Abstract
Aims
Concentrations of insulin‐like growth factor binding protein‐7 (IGFBP7) have been linked to abnormal cardiac structure and function in patients with chronic heart failure (HF), but cardiovascular correlates of the biomarker in patients with more acute presentations are lacking.
We aimed to determine the relationship between IGFBP7 concentrations and cardiac structure and to evaluate the impact of IGFBP7 on the diagnosis of acute HF among patients with acute dyspnoea.
Methods and results
In this pre‐specified subgroup analysis of the International Collaborative of N‐terminal pro‐B‐type Natriuretic Peptide Re‐evaluation of Acute Diagnostic Cut‐Offs in the Emergency Department (ICON‐RELOADED) study, we included 271 patients with and without acute HF. All patients presented to an emergency department with acute dyspnoea, had blood samples for IGFBP7 measurement, and detailed echocardiographic evaluation.
Higher IGFBP7 concentrations were associated with numerous cardiac abnormalities, including increased left atrial volume index (LAVi; r = 0.49, P < 0.001), lower left ventricular ejection fraction (r = −0.27, P < 0.001), lower right ventricular fractional area change (r = −0.31, P < 0.001), and higher tissue Doppler E/e′ ratio (r = 0.44, P < 0.001). In multivariable linear regression analyses, increased LAVi (P = 0.01), lower estimated glomerular filtration rate (P = 0.008), higher body mass index (P = 0.001), diabetes (P = 0.009), and higher concentrations of amino‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP, P = 0.02) were independently associated with higher IGFBP7 concentrations regardless of other variables. Furthermore, IGFBP7 (odds ratio = 12.08, 95% confidence interval 2.42–60.15, P = 0.02) was found to be independently associated with the diagnosis of acute HF in the multivariable logistic regression analysis.
Conclusions
Among acute dyspnoeic patients with and without acute HF, increased IGFBP7 concentrations are associated with a range of cardiac structure and function abnormalities. Independent association with increased LAVi suggests elevated left ventricular filling pressure is an important trigger for IGFBP7 expression and release. IGFBP7 may enhance the diagnosis of acute HF.
Keywords: Dyspnoea, Acute heart failure, Echocardiography, IGFBP7
Introduction
Acute dyspnoea is a common symptom among emergency department (ED) patients accounting for approximately 3–4 million visits (3%) in the ED each year in the United States. 1 Narrowing the diagnosis for patients with dyspnoea may be challenging as the symptom may be caused by both cardiac and non‐cardiac diseases. In this regard, biomarker testing and cardiac imaging studies can be helpful for reaching the correct diagnosis and subsequently guiding patient management. When a cardiac aetiology is suspected, echocardiography is often the first imaging study (after chest X‐ray) chosen to support clinical judgement, as it provides immediate information on left ventricular (LV) size and function, including ejection fraction (EF), diastolic parameters, and valve function. 2
In dyspnoeic patients, circulating biomarkers may assist not only in diagnosis but also in the understanding of disease pathophysiology and prognosis. For example, concentrations of BNP and its amino‐terminal precursor (NT‐proBNP) are associated with a broad range of echocardiographic parameters, including systolic and diastolic LV function, filling pressures, and chamber size. 3 , 4 , 5 While such associations render natriuretic peptides nonspecific for individual cardiac structure and functional correlates, they help to demonstrate why both BNP and NT‐proBNP are highly sensitive for a diagnosis of heart failure (HF) and contribute to prognostic understanding of such patients. Natriuretic peptides have insufficient specificity for diastolic function because other conditions including valvular abnormalities and change in LVEF may contribute to their concentrations. 6 Several biomarkers that reflect different pathophysiologic pathways have been proposed for the diagnosis, prognosis, and risk stratification of patients with acute HF when natriuretic peptide levels are inconclusive. In this context, new and emerging biomarkers may be helpful to better define distinct pathophysiology and guide targeted therapeutic strategies.
Insulin‐like growth factor binding protein‐7 (IGFBP7), a cell cycle arrest biomarker associated with the senescence associated secretory phenotype, where senescent cells express and release inflammatory cytokines, interleukins, and growth factors. IGFBP7 was originally identified as a candidate HF biomarker in proteomic scans performed in a murine model of cardiac failure. 7 Among patients with chronic HF, elevated concentrations of IGFBP7 predict major adverse cardiovascular events 8 and are correlated with multiple parameters associated with impaired myocardial relaxation, notably including left atrial volume index (LAVi). 9 However, to date, all available data regarding IGFBP7 have been derived from patients with chronic HF. It is well‐established that acute decompensated HF and chronic HF are distinct entities with different pathophysiology and treatment strategies. The goals of the present study were (i) to examine associations between IGFBP7 concentrations with clinically relevant characteristics and cardiac structural relationships (ii) to evaluate whether using IGFBP7 would optimize the diagnosis of acute HF in patients presenting to the ED with acute dyspnoea enrolled in the recent ICON‐RELOADED study. 10
Methods
Patient population and study design
This study was performed in compliance with the Declaration of Helsinki and with approval of each institutional review board, and all patients provided written, informed consent before enrolment. Patients provided additional written, informed consent prior to giving the biorepository blood sample. The ICON‐RELOADED study was a prospective, multicenter trial conducted at 19 sites in the United States and Canada. The design and primary results have been previously published. 10 , 11 In brief, patients aged 22 years or older presenting to EDs with complaints of dyspnoea (defined as subjective feeling of shortness of breath, difficult or labored breathing, arising or worsening over the course of no longer than several days) were eligible for inclusion. Major exclusion criteria included renal insufficiency requiring dialysis or known estimated glomerular filtration rate (eGFR) <15.0 mL/min/1.73 m2 prior to enrolment, dyspnoea after chest trauma, patients who are unable to donate up to 50 mL of blood at one time, and known pregnancy. Overall, 1461 patients presenting at the ED with dyspnoea were enrolled in a prospective trial examining the age‐stratified cut points of NT‐proBNP for the diagnosis of acute HF. Adjudicated diagnosis of the cause of dyspnoea was determined by a clinical events adjudication committee, blinded to NT‐proBNP results, who independently reviewed and adjudicated the diagnosis of acute HF.
The present analysis is a pre‐specified sub‐study of the ICON‐RELOADED trial, examining the subset of 271 patients from the overall cohort who underwent detailed echocardiographic examination during their index admission as part of standard of care; those with available echocardiograms and a blood sample for IGFBP7 measurement were included.
IGFBP7 analysis
At enrolment, a blood sample was collected into EDTA‐containing tubes, processed, and frozen at −80°C until measurement of IGFBP7 using a precommercial Elecsys assay (Roche Diagnostic, Penzberg, Germany) was performed. Assay of IGFBP7 was performed by Roche Diagnostics by laboratory personnel completely blinded to clinical information. Treating clinicians and those interpreting the echocardiograms were blinded to assay results.
Echocardiography
In the course of standard of care, echocardiography was determined to be indicated in 271 study participants either in the ED or during the index hospitalization. Full image sets of each echocardiogram were exported in DICOM format onto suitable media (CD, DVD, etc.) or online portal to the designated Echocardiogram Core Laboratory at Massachusetts General Hospital. The readers were blinded to clinical picture and biomarker results.
