output; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; mean PAP, mean pulmonary artery pressure; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; Sig., significance; Syst. BP, systolic blood pressure; TSAT, transferrin saturation; TSAT, transferrin saturation.
Smoker in the past or former alcohol abuse.
Most patients suffered from HFrEF (n = 1501), 260 patients had HFmrEF, and 443 patients had HFpEF. Four hundred sixty‐four patients (20.9%) presented with chronic kidney disease stage III, and 69 patients (3.1%) presented with an eGFR <30 mL/min/1.73 m2, that is, chronic kidney disease stage IV or worse.
Anaemia related to heart failure severity and outcome of patients
Anaemia was found in 17.7% (n = 393) of the patients with no differences between men (18.6%) and women (15.3%). Anaemic patients were older, presented with higher NYHA functional class, had a lower BMI, systolic blood pressure, eGFR and TSAT as well as lower serum iron concentrations, and showed significantly higher NT‐proBNP and CRP concentrations (Table 1 ). Accordingly, a stepwise decrease in haemoglobin levels was found with higher NYHA classes (I: 145 ± 15 g/L, II: 142 ± 17 g/L, III/IV: 138 ± 21 g/L, P < 0.001). Haemoglobin levels were negatively correlated with NT‐proBNP (r s = −0.203, P < 0.001) and CRP levels (r s = −0.167, P < 0.001). Moreover, haemoglobin levels positively correlated with ferritin levels (r s = 0.162, P < 0.001), serum iron levels (r s = 0.309, P = 0.006), and TSAT (r s = 0.359, P < 0.001).
During a median follow‐up of 84 months, 686 patients died, 197 patients underwent HTx, and 34 received a VAD implantation. Anaemia was a strong predictor of outcome in both men and women, as well as in patients with and without signs of systemic inflammation as reflected by normal or increased CRP levels (CRP > 0.5 mg/L) as well as in patients with HFpEF, HFmrEF, and HFrEF (Figure 3 A ). Cox regression analysis together with the multiplication term revealed a significantly interaction of anaemia with age (P < 0.001), sex (P = 0.034), and kidney function (P = 0.018). Anaemic patients had a more than two‐fold higher risk for an unfavourable event as compared with non‐anaemic patients (HR 2.164 (95% CI 1.865–2.512), P < 0.001, Figure 1 A ]. Cumulative 10 year event rate was 62.3% in anaemic patients and 37.2% in non‐anaemic patients. Accordingly, low haemoglobin levels were significantly linked to the combined endpoint [HR 0.119 (95% CI 0.075–0.189), P < 0.001].
Figure 3.
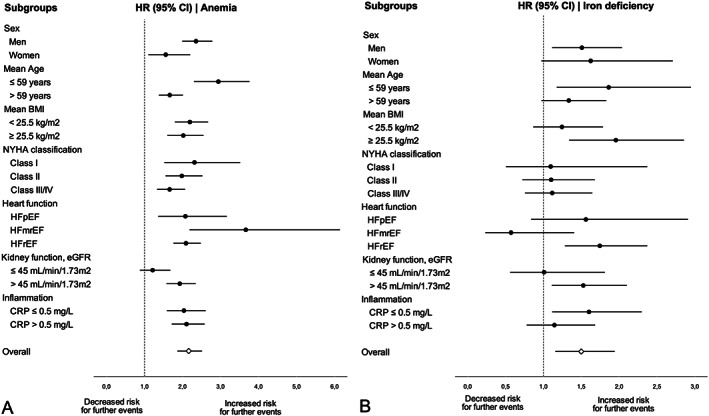
Linkage of anaemia with the combined endpoint in various subgroups of 2223 patients with CHF (A) and linkage of iron deficiency with the combined endpoint in various subgroups of 674 patients with CHF and available iron metabolism parameters (B). Hazard ratio with lower and upper 95% confidence interval is shown.
Figure 1.
