Abstract
Right ventricular (RV) failure remains a major complication after surgical implantation of a left ventricular assist device (LVAD). While the use of a percutaneous RV assist device has been described as a short‐term bridge to recovery in end‐stage heart failure patients with early post‐operative RV failure after index LVAD implant, management of refractory late RV failure remains challenging in these patients. We report the first successful case of extended Impella RP use as a safe and effective bridge to orthotopic heart transplant in an LVAD patient with refractory, haemodynamically significant late RV failure. The Impella RP provided support for 37 days. Haemodynamically intolerant arrhythmia precluded use of inotropic support. No adverse complications related to percutaneous Impella RP support were seen. We also review potential considerations for mechanical circulatory support strategies in this setting: central RV assist device cannulation requires sternotomy incision that can impact subsequent cardiac surgeries, while percutaneous Protek Duo insertion requires adequate vessel size and patency. With an LVAD in situ, veno‐arterial extracorporeal membrane oxygenation was not considered for isolated RV support in this case. The patient is currently over 6 months post‐orthotopic heart transplant.
Keywords: Right ventricular failure, Bridge to heart transplant, Percutaneous right ventricular assist device, Impella RP, Left ventricular assist device
Introduction
While de novo pump thrombosis has been considerably reduced with the HeartMate3 left ventricular assist device (LVAD), stroke, mucosal bleeding, aortic regurgitation, infection, and right ventricular (RV) failure still remain problematic. 1 The overall incidence of severe RV failure requiring mechanical support in MOMENTUM3 was low, but early and late RV failure that is at least moderate in nature is associated with increased post‐operative morbidity and mortality outcomes. 2 , 3 , 4 Short‐term RV assist device (RVAD) support has been used as a bridge to recovery in patients with early post‐operative RV failure after LVAD implant or with de novo cardiogenic shock. 5 , 6 , 7 , 8 , 9 , 10 Biventricular mechanical support with percutaneous short‐term devices was recently reported as a bridge to orthotopic heart transplant (OHT) in a patient with acute biventricular heart failure. 11 , 12 , 13 We report the first case of a patient with refractory late RV failure post‐LVAD implant who was safely supported for an extended period (37 days) as a bridge to OHT with an Impella RP.
Case report
A 64‐year‐old male with end‐stage ischaemic cardiomyopathy (ejection fraction, 20%; left ventricular end‐diastolic diameter, 6.3 cm) s/p six‐vessel coronary artery bypass graft and cardiac resynchronization therapy defibrillator underwent a HeartMate3 LVAD implant as bridge to OHT. His LVAD course was complicated by readmissions for decompensated heart failure and acute on chronic renal dysfunction. Past medical history includes a chronic methicillin‐sensitive Staphylococcus aureus driveline infection, ventricular tachycardia s/p epicardial ablation, type II diabetes, hypertension, dyslipidaemia, gout, obstructive sleep apnoea, veno‐thromboembolism, and remote smoking.
Persistent volume overload despite intravenous diuretics and progressive renal dysfunction prompted an intensive‐care unit (ICU) transfer for swan‐guided therapy. Haemodynamics revealed elevated biventricular filling pressures and RV failure [right atrial pressure (RAP), 22 mmHg; pulmonary artery pulsatility index, 1.05; mean pulmonary artery pressure, 40 mmHg; mean pulmonary capillary wedge pressure, 27 mmHg] with adequate cardiac output (cardiac index, 2.62 L/min/m2) and mean arterial pressure (74 mmHg) on LVAD support. The degree of RV failure as assessed by haemodynamics as well as significant RV distension (Figure 1 A ) and moderate aortic insufficiency observed by echocardiogram precluded any further LVAD speed (5600 rpm) increase. Haemodynamically intolerant arrhythmia prohibited inotropic support. Haemodynamics, diuretic‐resistant volume overload, and worsening renal function (peak serum creatinine, 2.89 mg/dL; estimated glomerular filtration rate, 22; blood urea nitrogen, 68 mg/dL; from baseline, <2 mg/dL; estimated glomerular filtration rate, >45 mL/min/1.73 m2; blood urea nitrogen, <24 mg/dL) prompted insertion of the Impella RP for RV support. The Impella RP was guided across a tricuspid valve ring and the pulmonic valve, so as to position the inlet within the inferior vena cava and the outlet within the main pulmonary artery (PA) (Figures 1 B and 2 ). With an LVAD and Impella RP in situ (Figure 2 ), the patient was listed as United Network for Organ Sharing status 2 for OHT based on the new listing criteria. Ultrafiltration and haemodialysis were felt not to be necessary due to adequate solute clearance and urine output.