Standard two‐dimensional and colour Doppler imaging was performed. Measurements were averaged over three cycles (five if atrial fibrillation was present). Structural indices included biplane LV end‐diastolic and end‐systolic volume indexed to body surface area, posterior wall thickness, LV mass by the modified American Society of Echocardiography formula indexed to body surface area, biplane LAVi, and RV end‐diastolic and end‐systolic area measured in the apical four‐chamber view. 12 The LVEF was determined using biplane modified Simpson's measurements. Diastolic indices included early and late transmitral diastolic velocities (E and A), early deceleration time (DT), pulmonary venous systolic and diastolic velocities (PVS and D), and diastolic tissue Doppler velocities at the septal and lateral mitral annulus. RV indices included RV fractional area change; RV hypokinesis was qualitatively graded as none, mild, moderate, and severe. Mitral regurgitation and TR severity were graded as none, trace, mild, moderate, and severe based on visual assessment of structural and Doppler parameters and calculation of echocardiographic equations. Systolic dysfunction was defined as an LVEF < 50%. Significant diastolic dysfunction was defined as E/e′ ≥ 15 while diastolic dysfunction stages 1, 2, or 3 were used based on established criteria. 13
Statistical analysis
Analyses in this sub‐study were pre‐specified in the protocol of the ICON‐RELOADED study. 11 Demographics and baseline characteristics were reported using frequencies and percentages for categorical variables and median and interquartile range for continuous variables, respectively. Comparisons between groups were performed using the chi‐square test for categorical variables and the Student's t‐test or Wilcoxon rank‐sum test for continuous variables. IGFBP7 and NT‐proBNP levels were log transformed because of their positively skewed distributions. For the purposes of analysing the relationship between IGFBP7, NT‐proBNP concentrations, and echocardiographic parameters, patients were dichotomized as a function of being above or below median IGFBP7 and NT‐proBNP values. Associations between IGFBP7, NT‐proBNP, and echocardiographic indices were assessed by Spearman's correlation coefficient.
Multivariable linear regression analysis was performed to determine the independent contributions of candidate variables to log IGFBP7 concentrations. Candidate predictor variables for the model were selected according to the clinical, biological plausibility, and literature based associations. To avoid multicollinearity, nearly identical echocardiographic indices were not included into the model. Multicollinearity was assessed using variance inflation factor. The model was constructed with log IGFBP7 as the dependent variable and the other candidate co‐variates [age (as continuous), gender, log NT‐proBNP levels (as continuous), body mass index (BMI, as continuous), eGFR (as continuous), LV mass index (as continuous), LVEF (as continuous), RV fractional area change (as continuous), LAVI (as continuous), grades of diastolic dysfunction (as categorical), atrial fibrillation, asthma, chronic obstructive pulmonary disease, history of diabetes, history of hypertension, history of HF, use of cardiovascular agents, and lung cancer]. Standardized β coefficients were generated and presented.
To identify the diagnostic value of IGFBP7 for acute HF, a multivariable logistic regression analysis was performed. The model was constructed to estimate multivariable odds ratios (ORs) and 95% confidence interval (CI) for IGFBP7 and the other covariates for predicting the risk of acute HF. Predictors were selected based on their relevance in previous literature. The main explanatory variable was IGFBP7 (log transformed), and established risk factors [NT‐proBNP (log transformed), age (as continuous), gender, history of diabetes, history of hypertension, atrial fibrillation, GFR (as continuous), prior coronary artery disease, BMI (as continuous), and LVEF (as continuous)] were included into the model to assess associations with acute HF. Receiver operating characteristic (ROC) analysis was used to measure and compare the performance of IGFBP7 and NT‐proBNP for the diagnosis of acute HF.
For all statistical analyses, P values reported are from two‐sided tests and considered as statistically significant with a value of less than 0.05. All data analyses were performed using the STATA version 15.1 (StataCorp LLC, College Station, TX, USA).
Results
Clinical characteristics
Two hundred and seventy‐one study participants (age 62.7 ± 13.9 and 57% male patients) underwent echocardiography with data sufficient for analysis in the Core Laboratory. Among those with echocardiographic data, 143 (52.7%) had adjudicated acute HF. Patients with supra‐median IGFBP7 levels had a 76% acute HF; patients less than the median value had a 28% acute HF (P < 0.001). NT‐proBNP (2700 [1247–5919] vs. 317 [82–1083] pg/mL, P < 0.001) and IGFBP7 (146 [116–188] vs. 96 [81–119] ng/mL, P < 0.001) concentrations were significantly higher in subjects with acute HF compared with subjects without HF.
IGFBP7 concentrations
The median IGFBP7 level in this sub‐analysis was 119 ng/mL (interquartile range = 91–157 ng/mL). A summary of baseline clinical characteristics, dichotomized by the median IGFBP7 value, is presented in Table 1 . Patients with an IGFBP7 level greater than the median value tended to be older, had worse kidney function, and more commonly had a history of diabetes mellitus (DM), HF, hypertension, prior coronary artery disease, prior myocardial infarction, atrial fibrillation, significant aortic and mitral valve diseases, and more frequent medication use for heart disease compared with those with an IGFBP7 level below the median value.
Table 1.
Clinical characteristics of the study population by the median value of insulin‐like growth factor binding protein‐7
| Patient characteristic | IGFBP7 < median (N = 135) | IGFBP7 ≥ median (N = 136) | P value |
|---|---|---|---|
| Age, yearsa | 59 (51–68) | 67 (56–76) | <0.001 |
| Female (%) | 46.6 (63/135) | 39.7 (54/136) | 0.24 |
| eGFR (mL/min/1.73m2)a | 79.4 (65.2–101.3) | 54 (42.5–74) | <0.001 |
| BMI (kg/m2)a | 30.5 (26.4–36.9) | 31.7 (26–37.7) | 0.43 |
| Body surface area (m2)a | 2.01 (1.79–2.21) | 2.04 (1.85–2.28) | 0.39 |
| Medical history | |||
| Diabetes mellitus (%) | 26.3 (35/133) | 46.3 (63/136) | 0.001 |
| Heart failure (%) | 22.3 (29/130) | 62.1 (82/132) | <0.001 |
| Hypertension (%) | 68.6 (92/134) | 82.2 (111/135) | 0.01 |
| Prior CAD (%) | 24.6 (33/134) | 40.3 (54/134) | 0.006 |
| Previous MI (%) | 11.4 (15/131) | 22.1 (29/131) | 0.02 |
| Significant aortic valve disease (%) | 0.8 (1/121) | 8.4 (10/119) | 0.005 |
| Significant mitral valve disease (%) | 3.25 (4/123) | 10.4 (13/125) | 0.03 |
| COPD (%) | 24.2 (32/132) | 26.4 (36/136) | 0.67 |
| Asthma (%) | 15.1 (20/132) | 19.8 (27/136) | 0.31 |
| Atrial Fibrillation (%) | 17.9 (24/134) | 36.3 (48/132) | 0.001 |
| Medications at discharge | |||
| Beta blockers (%) | 29.6 (40/135) | 45.5 (62/136) | 0.007 |
| Loop diuretic (%) | 31.1 (42/135) | 64.7 (88/136) | <0.001 |
| ACEi or ARB (%) | 25.9 (35/135) | 35.2 (48/136) | 0.12 |
| Vital signs | |||
| Pulse rate (bpm)a | 95 (80–109) | 87 (70–106.5) | 0.02 |
| SBP (mmHg)a | 140 (126–165) | 142 (127–165) | 0.49 |
| DBP (mmHg)a | 83 (74–94) | 81 (71–97) | 0.35 |
IQR, interquartile range; eGFR, estimated glomerular filtration rate; BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; ACEi, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; SBP, systolic blood pressure; DBP, diastolic blood pressure; IGFBP7, insulin‐like growth factor binding protein‐7.