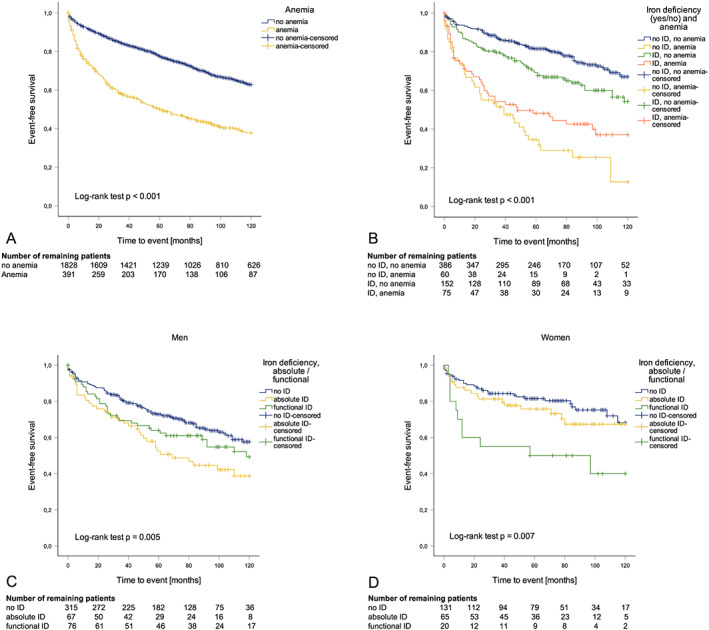
Kaplan–Meier plots of patients concerning anaemia, absolute/functional ID, and combined anaemia/ID classification. The number of remaining patients after 20, 40, 60, 80, 100, and 120 months is depicted below each figure. (A) Event‐free survival of patients with (n = 393, yellow) or without (n = 1830, blue) anaemia: the cumulative event rate within 10 years was 62.3% in anaemic and 37.2% in non‐anaemic patients (log‐rank test P < 0.001). (B) Patients with no ID and anaemia (n = 60, yellow) had the highest event rate after 10 years (87.3%). Also, patients with ID and anaemia (n = 75, orange) had a higher event rate (63.0%) compared with patients with no ID and no anaemia (n = 386, blue). Finally, patients with ID and no anaemia (n = 152, green) had a higher event rate compared with patients with no ID and no anaemia as well (45.8% vs. 33.0%). (C) Absolute ID was associated with the highest event rate after 10 years (61.3%) in men (n = 67, yellow), while men with functional ID (n = 76, green) or no ID (n = 315, blue) had a significantly lower cumulative event rate (50.7% and 42.4%, log‐rank test P = 0.005). (D) In contrast, functional ID was associated with the highest event rate (60.0%) in women (n = 20, green), while women with absolute ID (n = 65, yellow) or no ID (n = 131, blue) had a significantly lower cumulative event rate (32.7% and 31.8%, log‐rank test: P = 0.007).
Association of iron deficiency with inflammation, heart failure severity, and outcome of patients
Parameters of serum iron metabolism were only available in 674 patients (458 men and 216 women). From these patients, 228 [143 men (31.2%) and 85 women (39.4%)] presented with either absolute or functional ID (33.8%) according to the definition provided in the Methods section irrespective of the haemoglobin content. Patients with ID had significantly higher NT‐proBNP [1652 ng/L (496–3883) vs. 955 ng/L (204–2083), P = 0.002] and CRP levels (1.26 ± 2.87 mg/dL vs. 0.73 ± 2.41 mg/dL, P = 0.011) compared with patients without ID. Accordingly, the TSAT was negatively correlated with NT‐proBNP (r s = −0.273, P < 0.001, Figure 2 A ) and CRP levels (r s = −0.329, P < 0.001, Figure 2 B ) and positively correlated with the eGFR (r s = 0.201, P < 0.001). In contrast, ferritin levels were correlated with NT‐proBNP (Figure 2 C ) and CRP levels (Figure 2 D ) only in women but not in men.
Figure 2.