Figure 1.
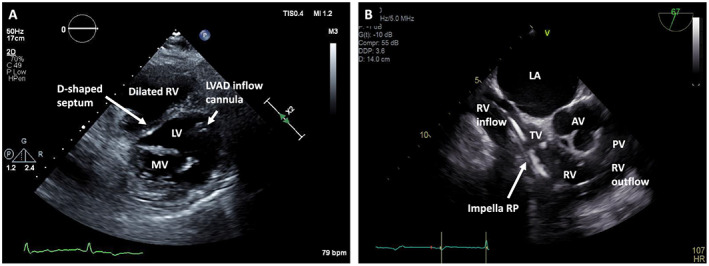
Echocardiogram of right ventricle with and without Impella RP in situ in patient on left ventricular assist device (LVAD) support. (A) Parasternal short‐axis transthoracic echocardiogram view shows a D‐shaped septum suggestive of right ventricular (RV) overload. Also seen are the left ventricle (LV), captured at the level of the mitral valve (MV), and the LVAD inflow cannula along with the dilated RV. (B) Mid‐oesophageal short‐axis RV inflow and outflow trans‐oesophageal echocardiogram view shows the Impella RP course across the tricuspid valve (TV) into the RV and heading towards the pulmonic valve (PV). Also seen are the left atrium (LA) and aortic valve (AV) abutting the RV inflow and outflow tracts, respectively.
Figure 2.
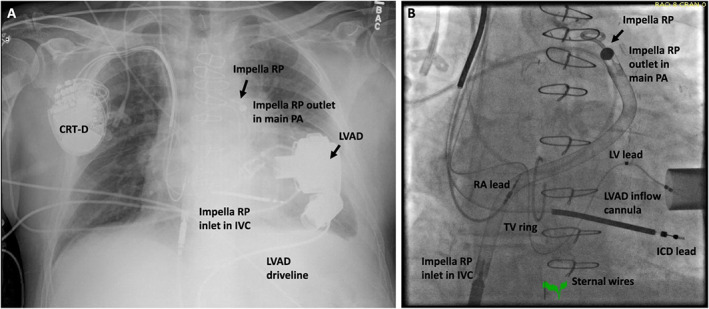
Impella RP and left ventricular assist device (LVAD) in situ. Shown on chest x‐ray (A) and fluoroscopy (B) are the Impella RP and the LVAD in situ, providing right and left ventricular support, respectively. The Impella RP inlet is located in the inferior vena cava (IVC), and the outlet is situated in the main pulmonary artery (PA) by having traversed across a tricuspid valve ring (TV ring) and the pulmonary valve in this case. CRT‐D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter defibrillator; LV, left ventricular; RA, right atrial.
The patient experienced significant improvement in haemodynamics and end‐organ function after Impella RP insertion. Responsiveness to diuretics also improved. Serum creatinine steadily decreased to 1.2–1.4 mg/dL after 20 days with Impella RP support. Haemodynamics improved to RAP (6 mmHg), pulmonary artery pulsatility index (1.83), mean pulmonary artery pressure (22 mmHg), pulmonary capillary wedge pressure (14 mmHg), and cardiac index (2.36 L/min/m2) with mean arterial pressure (80 mmHg) within 5 days of Impella RP support with concomitant diuresis. Impella RP was set to P6 with approximately 2.8–3.2 L/min calculated flow. The LVAD was kept at ~5600 rpm support, with approximately 4.4–4.9 L/min calculated flow. Attempts to wean the Impella RP resulted in recurrent RV failure with a rise in RAP (>20 mmHg) and renal dysfunction. Heparin was continued for the duration of Impella RP and LVAD support with an activated partial thromboplastin time goal of 50–60 s. The patient remained on 37 days of Impella RP support prior to undergoing OHT. There were no patient or device‐related complications during this time, including an absence of clinically significant haemolysis (lactic acid dehydrogenase throughout the hospital and Impella RP course was <380 U/L; no tea‐coloured urine was observed).