Those with a supra‐median IGFBP7 value were more likely to be older, have worse kidney function, and other chronic diseases.
Median and IQR are presented.
Higher IGFBP7 concentrations were associated with prevalent echocardiographic abnormalities. For example, higher LAVi was observed in patients with supra‐median IGFBP7 levels across the study population irrespective of the diagnosis of acute HF. Compared to patients with an inframedian level of IGFBP7, patients with a supra‐median IGFBP7 level with pulmonary diseases (COPD and asthma) but without acute HF had an increased tissue Doppler E/e′ ratio ( Table S1 ). Baseline characteristics, dichotomized by median NT‐proBNP values are summarized in Table S 2 .
Echocardiographic findings
A summary of echocardiographic findings of acutely dyspnoeic patients, dichotomized by the median IGFBP7 value of 119 ng/mL is shown in Table 2 . Cardiac structural and functional abnormalities were more common in those with higher IGFBP7 concentrations, including greater chamber sizes, worse systolic and diastolic function, higher filling pressures, and more severe valvular heart disease. A significant association was observed between increased IGFBP7 concentrations and worsening LV diastolic function (P < 0.001, Figure 1 ). For comparison, the distribution of NT‐proBNP concentrations according to LV diastolic dysfunction is presented in Figure S1 .
Table 2.
Echocardiographic indices of the study population by the median value of IGFBP7
| Echocardiographic indices | IGFBP7 < median (N = 135) | IGFBP7 ≥ median (N = 136) | P value |
|---|---|---|---|
| Left ventricle | |||
| Posterior wall thickness (mm) * | 10 (8–11.5) | 10 (9–12) | 0.04 |
| LV mass index (male, g/m2) a | 85.4 (67.9–106.2) | 111.6 (92.2–130.2) | <0.001 |
| LV mass index (female, g/m2) a | 77.9 (64.3–97.1) | 96.6(81.5–111.6) | 0.003 |
| LVEDVi (mL/m2) a | 53.3 (44.9–65.7) | 68.1 (51.2–86.5) | <0.001 |
| LVESVi (mL/m2) a | 20.3 (15.3–28.7) | 35.5 (18.3–56.3) | <0.001 |
| LVEF (%) a | 61.6 (51.9–69.2) | 50 (30.4–64.7) | <0.001 |
| LVEF |
<0.001 |
||
| LVEF a | 61.6 (51.9–69.2) | 50 (30.4–64.7) | |
| LVEF < 50 (%) | 21 (25/117) | 48 (53/110) | |
| LVEF ≥ 50 (%) | 79 (92/117) | 52 (57/110) | |
| Right ventricle | |||
| RV diastolic area (cm2) a | 19 (14.8–23.4) | 22.3 (18.6–27.4) | 0.001 |
| RV systolic area (cm2) a | 10.4 (8.1–13.9) | 14.5 (10.6–18.6) | <0.001 |
| RV fractional area change (%) a | 43.2 (36.8–51.3) | 36.3 (28.1–45.9) | <0.001 |
| Right ventricular hypokinesis | 0.003 | ||
| None (%) | 85.6 (107/125) | 64.6 (73/113) | |
| Mild (%) | 5.6 (7/125) | 15 (17/113) | |
| Moderate (%) | 4.8 (6/125) | 11.5 (13/113) | |
| Severe (%) | 4 (5/125) | 8.6 (10/113) | |
| LAVi (mL/m2) a | 28.9 (21.9–36.2) | 41.9 (33.1–51.2) | <0.001 |
| Trans‐mitral Doppler | |||
| E (cm/s) a | 72 (61–92) | 97.5 (80–121) | <0.001 |
| A (cm/s) a | 67 (55–85) | 65.5 (46–87) | 0.45 |
| E/A* | 1.04 (0.8–1.3) | 1.3 (0.9–1.9) | <0.001 |
| DT (ms) a | 177 (145–210.5) | 167 (129.4–199) | 0.04 |
| Pulmonary vein flow | |||
| S (cm/s) a | 45 (38–57) | 35.5 (24–47.5) | 0.001 |
| D (cm/s) a | 43 (32–52) | 55.5 (42.5–68.5) | <0.001 |
| S/D a | 1.1 (0.8–1.4) | 0.6 (0.5–0.9) | <0.001 |
| Tissue Doppler | |||
| Mitral annular e′ septal (cm/s) a | 7 (6–9) | 6 (5–7) | <0.001 |
| Mitral annular e′ lateral (cm/s) a | 9 (6–11) | 8 (6–10) | 0.09 |
| Mean mitral annular e′ (cm/s) a | 8 (6–10) | 7 (5.2–9) | 0.01 |
| E/e′ a | 9.4 (7.4–13.6) | 13.4 (10.7–19.6) | <0.001 |
| E/e′ ≥ 15 (%) | 19.6 (23/117) | 42.5 (46/108) | <0.001 |
| Grades of diastolic dysfunction | <0.001 | ||
| Normal (%) | 59 (65/110) | 19.8 (18/91) | |
| Grade I (%) | 15.4 (17/110) | 11 (10/91) | |
| Grade II (%) | 18.1 (20/110) | 36.2 (33/91) | |
| Grade III (%) | 6.4 (7/110) | 32.9 (30/91) | |
| Indeterminate (%) | 0.9 (1/110) | 0 (0/91) | |
| TR severity | 0.001 | ||
| None (%) | 2.4 (3/127) | 2.3 (3/130) | |
| Trace (%) | 61.4 (78/127) | 40 (52/130) | |
| Mild (%) | 28.3 (36/127) | 33 (43/130) | |
| Moderate (%) | 7.9 (10/127) | 21.5 (28/130) | |
| Severe (%) | 0 (0/127) | 3 (4/130) | |
| MR severity | <0.001 | ||
| None (%) | 11.8 (15/127) | 3.1 (4/129) | |
| Trace (%) | 59 (75/127) | 36.4 (47/129) | |
| Mild (%) | 19.7 (25/127) | 40.3 (52/129) | |
| Moderate (%) | 7.9 (10/127) | 17 (22/129) | |
| Severe (%) | 1.6 (2/127) | 3.1 (4/129) |
IQR, interquartile range; LV, left ventricle; LVEDVi, left ventricular end‐diastolic volume index; LVESVi, left ventricular end‐systolic volume index; LVEF, left ventricular ejection fraction; RV, right ventricle; LAVi, left atrial volume index; E, early transmitral diastolic velocities; A, late transmitral diastolic velocities; E/A, the ratio of early to late transmitral diastolic velocities; DT, early deceleration time; S, pulmonary venous systolic velocities; D, pulmonary venous diastolic velocities; S/D, the ratio of pulmonary venous systolic to diastolic velocities; E/e′, the ratio between early mitral inflow velocity and mitral annular early diastolic velocity; TR, tricuspid regurgitation; MR, mitral regurgitation; IGFBP7, insulin‐like growth factor binding protein‐7.