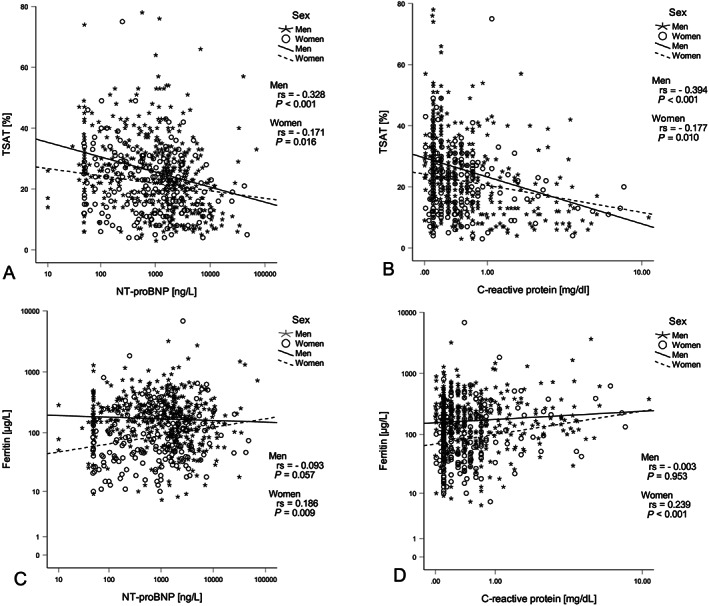
Spearman rank correlations among selected parameters in CHF patients (n = 674). TSAT correlated with NT‐proBNP (A) (r s = −0.271, P < 0.001) and CRP (B) (r s = −0.239, P < 0.001) independent of sex, while ferritin levels correlated with NT‐proBNP (C) (r s = 0.186, P = 0.009) and CRP (D) (r s = 0.239, P < 0.001) only in women (n = 216).
The median follow‐up of patients with available iron parameters was 68 months. During follow‐up, 176 patients died, and a total of 61 patients underwent HTx (n = 53) or VAD implantation (n = 8). ID was related to a significantly higher event rate (Table 2 ). Subgroup analysis revealed that ID was only significant for adverse events in men, patients under the age of 59, patients with an BMI < 25.5 kg/m2, patients with HFrEF, patients with an eGFR >45 mL/min/1.73 m2 as well as in patients with normal CRP concentrations ≤0.5 mg/L (Figure 3 B ). However, when analysing for interactions by including the multiplication term into the Cox regression analysis, no significantly interactions were found between ID and subgroups.
Table 2.
Univariate and multivariate Cox regression analysis for the combined endpoint in the subpopulation with available iron metabolism parameters
| Univariate model | Multivariate model a | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Anaemia yes vs. no | 3.127 | 2.390–4.090 | <0.001 | 1.646 | 1.132–2.394 | 0.009 |
| ID yes vs. no | 1.499 | 1.158–1.940 | 0.002 | 1.044 | 0.750–1.451 | 0.800 |
| TSAT <20% vs. ≥20% | 1.771 | 1.373–2.285 | <0.001 | |||
| Ferritin 100–300 μg/L vs. <100 μg/L | 0.857 | 0.643–1.144 | 0.298 | |||
| Ferritin >300 μg/L vs. <100 μg/L | 1.121 | 0.792–1.585 | 0.519 | |||
| NYHA class II vs. I | 2.596 | 1.743–3.867 | <0.001 | 1.538 | 0.941–2.513 | 0.086 |
| NYHA class III/IV vs. I | 7.446 | 4.998–11.092 | <0.001 | 2.763 | 1.610–4.741 | <0.001 |
| NT‐proBNP [ng/L]_Ln | 1.970 | 1.770–2.192 | <0.001 | 1.658 | 1.394–1.973 | <0.001 |
| eGFR [mL/min/1.73m2]_Ln | 0.392 | 0.311–0.494 | <0.001 | 1.074 | 0.748–1.542 | 0.700 |
| CRP [mg/L]_Ln | 1.276 | 1.159–1.405 | <0.001 | 0.938 | 0.811–1.085 | 0.390 |
| LVEF [%]_Ln | 0.434 | 0.328–0.573 | <0.001 | 1.147 | 0.774–1.699 | 0.494 |
| Age [years]_Ln | 6.511 | 3.717–11.405 | <0.001 | 3.789 | 1.664–8.628 | 0.002 |
| Sex men vs. women | 1.379 | 1.027–1.851 | 0.033 | |||
| BMI [kg/m2]_Ln | 0.614 | 0.291–1.298 | 0.202 | |||
Multivariate Cox regression analysis is stratified for sex.
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association.
After a Bonferroni correction for multiple comparisons in univariate Cox regression analysis, a P‐value <0.0036 was considered significant.
Absolute and functional iron deficiency in patients with heart failure
Specification of ID revealed that absolute ID was prevalent in 132 patients and functional ID in 96 patients. In the subpopulation, the prevalence of absolute ID was twice as high in women (n = 65, 30.1%) as compared with men (n = 67, 14.6%), while the prevalence of functional ID was higher in men (n = 76, 16.6%) as compared with women (n = 20, 9.3%). Patients with functional ID had significantly higher CRP levels compared with patients with absolute ID (1.98 ± 4.16 mg/dL vs. 0.73 ± 0.97 mg/dL, P = 0.004).