His post‐transplant course was complicated by an acute right internal jugular thrombus, hepatitis C virus (HCV) seroconversion in setting of an HCV‐positive donor requiring HCV therapy, and grade 2R acute cellular rejection in the setting of a non‐human leucocyte antigen‐positive cross‐match requiring high‐dose steroid therapy. The patient was discharged home after a total of 80 days in hospital (with only 14 post‐op days and 8 post‐op ICU days). His post‐discharge course has been complicated by cytomegalovirus viremia and recurrent grade 2R acute cellular rejection. He has been successfully treated for these complications and is currently over 6 months post‐OHT.
Discussion
Right ventricular failure after LVAD implant remains a major and common complication. 1 , 2 , 3 If inotropic support is insufficient or not possible due to medication‐related complications such as arrhythmias, an RVAD should be considered. In patients with early post‐operative RV failure after LVAD implant, percutaneous and central RVAD cannulation has been described to support the RV as a short‐term bridge to recovery. 6 , 12 Refractory late RV failure remains challenging in patients with durable LVADs.
The Impella RP is an axial flow device designed for short‐term RV support. It is Food and Drug Administration approved for use up to 14 days. 12 We report the safe and efficacious use of the Impella RP in our patient with late RV failure post‐LVAD implant over an extended period of 37 days. The patient did not experience any device‐related complications, which can include haemolysis, vascular injury to the PA or femoral venous systems, device migration, and pulmonic insufficiency. The patient was successfully bridged to OHT with percutaneous RV support. He experienced haemodynamic and renal improvement after Impella RP insertion. To our knowledge, this case represents the first‐in‐the‐world use of an Impella RP as a successful extended bridge to OHT in an LVAD patient with refractory late RV failure.
The Impella RP is currently one option for bridge to OHT in LVAD patients with haemodynamically significant RV failure. Its major benefit is percutaneous insertion, sized to be implanted in the catheterization lab, which reduces procedural morbidity and bleeding risk in comparison with more invasive strategies. Because femoral cannulation is required, patients with an Impella RP cannot ambulate. RVAD cannulation of the right heart and PA via sternotomy is another option. Patients may be ambulatory with this approach after recovery from device implant. Sternotomy unfortunately increases risk for thoracic adhesions and fibrosis that may complicate subsequent surgeries. Percutaneous Protek Duo insertion via the right internal jugular vein is a third option. Protek Duo (a dual‐lumen catheter) allows for removal of blood from the right heart and reintroduction into the PA with flow generated via bypass circuit. This configuration allows for both percutaneous access and potential patient ambulation, although it requires adequate vessel size and patency for insertion. 6 There are other variations on the configuration of percutaneous RVAD insertion that are less suitable for ambulation. 6 Given that the patient had a functioning LVAD in situ for left ventricular support, veno‐arterial extracorporeal membrane oxygenation was not considered in this case.
In conclusion, we present a case of haemodynamically significant RV failure in an LVAD patient that was treated successfully by Impella RP and ICU care as a bridge to cardiac transplantation. The Impella RP remained in the patient for 37 days without complication until its removal at the time of OHT. Additional long‐term experience with the Impella RP is necessary.
Conflict of interest
Dr Perez is an advisory board member for Abiomed. Dr Soltesz provides training on behalf of Abbott and is a speaker for Abiomed. Dr Tong serves as consultant and speaker for Abbott and Abiomed. Dr Estep is a consultant for Abbott and a medical advisor for Medtronic Inc. The remainder of the authors have no relevant disclosures of interest.
Funding
None.