Study participants with an IGFBP7 ≥ 119 ng/mL had prevalent cardiac abnormalities.
Median and IQR are presented.
Figure 1.
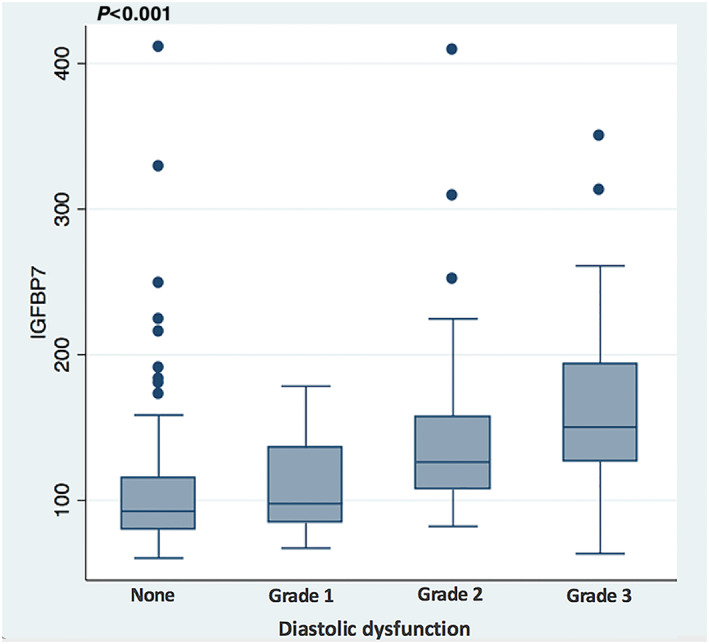
Box plots of IGFBP7 concentrations according to the left venticular diastolic dysfunction grading (P < 0.001) (diastolic dysfunction, n = 242). IGFBP7, insulin‐like growth factor binding protein‐7.
Bivariate Spearman correlation analysis of echocardiographic indices and IGFBP7 concentrations are presented in Table 3 . This shows modest correlation with a broad array of cardiac abnormalities. Notably, higher IGFBP7 was associated with an increased LAVi (r = 0.49, P < 0.001), a reduced LVEF (r = −0.27, P < 0.001), a lower RV fractional area change (r = −0.31, P < 0.001), and a higher tissue Doppler E/e′ ratio (r = 0.44, P < 0.001). For comparison, echocardiographic indices, dichotomized by the median NT‐proBNP value, are summarized in Table S 3 .
Table 3.
Univariable correlations of echocardiographic indices with insulin‐like growth factor binding protein‐7 levels
| Echocardiographic indices | Spearman correlation coefficient (ρ) | P value |
|---|---|---|
| Left ventricle | ||
| Posterior wall thickness (mm) | 0.187 | 0.002 |
| LV mass index (g/m2) | 0.342 | <0.001 |
| LVEDVi (mL/m2) | 0.285 | <0.001 |
| LVESVi (mL/m2) | 0.307 | <0.001 |
| LVEF (%) | −0.278 | <0.001 |
| Right ventricle | ||
| RV diastolic area (cm2) | 0.342 | <0.001 |
| RV systolic area (cm2) | 0.391 | <0.001 |
| RV fractional area change (%) | −0.310 | <0.001 |
| LAVi (mL/m2) | 0.491 | <0.001 |
| Grades of diastolic dysfunction | 0.459 | <0.001 |
| Transmitral doppler | ||
| E (cm/s) | 0.435 | <0.001 |
| A (cm/s) | −0.084 | 0.25 |
| E/A | 0.311 | <0.001 |
| DT (ms) | −0.137 | 0.04 |
| Pulmonary vein flow | ||
| S (cm/s) | −0.353 | <0.001 |
| D (cm/s) | 0.281 | <0.001 |
| S/D | −0.480 | <0.001 |
| Tissue doppler | ||
| Mitral annular e′ septal (cm/s) | −0.279 | <0.001 |
| Mitral annular e′ lateral (cm/s) | −0.101 | 0.15 |
| Mean mitral annular e′ (cm/s) | −0.182 | 0.01 |
| E/e′ | 0.442 | <0.001 |
| MR severity | 0.320 | <0.001 |
LV, left ventricle; LVEDVI, left ventricular end‐diastolic volume index; LVESVi, left ventricular end‐systolic volume index; LVEF, left ventricular ejection fraction; RV, right ventricle; LAVi, left atrial volume index; E, early transmitral diastolic velocities; A, late transmitral diastolic velocities; E/A, the ratio of early to late transmitral diastolic velocities; DT, early deceleration time; S, pulmonary venous systolic velocities; D, pulmonary venous diastolic velocities; S/D, the ratio of pulmonary venous systolic to diastolic velocities; E/e′, the ratio between early mitral inflow velocity and mitral annular early diastolic velocity; TR, tricuspid regurgitation; MR, mitral regurgitation; IGFBP7, insulin‐like growth factor binding protein‐7.
Independent predictors of log IGFBP7
Multivariable linear regression analysis was performed in order to determine the independent contributions of covariates to log‐transformed IGFBP7 concentration. Diabetes (P = 0.009), log‐NT‐proBNP (P = 0.02), and BMI (P = 0.001), eGFR (P = 0.008), and LAVi (P = 0.01) were significantly associated with log‐IGFBP7 concentrations, regardless of other variables (Table 4 ; Figure 2 ). Diabetes, log NT‐proBNP, and BMI showed a positive association with IGFBP7 as opposed to the negative association of GFR.
Table 4.
Multivariable correlations of clinical and echocardiographic indices with log‐transformed insulin‐like growth factor binding protein‐7 levels
| Patient characteristic | Estimated β | Standard error | P value |
|---|---|---|---|
| Age | 0.003 | 0.002 | 0.14 |
| Gender (female vs. male) | −0.037 | 0.050 | 0.46 |
| Log‐NT‐proBNP | 0.048 | 0.020 | 0.02 |
| BMI | 0.010 | 0.003 | 0.001 |
| eGFR (mL/min/1.73 m) | −0.003 | 0.001 | 0.008 |
| LV mass index | −0.001 | 0.001 | 0.09 |
| LVEF | −0.001 | 0.002 | 0.59 |
| RV fractional area change | −0.001 | 0.002 | 0.62 |
| LAVI | 0.005 | 0.002 | 0.01 |
| Grades of diastolic dysfunction | 0.035 | 0.020 | 0.08 |
| Atrial fibrillation | 0.020 | 0.060 | 0.73 |
| Asthma | −0.090 | 0.077 | 0.24 |
| COPD | −0.034 | 0.059 | 0.56 |
| Diabetes | 0.139 | 0.053 | 0.009 |
| Hypertension | −0.038 | 0.058 | 0.50 |
| Heart failure | 0.073 | 0.054 | 0.18 |
| Loop diuretics | 0.003 | 0.056 | 0.95 |
| Beta blockers | 0.062 | 0.055 | 0.25 |
| ACEI/ARBs | −0.086 | 0.056 | 0.12 |
| Lung cancer | −0.001 | 0.112 | 0.99 |
NT‐proBNP, N‐terminal pro b‐type natriuretic peptide; BMI, body mass index; eGFR, estimated glomerular filtration rate; LV, left ventricle; LVEF, left ventricular ejection fraction; RV, right ventricle; LAVi, left atrial volume index; COPD, chronic obstructive pulmonary disease; ACEI/ARB, angiotensin‐converting‐enzyme inhibitors/angiotensin receptor blockers.