In age‐adjusted and sex‐stratified Cox regression analysis of the subpopulation, there was no difference regarding outcome whether absolute or functional ID was present [HR 0.900 for absolute compared with functional ID (95% CI 0.603–1.342), P = 0.605]. However, in patients with HFpEF, functional ID, but not absolute ID, was related to a higher event rate [n = 25; HR 2.252 (95% CI 1.027–4.937), P = 0.043] independent of sex.
Univariate Cox regression analysis revealed that absolute ID, but not functional ID, was associated with worse outcome in men [HR 1.761 (95% CI 1.206–2.570), P = 0.003], while functional ID, but not absolute ID, was associated with adverse outcome in women [HR 2.778 (95% CI 1.390–5.555), P = 0.004] (Figure 1 C and 1 D ). Interestingly, women had significantly lower serum CRP (0.89 mg/dL vs. 0.99 mg/dL, P = 0.011) and ferritin (171 μg/L vs. 259 μg/L, P < 0.001) concentrations compared with men.
When studying single indicators of iron homeostasis, we found that patients with a TSAT <20% (irrespective of CRP levels) had a higher risk for further events compared with patients with a TSAT ≥20% (Table 2 ). Accordingly, a low TSAT was significantly linked to the combined endpoint [HR 0.608 (95% CI 0.498–0.741), P < 0.001]. This association was found in both, men and women, patients with HFrEF and HFpEF as well as in patients with reduced and preserved kidney function. Contrary, ferritin levels were not predictive for a worse outcome (Table 2 ).
Anaemia combined with iron deficiency in the prediction of outcome
In the subpopulation of patients with available iron metabolism parameters (n = 674), 228 had absolute or functional ID. Anaemia was also present in 135 patients of whom 51 had absolute and 24 had functional ID. Therefore, the prevalence of ID was twice as high in patients with anaemia as compared with non‐anaemic patients (55.6% vs. 28.4%, P < 0.001) and equally distributed between men (18.6%) and women (15.3%). NT‐proBNP levels were highest in patients with both, anaemia and ID, and these patients had the lowest TSAT, iron, ferritin, and haemoglobin levels. Interestingly, we found no differences in haemodynamic parameters between the different groups (Supporting Information, Table S1 ).
In Cox regression analysis adjusted for age and stratified for sex, patients with anaemia and no ID (n = 60) had the highest risk for further events [HR 3.662 (95% CI 2.510–5.342), P < 0.001] as compared with subjects without anaemia and no ID (n = 386). Also, patients with anaemia and ID and (n = 75) had a significantly higher risk for an event [HR 2.711 (95% CI 1.876–3.919), P < 0.001] compared with patients without anaemia and no ID. Finally, patients with ID and no anaemia (n = 152) had a higher event rate [HR 1.622 (95% CI 1.170–2.250), P = 0.004] compared with patients without anaemia and no ID (Figure 1 B ).
Interestingly, anaemic patients without ID tended to have a higher event rate compared with anaemic patients with ID [HR 1.523 (95% CI 0.988–2.349), P = 0.057, Figure 1 B ]. However, anaemic patients without ID were significantly older, had the lowest BMI, eGFR and cardiac output of all groups, but presented with the highest CRP and ferritin levels (Supporting Information, Table S1 ). Moreover, the worst prognosis was found in patients with ferritin >300 μg/L and TSAT <20%, which is the typical pattern of ACD 21 (Supporting Information, Figure S1 ).
When adjusting for sex, age and established predictors in HF (including NYHA class, NT‐proBNP, eGFR, and left ventricular ejection fraction) in the multivariate Cox regression model anaemia but not ID was predictive for further adverse events (Table 2 ).