Randhawa, V. K. , Hoffman, K. , Bock, A. , Bhat, P. , Young, L. , Rossi, J. , Campbell, J. , Bott‐Silverman, C. , Soltesz, E. G. , Tong, M. Z. Y. , Unai, S. , Nair, R. , Estep, J. D. , and Perez, A. L. (2020) Impella RP as a bridge to cardiac transplant for refractory late right ventricular failure in setting of left ventricular assist device. ESC Heart Failure, 7: 1972–1975. 10.1002/ehf2.12685.
References
- 1. Michaels A, Cowger J. Patient selection for destination LVAD therapy: predicting success in the short and long term. J Curr Heart Fail Rep 2019; 16: 140–149. [DOI] [PubMed] [Google Scholar]
- 2. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ, MOMENTUM 3 Investigators . A fully magnetically levitated left ventricular assist device—final report. N Engl J Med 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 3. Morgan JA, Paone G, Nemeh HW, Murthy R, Williams CT, Lanfear DE, Tita C, Brewer RJ. Impact of continuous‐flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant 2013; 32: 398–403. [DOI] [PubMed] [Google Scholar]
- 4. Tsiouris A, Paone C, Brewer RJ, Nemeh HW, Borgi J, Morgan JA. Outcomes of patients with right ventricular failure on milrinone after left ventricular assist device implantation. ASAIO J 2015; 61: 133–138. [DOI] [PubMed] [Google Scholar]
- 5. Morgan JA, O'Neill W. Percutaneous right ventricular assist device support in a patient supported by an LVAD. ASAIO J 2016: e41–e42. [DOI] [PubMed] [Google Scholar]
- 6. Bhama JK, Kormos RL, Toyoda Y, Teuteberg JJ, McCurry KR, Siegenthaler MP. Clinical experience using the Levitronix CentriMag system for temporary right ventricular mechanical circulatory support. J Heart Lung Transplant 2009; 28: 971–976. [DOI] [PubMed] [Google Scholar]
- 7. Moazami N, Pasque MK, Moon MR, Herren RL, Bailey MS, Lawton JS, Damiano RJ Jr. Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant 2004; 23: 1371–1375. [DOI] [PubMed] [Google Scholar]
- 8. Cheung AW, White CW, Davis MK, Freed DH. Short‐term mechanical circulatory support for recovery from acute right ventricular failure: clinical outcomes. J Heart Lung Transplant 2014; 33: 794–799. [DOI] [PubMed] [Google Scholar]
- 9. Alkhawam H, Rafeedheen R, Abo‐Salem E. Right ventricular failure following placement of a percutaneous left ventricular assist device. Heart Lung 2019; 48: 111–113. [DOI] [PubMed] [Google Scholar]
- 10. Anderson M, Morris DL, Tang D, Batsides G, Kirtane A, Hanson I, Meraj P, Kapur NK, O'Neill W. Outcomes of patients with right ventricular failure requiring short‐term hemodynamic support with the Impella RP device. J Heart Lung Transplant 2018; 37: 1448–1458. [DOI] [PubMed] [Google Scholar]
- 11. Lasa JJ, Castellanos DA, Denfield SW, Dreyer WJ, Tume SC, Justino H, Qureshi AM. First report of biventricular percutaneous Impella ventricular assist device use in pediatric patients. ASAIO J 2018; 64: e134–e137. [DOI] [PubMed] [Google Scholar]
- 12. Anderson MB, Goldstein J, Milano C, Morris LD, Kormos RL, Bhama J, Kapur NK, Bansal A, Garcia J, Baker JN, Silvestry S, Holman WL, Douglas PS, O'Neill W. Benefits of a novel percutaneous ventricular assist device for right heart failure: the prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant 2015; 34: 1549–1560. [DOI] [PubMed] [Google Scholar]
- 13. Varian K, Xu WD, Lin W, Unai S, Tong MZ, Soltesz E, Krishnaswamy A, Kapadia S, Feitell S, Hanna M, Joyce E, Schoenhagen P, Starling RC, Taylor DO, Perez AL. Minimally invasive biventricular mechanical circulatory support with Impella pumps as a bridge to heart transplantation: a first‐in‐the‐world case report. ESC Heart Fail 2019; 6: 552–554. [DOI] [PMC free article] [PubMed] [Google Scholar]