The association between IGFBP7 concentrations and LAVi remained significant, even after adjusting for atrial fibrillation, diastolic abnormalities, LVEF, and NT‐proBNP levels. (LV mass index, n = 258; LVEF, n = 229; RV fractional area change, n = 195; LAVI, n = 238; diastology grade, n = 242)
Figure 2.
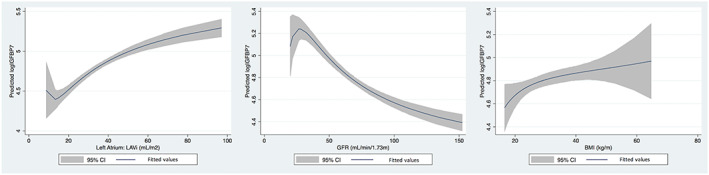
Associations between predicted log IGFBP7 and LAVi, GFR, and BMI were plotted with smooth curves. IGFBP7, insulin‐like growth factor binding protein‐7; LAVi, left atrial volume index; eGFR, estimated glomerular filtration rate; BMI, body mass index.
Diagnostic performance of IGFBP7
Out of 271 patients, 143 were diagnosed with acute HF and 128 were controls. Patients with supra‐median IGFBP7 levels had a 76% acute HF; patients less than the median value had a 28% acute HF (P < 0.001). To determine the diagnostic value of IGFBP7 for acute HF, we conducted a multivariable logistic regression analysis. As a result, log IGFBP7 (OR = 12.08, 95% CI 2.42–60.15, P = 0.02) was found to be independently associated with the diagnosis of acute HF (Table 5 ). The relationship between log IGFBP7 and predicted probability of acute HF was illustrated by a Lowess curve in Figure 3 . The diagnostic performance of NT‐proBNP and IGFBP7 for acute HF was measured by ROC analysis. ROC curve analysis revealed 72% sensitivity and 79% specificity in the prediction of the risk for acute HF with the cut‐off value of 121 pg/mL for IGFBP7 (area under the curve: 0.81, 0.75–0.86). The diagnostic performances of NT‐proBNP and IGFBP7 for acute HF were comparable in the ROC curve comparison analysis (P = 0.428, Figure 4 ).
Table 5.
Adjusted odds ratios for individual predictors included in logistic regression model
| Variables | Multivariable OR, 95% CI | P value |
|---|---|---|
| log IGFBP7 level | 12.08 (2.42 to 60.15) | 0.002 |
| log NT‐proBNP level | 2.20 (1.49 to 3.25) | <0.001 |
| Age (each increases in 1 year) | 0.99 (0.96 to 1.03) | 0.71 |
| Gender (female vs. male) | 1.11 (0.49 to 2.50) | 0.80 |
| History of diabetes (yes vs. no) | 1.41 (0.55 to 3.65) | 0.47 |
| GFR (each increases in 1 mg/dL) | 1.001 (0.98 to 1.02) | 0.95 |
| History of hypertension (yes vs. no) | 1.52 (0.53 to 4.35) | 0.43 |
| Atrial fibrillation (yes vs. no) | 0.85 (0.32 to 2.30) | 0.75 |
| Prior CAD (yes vs. no) | 0.63 (0.23 to 1.75) | 0.38 |
| BMI (each increases in 1 kg/m2) | 1.07 (1.01 to 1.13) | 0.02 |
| LVEF (each increase in %) | 0.97 (0.95 to 1.001) | 0.06 |
OR, odds ratio; CI, confidence interval; IGFBP7, insulin‐like growth factor‐binding protein‐7; NT‐proBNP,; N‐terminal pro b‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; CAD, coronary artery disease; BMI, body mass index; LVEF, left ventricular ejection fraction
Figure 3.
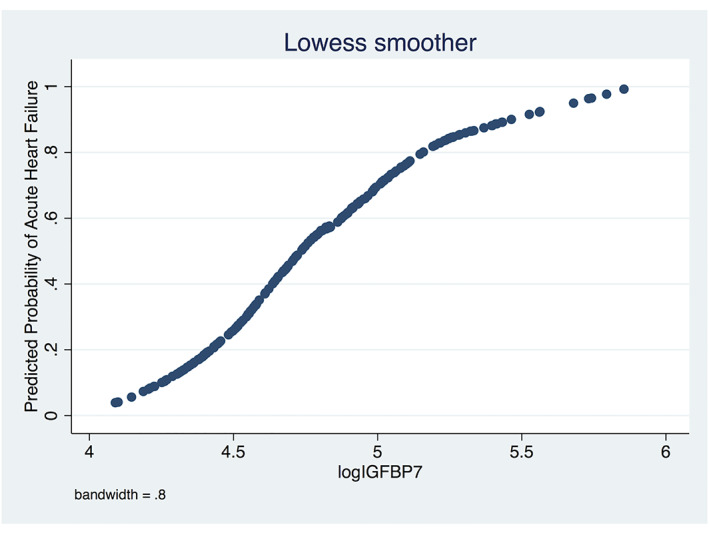
Association between predicted risk of acute heart failure and insulin‐like growth factor‐binding protein‐7 was plotted with smooth curves. IGFBP7, insulin‐like growth factor binding protein‐7.
Figure 4.
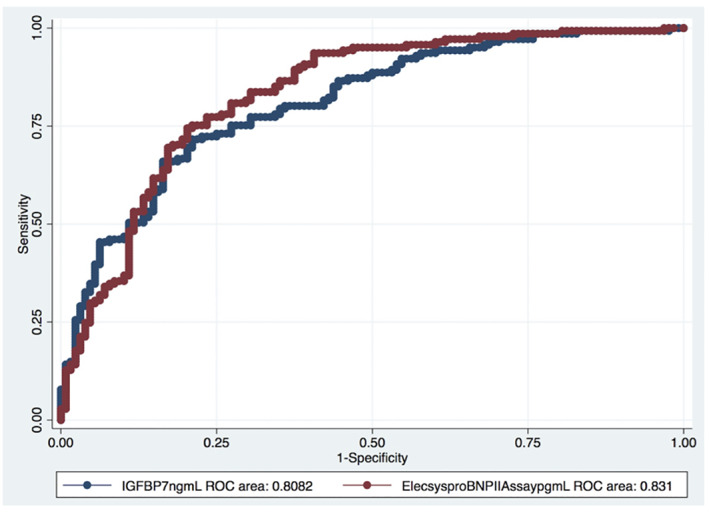
Comparison of ROC curves for IGFBP7 and NT‐proBNP for the diagnosis of acute heart failure. ROC, receiver operating characteristic; IGFBP7, insulin‐like growth factor binding protein‐7; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide.