Discussion
In this observational study including 2223 patients with CHF, the impacts of anaemia and the contribution of co‐factors including age, BMI, and gender were investigated in regard to patients' outcome. Our finding that anaemia is associated with a worse outcome in patients with HF are well in line with earlier studies. 6 , 7 , 8 While in a subgroup with available parameters of iron metabolism both anaemia and ID with and without anaemia were strong predictors of an adverse outcome of patients, we found that anaemia was a better predictor for outcome than ID. This appears to be in contrast to recent studies, 12 , 13 where ID was an even stronger predictor of mortality than anaemia without ID. Differences from those observations may be due to the fact that in our study ID was not related to an adverse outcome in patients with HFpEF and the relative numbers of patients with HFrEF differed between the study by Klip and co‐workers 13 and our cohort (87.0% patients with HFrEF vs. 55.6% in our study). Also the fact that in our subpopulation only 23.6% of the patients had an ischaemic cause of CHF (vs. 60.0% in the study by Klip and co‐workers 13 or 64% in the investigation by Okonko et al. 11 ) and that patients were significantly older in those two other studies 11 , 13 in which additional factors contributing to anaemia and imbalances of iron homeostasis 33 might likewise have contributed to these differences. Finally, the fact that parameters of iron metabolism were not available from all patients that were initially included in the study must be taken into consideration. The definition of ID in general may also play a role because some studies only use ferritin levels <100 μg/mL as definition of ID whereas others also take a low transferrin saturation into account, because the latter parameter reflects iron availability for the bone marrow and tissue. 12 , 13 , 14 , 15
In our study, ID was the best predictor of subsequent adverse events in younger male patients with preserved kidney function and no inflammation. Of interest, we also observed that low tissue iron availability as reflected by TSAT <20% rather than low iron stores as estimated from ferritin levels were predicting poor prognosis in CHF. This concurs with a recent study where bone marrow iron staining was used to define ID 34 as well as with a publication from the European Iron Consortium cohort. 35 However, our results partly contrasts with results from the BIOSTAT‐CHF study (n = 2357) showing that defective iron utilization in combination with low tissue iron storage (but not normal tissue iron storage) is associated with a worse prognosis. 36 These differences might be due to the higher prevalence of ID in the BIOSTAT‐CHF study (61.6% vs. 33.8%) but also attributed to the fact that the BIOSTAT‐CHF study comprise more than three times more CHF patients than our cohort, which might explain this disagreement. Ferritin levels, however, are not only affected by body iron availability but also by inflammation. Therefore, its diagnostic utility to indicate ID is limited in subjects with chronic diseases. 23
Interestingly, while absolute ID was associated with a higher event rate in men, functional ID was associated with a higher event rate in women. These findings suggest that the mechanism of iron retention might be different in male and female patients with CHF. The background for this observation is unclear but may be linked to gender‐specific differences in regulation of iron status by hormones such as oestrogens 37 and testosterone 38 or altered immune regulation and inflammatory profiles between men and women based on genetic and hormonal factors. 39 The latter is supported by our finding that women had significantly lower CRP and ferritin levels than men. This goes along with our observations that women had a worse outcome with ferritin levels >300 μg/L and with advanced inflammation. On the other hand, also the fact that women comprised only about a third of our study population might explain this finding.
Of interest, functional ID was related to an increased event rate in patients with HFpEF, while absolute ID was not. This would suggest that inflammation in association with ID are linked to a poor prognosis in such patients. 21 A recent meta‐analysis showed that ID is associated with worse exercise capacity and functional outcome but not with mortality or hospitalization in patients with HFpEF. 40 However, no differentiation between absolute and functional ID was made in that outcome analysis.
In fact, inflammation is likely to induce the development of anaemia and thus be a major driver of worse outcome. This is in line with our observation that patients with anaemia, inflammation, and high ferritin levels had the poorest prognosis. The latter two parameters together would suggest the presence of advanced ACD characterized by higher ferritin levels and massive iron restriction for tissues such as the heart. 21 Elderly patients often have a chronic inflammatory status, 41 which contributes to multifactorial anaemia in such subjects 33 along with the negative effects of nutritional deficiency and impaired renal function on iron homeostasis and erythropoiesis. 42 Thus, further prospective studies with large cohorts are needed to clarify the impact of any cause of ID and/or anaemia for the outcome of patients with CHF to identify the nature of gender‐specific differences and to identify good predictive markers for outcome and for guiding therapy including iron supplementation.
Limitations
This was a retrospective explorative analysis with a frequent but specific group of patients with CHF. In addition, iron metabolism parameters were not available of all patients initially included in the study, which may be unmeasured bias in who/why some patients had these measures and not others. This depict a risk for the possibility of type I and II errors. The use of the Bonferroni correction not only limits the risk of false‐positive results but also carries the risk of increasing false‐negative results. Therefore, the findings in the final patient cohort do not allow unrestricted generalization for all CHF patients.
Conclusion
This study shows that anaemia and also low tissue iron availability (TSAT <20%) are associated with an adverse outcome in patients with CHF. In addition, our investigation identified risk factors in anaemic patients linked to a poor outcome that are older age, male gender, reduced kidney function, low BMI, high ferritin levels and more advanced inflammation. Moreover, this study provides novel information in regard to gender‐specific effects of ID toward outcome. While the presence of ID in general is linked to a worse prognosis, absolute ID appears to be an indicator for poor outcome in men, whereas in women, inflammation‐driven functional ID is linked to an unfavourable prognosis. Our study thus indicates gender differences in CHF disease outcomes as a function of iron homeostasis and inflammatory status that may impact on diagnostic and therapeutic algorithm in the clinical management of such patients.
Conflict of interest
The authors declare no conflict of interest.
Funding
This research was supported by the Christian Doppler Society (CDG to G.W.).
Supporting information
Figure S1 Association of iron status with outcome. Patients with ferritin levels ≥300 μg/L and a TSAT >20% (n = 29, green) had the highest event rate after 10 years (84.6%). Patients with ferritin levels 100–300 μg/L and a TSAT <20% (n = 96, yellow) as well as patients with ferritin levels <100 μg/L and TSAT <20% (n = 132, blue) had a lower event‐rate of 52.9% and 49.0%. Ferritin levels >300 μg/L in combination with a TSAT >20% (n = 103, red) and Ferritin levels <100 μg/L in combination with a TSAT >20% (n = 102, orange) had an event‐rate of 40.9% and 41.3%. The lowest event‐rate with 31.4% had patients with ferritin levels 100–300 μg/L and a TSAT >20% (n = 212, violet).
Table S1 Demographic and clinical characteristics and laboratory measurements according to the anaemia/ID classification
Acknowledgement
We hereby thank Katie Bates, PhD from the Department of Medical Statistics, Informatics and Health Economics of the Innsbruck Medical University for statistical support.
Kurz, K. , Lanser, L. , Seifert, M. , Kocher, F. , Pölzl, G. , and Weiss, G. (2020) Anaemia, iron status, and gender predict the outcome in patients with chronic heart failure. ESC Heart Failure, 7: 1880–1890. 10.1002/ehf2.12755.
Contributor Information
Katharina Kurz, Email: katharina.kurz@i-med.ac.at.
Günter Weiss, Email: guenter.weiss@i-med.ac.at.
References
- 1. Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation 2003; 107: 223–225. [DOI] [PubMed] [Google Scholar]
- 2. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 3. McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen‐Solal A, Kindermann I, Manito N, Pfister O, Pohjantahti‐Maaroos H, Taylor J, Comin‐Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39: 1780–1786. [DOI] [PubMed] [Google Scholar]
- 5. Kraai IH, Luttik ML, Johansson P, De Jong RM, Van Veldhuisen DJ, Hillege HL, Jaarsma T. Health‐related quality of life and anemia in hospitalized patients with heart failure. Int J Cardiol 2012; 161: 151–155. [DOI] [PubMed] [Google Scholar]
- 6. Szachniewicz J, Petruk‐Kowalczyk J, Majda J, Kaczmarek A, Reczuch K, Kalra PR, Piepoli MF, Anker SD, Banasiak W, Ponikowski P. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol 2003; 90: 303–308. [DOI] [PubMed] [Google Scholar]
- 7. Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, Havranek EP, Krumholz HM. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med 2005; 165: 2237–2244. [DOI] [PubMed] [Google Scholar]
- 8. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta‐analysis. J Am Coll Cardiol 2008; 52: 818–827. [DOI] [PubMed] [Google Scholar]
- 9. Jankowska EA, Malyszko J, Ardehali H, Koc‐Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17: 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011; 58: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 12. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34: 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582 e573. [DOI] [PubMed] [Google Scholar]
- 14. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood 2019; 133: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Camaschella C. Iron deficiency. Blood 2019; 133: 30–39. [DOI] [PubMed] [Google Scholar]
- 16. Niethammer M, Sieber M, von Haehling S, Anker SD, Munzel T, Horstick G, Genth‐Zotz S. Inflammatory pathways in patients with heart failure and preserved ejection fraction. Int J Cardiol 2008; 129: 111–117. [DOI] [PubMed] [Google Scholar]