Discussion
IGFBP7 is an emerging biomarker that is strongly associated with cardiac structure, diastolic function, filling pressures, and prognosis. 14 , 15 In our analysis of ED patients with acute dyspnoea, we have identified that higher concentrations of IGFBP7 are associated with an increased indexed LA volume, worse kidney function, obesity, diabetes, and higher NT‐proBNP concentrations. In addition, higher concentrations of IGFBP7 were clearly associated with more severe structural heart disease and might be a predictor for the diagnosis of acute HF. Although associations between IGFBP7 and cardiac structural correlates have been examined in chronic HF 8 , 9 , 14 , 15 , 16 , 17 , 18 , our study is the first to examine the biomarker in patients with acute dyspnoea; our results further solidify IGFBP7 as a plausible candidate cardiac marker.
The role of IGFBP7 in HF was initially described in HF patients with reduced EF. Subsequently, robust relationships have been reported with the presence and severity of echocardiographic parameters of abnormal diastolic function in chronic HF. 10 IGFBP7 is basically associated with cardiac hypertrophy 8 and is expressed at high levels in HF patients. Cardiac hypertrophy is an adaptive response to physiological and pathological overload. These hypertrophic responses increase oxygen demand and promote myocardial angiogenesis to compensate for hypoxic situation and to maintain cardiac contractile function. 19 It is known that IGFBP7 is highly expressed in the vasculature 20 , where it has apparently a role in the regulation of the angiogenesis in conjunction with other factors. At this point, IGFBP7 may play a pivotal role in the response to increased myocardial hypoxia and the need for angiogenesis during pressure and volume overload, including being an important part of LV and atrial remodelling. Accordingly, the findings of the present study indicate the diagnostic capability of IGFBP7 in distinguishing acute HF.
In many ways, higher IGFBP7 concentrations portray a commonly encountered HF phenotype, namely, the diabetic, obese patient with severe cardiac congestion and kidney dysfunction; the intersection between cellular senescence and these abnormalities has been recently highlighted, 14 with some suggesting a specific cardiovascular phenotype of ‘metabolic senescent HF'. 15 Indeed, in recent studies of chronic HF, higher concentrations of IGFBP7 were linked to ageing, diabetes mellitus, and obesity. 16 , 17 , 18 , 21 , 22 Diabetes is presumed to have a role in increased myocardial stiffness through deposition of collagen and advanced glycation end products. 23 Diastolic dysfunction may be the earliest manifestation of diabetes‐induced myocardial disease that leads to the progressive development of HF. 24 Previous studies reported an association of serum IGFBP7 concentrations with insulin resistance and diabetes. 25 , 26 Obesity is also a contributor to senescence, and expression of increased IGFBP7 has been found to be positively correlated with increase in BMI in HF patients. 14 , 15 Consistent with these findings, our data also demonstrate that higher levels of BMI and older age are independent predictors for increased IGFBP7 concentrations.
IGFBP7 has been defined as a biomarker of premature tissue ageing and fibrosis, 27 , 28 suggesting a contribution to increased myocardial stiffness and noncompliance. Higher concentrations of IGFBP7 may result in premature ageing of the myocardium with consequent myocardial fibrosis. 15 Given the link between cardiac fibrosis, hypertrophy, and HF, several studies have demonstrated IGFBP7 as a novel biomarker of impaired myocardial relaxation in chronic HF with both reduced and preserved EF. 9 , 15 , 29 We found similar associations between IGFBP7 and measures of impaired relaxation; however the extent of association between higher IGFBP7 and other abnormalities of cardiac structure and function was greater in our subjects with more acute presentations. This might also imply acute expression of IGFBP7 in the setting of acute pressure and volume overload states. Whether specific targeting of IGFBP7 to prevent or ameliorate adverse cardiac events in acute or chronic HF remains a speculative concept; it is worth noting that neprilysin inhibition reduced IGFBP7 concentrations in a randomized trial of patients with HF with preserved EF. 14
Compared with prior studies of IGFBP7, we found differences in the patterns of imaging correlation. In stable chronic HF patients IGFBP7 largely predicted variables consistent with impaired myocardial relaxation such as LAVi, transmitral E/A ratio, E/e′ ratio, and estimated right ventricular systolic pressure. 15 , 30 In the present analysis IGFBP7 concentrations were associated with a broader range of cardiac abnormalities, including LV and RV systolic dysfunction and worse valve disease. We observed relationships between higher IGFBP7 levels and greater LV mass, higher LV filling pressures (E/e′ ratio), impaired trans‐mitral inflow velocities (mitral E/A ratio), as well as more extensive diastolic relaxation abnormalities such as lower mean mitral annular e′ velocity and right ventricular dysfunction. Impaired LV relaxation and increased LV diastolic stiffness are major causes of diastolic dysfunction that lead to elevated LV filling pressure and LA stretch. 31 Prior studies have reported that systolic RV shortening is highly sensitive to afterload which is typically elevated in diastolic dysfunction. 32 , 33 In the present study, RV fractional area change was negatively correlated with IGFBP7 concentrations showing an increased afterload because of pressure and volume overloaded LV and LA. On the other hand, increased RV afterload may also occur because of lung diseases as it is highly dependent on the distribution of blood flow in the lung and increased alveolar pressure. 34 Furthermore, severe mitral regurgitation was found to be correlated with higher IGFBP7 concentrations suggesting the regurgitant volume causes volume and pressure overload of LA and LV. Our study has demonstrated that acute elevated LV filling pressure and volume overload, diastolic stiffness as well as increased RV afterload are correlated with increased IGFBP7 concentrations.
In adjusted analyses, the association between LAVi and IGFBP7 remained robust, much as in prior studies. 14 , 15 Indexed LA volume has emerged as a prime imaging marker in HF given its very significant association with elevated LV end diastolic pressures with impaired myocardial relaxation. 29 , 30 , 35 Indeed, LAVi has been suggested as a marker of the severity and duration of diastolic dysfunction. 30 , 35 , 36 , 37 Upregulation of several senescence‐associated biomarkers in cardiac fibroblasts and atrial myocytes along with notable atrial fibrosis has been observed in atrial tissue extracts of atrial fibrillation patients, indicating tissue ageing associated with atrial enlargement. 38 These observations are consistent with a potential role for IGFBP7 in mediating cardiac stiffness and potentially impeding diastolic filling resulting in a strong association with LAVi. With these strong associations, IGFBP7 may provide options for diagnosis, prognosis, and possibly even management strategies for patients whose primary mechanism of HF is abnormal diastolic function. 21
Strengths of this analysis include the fact it is derived from a multicenter clinical trial population with diagnoses assigned with strict adjudication, and the echocardiograms were blindly interpreted in a core lab fashion. Nonetheless, our work has limitations. First, our study has a relatively small size; however, for imaging studies larger numbers of study participants are not as required as they are in outcome studies. Though IGFBP7 was elevated in a manner consistent with a cardiac biomarker, it is important to concede abnormal concentrations of cardiac biomarkers may be seen in non‐cardiovascular presentations, presumably because of comorbid myocardial injury and stress. Indeed, Ruan et al identified that serum IGFBP7 levels are modestly increased during acute exacerbation of COPD. 39 Our data also demonstrated that among patients with asthma and COPD but without acute HF, those with higher IGFBP7 levels had higher LV filling pressures. Given our data includes acute dyspnoeic patients with suspected acute HF, we speculate that IGFBP7 concentrations might play a pivotal role in the response to acutely elevated LA and LV pressure and volume overload regardless of the underlying mechanism. The results of the present study have demonstrated that the biomarker may be a plausible candidate for use as a diagnostic test for HF, comparable with NT‐proBNP. Therefore, a potential incremental benefit of adding IGFBP7 to NT‐proBNP as a diagnostic tool for identifying acute HF as the cause of dyspnoea is currently being further evaluated in the larger ICON‐RELOADED data set.
Conclusions
In this cohort of acutely dyspnoeic patients presenting to the ED, we found that concentrations of IGFBP7 were strongly associated with a broad range of cardiac abnormalities, most notably including increased LAVi (a marker of elevated LV filling pressure and myocardial stiffness). IGFBP7 may enhance the diagnosis of acute HF. The role of IGFBP7 as a diagnostic and prognostic biomarker for acute HF is being further explored.
Conflict of interest
Dr Peacock has received grant support from Abbott, Boehringer Ingelheim, Braincheck, CSL Behring, Daiichi‐Sankyo, Immunarray, Janssen, Ortho Clinical Diagnostics, Portola, Relypsa, and Roche and has provided consulting for Abbott, Astra‐Zeneca, Bayer, Beckman, Boehrhinger‐Ingelheim, Ischemia Care, Dx, Immunarray, Instrument Labs, Janssen, Nabriva, Ortho Clinical Diagnostics, Relypsa, Roche, Quidel, and Siemens. He has provided expert testimony for Johnson and Johnson. He also reports stock/ownership interests for AseptiScope Inc, Brainbox Inc, Comprehensive Research Associates LLC, Emergencies in Medicine LLC, and Ischemia DX LLC. Dr Christenson has provided consulting for Roche Diagnostics, Siemens Heatlineers, Beckman Coulter Diagnostics, and BD Diagnostics and has received grant support from these entities. Dr Pang has provided consulting for Baxter, BMS during the past year and has received grant support from BMS, Roche, Novartis, PCORI, AHA, NHLBI, AHRQ, Ortho‐Diagnostics, and Abbott. Dr Kastner and Dr Masson are employees of Roche Diagnostics. Dr Gibson has received grant support from Portola Pharmaceuticals, Johnson & Johnson, and Bayer and has provided consulting for Portola Pharmaceuticals. Dr Gaggin has received research grant support from Roche Diagnostics, Jana Care, Ortho Clinical, and Novartis; consulting income from Merck, Roche Diagnostics; and research payments for clinical endpoint committees from Radiometer. Dr Januzzi has received grant support from Roche Diagnostics, Abbott, Cleveland Heart Labs, Singulex, and Prevencio, has received consulting income from Roche Diagnostics, Abbott, MyoKardia, and Novartis, has received funding as a member of the Board of Trustees of the American College of Cardiology, and has participated in clinical endpoint committees/data or safety monitoring boards for Novartis, Amgen, GE, Janssen, Pfizer, and Boehringer Ingelheim.
Funding
This study was partially funded by Roche Diagnostics (Rotkreuz, Switzerland). Dr Januzzi is supported in part by the Hutter Family Professorship.
Supporting information
Table S1. Echocardiographic Indices of Patients with Asthma and COPD, without Acute Heart Failure by the Median Value of IGFBP7.
Table S2. Clinical characteristics of the study population by the median value of NT‐proBNP.
Table S3. Echocardiographic indices of the study population by the median value of NT‐proBNP.
Table S4. Diagnostic performance of IGFBP7 across subgroups.
Figure S1. Box‐plots of NT‐proBNP concentrations according to the left ventricular diastolic dysfunction grading.
Kalayci, A. , Peacock, W. F. , Nagurney, J. T. , Hollander, J. E. , Levy, P. D. , Singer, A. J. , Shapiro, N. I. , Cheng, R. K. , Cannon, C. M. , Blomkalns, A. L. , Walters, E. L. , Christenson, R. H. , Chen‐Tournoux, A. , Nowak, R. M. , Lurie, M. D. , Pang, P. S. , Kastner, P. , Masson, S. , Gibson, C. M. , Gaggin, H. K. , and Januzzi Jr, J. L. (2020) Echocardiographic assessment of insulin‐like growth factor binding protein‐7 and early identification of acute heart failure. ESC Heart Failure, 7: 1664–1675. 10.1002/ehf2.12722.
References
- 1. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O'Donnell DE, American Thoracic Society Committee on Dyspnea . An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 3. Tschope C, Kasner M, Westermann D, Gaub R, Poller WC, Schultheiss HP. The role of NT‐proBNP in the diagnostics of isolated diastolic dysfunction: correlation with echocardiographic and invasive measurements. Eur Heart J 2005; 26: 2277–2284. [DOI] [PubMed] [Google Scholar]
- 4. Chen AA, Wood MJ, Krauser DG, Baggish AL, Tung R, Anwaruddin S, Picard MH, Januzzi JL. NT‐proBNP levels, echocardiographic findings, and outcomes in breathless patients: results from the ProBNP Investigation of Dyspnoea in the Emergency Department (PRIDE) echocardiographic substudy. Eur Heart J 2006; 27: 839–845. [DOI] [PubMed] [Google Scholar]
- 5. Grewal J, McKelvie R, Lonn E, Tait P, Carlsson J, Gianni M, Jarnert C, Persson H. BNP and NT‐proBNP predict echocardiographic severity of diastolic dysfunction. Eur J Heart Fail 2008; 10: 252–259. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi PU, Chow SL, Rector TS, Krum H, Gaggin HK, McMurray JJ, Zile MR, Komajda M, McKelvie RS, Carson PE, Januzzi JL Jr, Anand IS. Prognostic value of insulin‐like growth factor‐binding protein 7 in patients with heart failure and preserved ejection fraction. J Card Fail 2017; 23: 20–28. [DOI] [PubMed] [Google Scholar]
- 7. Chugh S, Ouzounian M, Lu Z, Mohamed S, Li W, Bousette N, Liu PP, Gramolini AO. Pilot study identifying myosin heavy chain 7, desmin, insulin‐like growth factor 7, and annexin A2 as circulating biomarkers of human heart failure. Proteomics 2013; 13: 2324–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motiwala SR, Szymonifka J, Belcher A, Weiner RB, Baggish AL, Gaggin HK, Bhardwaj A, Januzzi JL Jr. Measurement of novel biomarkers to predict chronic heart failure outcomes and left ventricular remodeling. J Cardiovasc Transl Res 2014; 7: 250–261. [DOI] [PubMed] [Google Scholar]
- 9. Gandhi PU, Gaggin HK, Sheftel AD, Belcher AM, Weiner RB, Baggish AL, Motiwala SR, Liu PP, Januzzi JL Jr. Prognostic usefulness of insulin‐like growth factor‐binding protein 7 in heart failure with reduced ejection fraction: a novel biomarker of myocardial diastolic function? Am J Cardiol 2014; 114: 1543–1549. [DOI] [PubMed] [Google Scholar]
- 10. Januzzi JL Jr, Chen‐Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, Nagurney JT, Nowak RM, Pang PS, Patel D, Peacock WF, Rivers EJ, Walters EL, Gaggin HK, Investigators ICON‐RELOADED. N‐terminal pro‐b‐type natriuretic peptide in the emergency department: the ICON‐RELOADED study. J Am Coll Cardiol 2018; 71: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 11. Gaggin HK, Chen‐Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, Nagurney JT, Nowak RM, Pang PS, Patel D, Peacock WF, Walters EL, Januzzi JL, ICON‐RELOADED Investigators . Rationale and design of the ICON‐RELOADED study: international collaborative of n‐terminal pro‐b‐type natriuretic peptide re‐evaluation of acute diagnostic cut‐offs in the emergency department. Am Heart J 2017; 192: 26–37. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 13. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 14. Januzzi JL Jr, Packer M, Claggett B, Liu J, Shah AM, Zile MR, Pieske B, Voors A, Gandhi PU, Prescott MF, Shi V, Lefkowitz MP, McMurray JJV, Solomon SD. IGFBP7 (insulin‐like growth factor‐binding protein‐7) and neprilysin inhibition in patients with heart failure. Circ Heart Fail 2018; 11: e005133. [DOI] [PubMed] [Google Scholar]