- 17. Lanser L, Polzl G, Fuchs D, Weiss G, Kurz K. Neopterin is associated with disease severity and outcome in patients with non‐ischaemic heart failure. J Clin Med 2019; 17: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT, Wroblewski VJ, Wurz E, Datz C, Weiss G. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 2009; 113: 5277–5286. [DOI] [PubMed] [Google Scholar]
- 19. Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 2015; 15: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood 2016; 127: 2809–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352: 1011–1023. [DOI] [PubMed] [Google Scholar]
- 22. Nairz M, Haschka D, Demetz E, Weiss G. Iron at the interface of immunity and infection. Front Pharmacol 2014; 5: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiss G. Anemia of chronic disorders: new diagnostic tools and new treatment strategies. Semin Hematol. [Review] 2015; 52: 313–320. [DOI] [PubMed] [Google Scholar]
- 24. van Santen S, de Mast Q, Oosting JD, van Ede A, Swinkels DW, van der Ven AJ. Hematologic parameters predicting a response to oral iron therapy in chronic inflammation. Haematologica 2014; 99: e171–e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018; 20: 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oexle H, Gnaiger E, Weiss G. Iron‐dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta 1999; 1413: 99–107. [DOI] [PubMed] [Google Scholar]
- 27. Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Col Cardiol 2013; 61: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 29. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 30. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P, Investigators F‐HT. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 31. Comin‐Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health‐related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR‐HF study. Eur Heart J 2013; 34: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 33. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood 2018; 131: 505–514. [DOI] [PubMed] [Google Scholar]
- 34. Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, van der Wal HH, Swinkels DW, van Pelt J, Mulder AB, Bulstra SK, Vellenga E, Mariani MA, de Boer RA, van Veldhuisen DJ, van der Meer P. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 2018; 11 e004519‐e004519. [DOI] [PubMed] [Google Scholar]
- 35. Moliner P, Jankowska EA, van Veldhuisen DJ, Farre N, Rozentryt P, Enjuanes C, Polonski L, Meroño O, Voors AA, Ponikowski P, Van der Meer P, Comin‐Colet J. Clinical correlates and prognostic impact of impaired iron storage versus impaired iron transport in an international cohort of 1821 patients with chronic heart failure. Int J Cardiol 2017; 243: 360–366. [DOI] [PubMed] [Google Scholar]
- 36. Grote Beverborg N, van der Wal HH, Klip IT, Anker SD, Cleland J, Dickstein K, van Veldhuisen DJ, Voors AA, van der Meer P. Differences in clinical profile and outcomes of low iron storage vs defective iron utilization in patients with heart failure: results from the DEFINE‐HF and BIOSTAT‐CHF studies. JAMA Cardiol 2019; 4: 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 2012; 511: 398–403. [DOI] [PubMed] [Google Scholar]
- 38. Latour C, Kautz L, Besson‐Fournier C, Island ML, Canonne‐Hergaux F, Loreal O, Ganz T, Coppin H, Roth MP. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 2014; 59: 683–694. [DOI] [PubMed] [Google Scholar]
- 39. Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis 2014; 209: S93–S99. [DOI] [PubMed] [Google Scholar]
- 40. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Open Heart 2019; 6: e001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger‐Gateau P, Layé S, Fuchs D. Chronic low‐grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry 2011; 70: 175–182. [DOI] [PubMed] [Google Scholar]
- 42. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol: JASN 2012; 23: 1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Association of iron status with outcome. Patients with ferritin levels ≥300 μg/L and a TSAT >20% (n = 29, green) had the highest event rate after 10 years (84.6%). Patients with ferritin levels 100–300 μg/L and a TSAT <20% (n = 96, yellow) as well as patients with ferritin levels <100 μg/L and TSAT <20% (n = 132, blue) had a lower event‐rate of 52.9% and 49.0%. Ferritin levels >300 μg/L in combination with a TSAT >20% (n = 103, red) and Ferritin levels <100 μg/L in combination with a TSAT >20% (n = 102, orange) had an event‐rate of 40.9% and 41.3%. The lowest event‐rate with 31.4% had patients with ferritin levels 100–300 μg/L and a TSAT >20% (n = 212, violet).
Table S1 Demographic and clinical characteristics and laboratory measurements according to the anaemia/ID classification