- 15. Gandhi PU, Gaggin HK, Redfield MM, Chen HH, Stevens SR, Anstrom KJ, Semigran MJ, Liu P, Januzzi JL Jr. Insulin‐like growth factor‐binding protein‐7 as a biomarker of diastolic dysfunction and functional capacity in heart failure with preserved ejection fraction: results from the RELAX trial. JACC Heart Fail 2016; 4: 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shakeri H, Lemmens K, Gevaert AB, De Meyer GRY, Segers VFM. Cellular senescence links aging and diabetes in cardiovascular disease. Am J Physiol Heart Circ Physiol 2018; 315: H448–H462. [DOI] [PubMed] [Google Scholar]
- 17. Morris AA, Butler J, Konstam MA. Heart failure with normal ejection fraction, heart failure with preserved ejection fraction, diastolic heart failure, or huff‐puff: time for a new taxonomy for hypertensive‐metabolic heart failure. J Card Fail 2014; 20: 779–781. [DOI] [PubMed] [Google Scholar]
- 18. Kashani K, Al‐Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes‐Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014; 114: 565–571. [DOI] [PubMed] [Google Scholar]
- 20. van Breevoort D, van Agtmaal EL, Dragt BS, Gebbinck JK, Dienava‐Verdoold I, Kragt A, Bierings R, Horrevoets AJ, Valentijn KM, Eikenboom JC, Fernandez‐Borja M, Meijer AB, Voorberg J. Proteomic screen identifies IGFBP7 as a novel component of endothelial cell‐specific Weibel‐Palade bodies. J Proteome Res 2012; 11: 2925–2936. [DOI] [PubMed] [Google Scholar]
- 21. Barroso MC, Kramer F, Greene SJ, Scheyer D, Köhler T, Karoff M, Seyfarth M, Gheorghiade M, Dinh W. Serum insulin‐like growth factor‐1 and its binding protein‐7: potential novel biomarkers for heart failure with preserved ejection fraction. BMC Cardiovasc Disord 2016; 16: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez‐Bermejo A, Khosravi J, Fern.ndez‐Real JM, Hwa V, Pratt KL, Casamitjana R, Garcia‐Gil MM, Rosenfeld RG, Ricart W. Insulin resistance is associated with increased serum concentration of IGF‐binding protein‐related protein 1 (IGFBP‐rP1/MAC25). Diabetes 2006; 55: 2333–2339. [DOI] [PubMed] [Google Scholar]
- 23. van Heerebeek L, Hamdani N, Handoko ML, Falcao‐Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008; 117: 43–51. [DOI] [PubMed] [Google Scholar]
- 24. Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004; 25: 543–567. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Wu M, Ling J, Cai L, Zhang D, Gu HF, Wang H, Zhu Y, Lai M. Serum IGFBP7 levels associate with insulin resistance and the risk of metabolic syndrome in a Chinese population. Sci Rep 2015; 5: 10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kutsukake M, Ishihara R, Momose K, Isaka K, Itokazu O, Higuma C, Matsutani T, Matsuda A, Sasajima K, Hara T, Tamura K. Circulating IGF‐binding protein 7 (IGFBP7) levels are elevated in patients with endometriosis or undergoing diabetic hemodialysis. Reprod Biol Endocrinol 2008; 19: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin‐like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 2013; 4: e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo XH, Liu LX, Zhang HY, Zhang QQ, Li Y, Tian XX, Qiu ZH. Insulin‐like growth factor binding protein‐related protein 1 contributes to hepatic fibrogenesis. J Dig Dis 2014; 15: 202–210. [DOI] [PubMed] [Google Scholar]
- 29. Hage C, Bjerre M, Frystyk J, Gu HF, Brismar K, Donal E, Daubert JC, Linde C, Lund LH. Comparison of prognostic usefulness of serum insulin‐like growth factor‐binding protein 7 in patients with heart failure and preserved versus reduced left ventricular ejection fraction. Am J Cardiol 2018; 121: 1558–1566. [DOI] [PubMed] [Google Scholar]
- 30. Issa O, Peguero JG, Podesta C, Diaz D, De La Cruz J, Pirela D, Brenes JC. Left atrial size and heart failure hospitalization in patients with diastolic dysfunction and preserved ejection fraction. J Cardiovasc Echogr 2017; 27: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 32. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB, National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure . Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006; 114: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 33. Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012; 126: 975–990. [DOI] [PubMed] [Google Scholar]
- 34. Permutt S, Bromberger‐Barnea B, Bane HN. Alveolar pressure, pulmonary venous pressure, and the vascular waterfall. Med Thorac 1962; 19: 239–260. [DOI] [PubMed] [Google Scholar]
- 35. Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol 2003; 42: 1206–1207. [DOI] [PubMed] [Google Scholar]
- 36. Lupu S, Mitre A, Dobreanu D. Left atrium function assessment by echocardiography‐physiological and clinical implications. Med Ultrason 2014; 16: 152–159. [DOI] [PubMed] [Google Scholar]
- 37. Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, Oh JK, Leibson C, Montgomery SC, Seward JB. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002; 40: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 38. Xie J, Chen Y, Hu C, Pan Q, Wang B, Li X, Geng J, Xu B. Premature senescence of cardiac fibroblasts and atrial fibrosis in patients with atrial fibrillation. Oncotarget 2017; 8: 57981–57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruan W, Wu M, Shi L, Li F, Dong L, Qiu Y, Wu X, Ying K. Serum levels of IGFBP7 are elevated during acute exacerbation in COPD patients. Int J Chron Obstruct Pulmon Dis 2017; 12: 1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Echocardiographic Indices of Patients with Asthma and COPD, without Acute Heart Failure by the Median Value of IGFBP7.
Table S2. Clinical characteristics of the study population by the median value of NT‐proBNP.
Table S3. Echocardiographic indices of the study population by the median value of NT‐proBNP.
Table S4. Diagnostic performance of IGFBP7 across subgroups.
Figure S1. Box‐plots of NT‐proBNP concentrations according to the left ventricular diastolic dysfunction grading.


