Abstract
Aims
Hyponatremia is associated with poorer outcomes and diuretic response in patients hospitalized for heart failure. This study compared a tolvaptan‐based vs. furosemide‐based diuretic regimen on short‐term clinical responses in hyponatremic acute heart failure.
Methods and results
Prospective, randomized, open‐label, parallel‐group, single‐centre study comparing oral tolvaptan vs. continuous infusion furosemide. Thirty‐three subjects requiring hospitalization for acute congestive heart failure, and a serum sodium < 135 mmol/L, were randomized to tolvaptan 30 mg orally daily or furosemide 5 mg/h intravenously for initial 24 h, after which treatments could be escalated. Median daily dose throughout was tolvaptan 30 mg and furosemide 120 mg, with four subjects in each group requiring dose escalation. Urine output and net fluid balance were not different between groups at 24 h or subsequent time points up to 96 h. Changes in estimated glomerular filtration rate were comparable. Cystatin C improved at 24 h with tolvaptan compared with furosemide (−6.4 ± 11.8 vs. 4.1 ± 17.2% change, P = 0.036), but the effect was transient. No significant between group differences were seen for NT‐proBNP, plasma renin activity, or urinary neutrophil gelatinase‐associated lipocalin:Cr. Serum sodium, as well as copeptin levels, increased with tolvaptan compared with furosemide.
Conclusions
Oral tolvaptan was associated with similar, but not superior, diuresis compared with intravenous furosemide for acute heart failure with concomitant hyponatremia.
Keywords: Vasopressin, Diuretics, Heart failure, Hyponatremia
Introduction
Hyponatremia is the most common electrolyte abnormality in the hospital setting and is associated with higher in‐hospital mortality, 60‐day mortality, and longer length of hospitalization. 1 , 2 , 3 , 4 , 5 These findings have also been shown for patients hospitalized with heart failure (HF). 6 , 7 , 8 , 9 , 10 Despite these associations, the importance of hyponatremia as an influencing factor for acute treatment decisions in acute HF remains equivocal because of a paucity of prospective data. Notably, a previous study found moderate to severe hyponatremia, especially when <130 mmol/L, was associated with higher loop diuretic dose requirements and more frequent need for diuretic regimen escalation to achieve the same level of urine output as in normonatremic patients. 11 Severity of hyponatremia was also associated with a greater than two‐fold increase in the incidence of diuretic resistance, acute increases in serum creatinine, sustained hypotension, increased length of stay, and in‐hospital mortality. The study raised important questions, including whether alternative treatments such as aquaretics (i.e. vasopressin receptor antagonists) might represent a superior treatment modality for selected patients. Data from subgroups of hyponatremic patients in the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan and Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure studies indicated that tolvaptan is effective at raising serum sodium and urine output in these patients. 12 , 13 , 14 In addition, tolvaptan monotherapy was shown to exert a greater effect on urine volume and weight loss than a fixed dose of furosemide in stable New York Heart Association functional class II–III systolic HF patients. 15 However, these were not acutely decompensated patients, and tolvaptan was being compared with oral furosemide. Therefore, there is a need to evaluate the role of aquaresis with tolvaptan as an acute treatment modality for acute HF complicated by hyponatremia. These are clinically challenging patients because of their poor response to loop diuretics and their propensity for adverse effects, and may represent an important niche for alternative diuretic strategies. Therefore, the Aquaresis Utility for Hyponatremic Acute Heart Failure study (NCT02183792) was undertaken to prospectively evaluate the comparative efficacy and safety of a tolvaptan‐based diuretic regimen compared with conventional diuresis with a furosemide‐based regimen on short‐term clinical and treatment outcomes in patients hospitalized for acute HF with concomitant hyponatremia.
Methods
Study design and patient population
A prospective, open‐label, parallel‐group, single‐centre, randomized study comparing a tolvaptan‐based regimen with a conventional continuous infusion loop diuretic‐based regimen of furosemide. Adult patients admitted for acute HF were screened for inclusion from January 2015 to February 2018. They were included if they required hospitalization for acute HF with signs or symptoms of volume overload (i.e. elevated jugular venous pressure, rales, and oedema), a serum sodium < 135 mmol/L at time of or within first 48 h of hospitalization, and provided informed consent.
Exclusion criteria included severe symptomatic hyponatremia requiring acute treatment, pseudohyponatremia, moderate to severe liver impairment, severe renal impairment upon admission [estimated glomerular filtration rate (GFR) < 20 mL/min/m2], renal replacement therapy dependent or required upon admission, acute coronary syndrome on admission, evidence of cardiogenic shock or requiring intravenous vasopressors, pregnant, or concomitant use of strong CYP3A4 inhibitors (clarithromycin, ketoconazole, itraconazole, ritonavir, indinavir, nelfinavir, saquinavir, nefazodone, and telithromycin). The study was approved by the university institutional review board and registered on clinicaltrials.gov (NCT02183792).
Study procedures
Patients were assigned to tolvaptan or continuous infusion furosemide‐based treatment groups based on a computer‐generated 10‐block randomization scheme, stratified for level of hyponatremia (130–134 and <130 mmol/L). All patients received a bolus of furosemide 20 mg intravenously at study entry if they were diuretic naïve. Patients randomized to tolvaptan were initiated on 30 mg orally once daily (every 24 h). Patients randomized to furosemide were initiated on 5 mg/h administered via continuous intravenous infusion. This fixed dose was chosen as it has previously been shown to be an effective dose for our patient population, 16 and the study was designed prior to the results of the Diuretic Optimization Strategies Evaluation trial being reported 17 . Baseline thiazide diuretics were discontinued during the protocol‐guided diuretic treatment. After the initial 24‐h monotherapy period, escalation of the study treatment regimen for either group was at the discretion of the primary treating physician (encouraged to achieve a minimum urine output of 100 mL/h). Escalation of therapy excluded the initiation of tolvaptan or loop diuretic in the respective contralateral study group. The treating physician was encouraged to increase the dose of the respective study regimen if greater diuresis was required, with the possible addition of metolazone if desired diuresis was not achieved with tolvaptan 60 mg or furosemide 20 mg/h. The doses could also be reduced, however, if the treating physician wished to discontinue tolvaptan or the continuous infusion of furosemide for clinical de‐escalation of diuresis or a switch to bumetanide, this was considered the end of per protocol study treatment.
At the time of randomization, prior diuretic and urine output volume were documented. Fluid balance was extracted from the electronic health records on a daily basis, as nurses in the cardiac intensive care unit were deemed trained and experienced in accurate collection. Baseline vital statistics, basic metabolic panel, as well as blood and urine samples for biomarker analysis (described hereafter) were collected. Body weight was documented. However, because of unreliability of weight assessments at our institution, changes in body weight were not used as a formal endpoint. After the study regimen was initiated, urine output and fluid balance, vital statistics, labs, and samples for biomarkers were obtained at 8, 24, 48, 72, and 96 h, respectively. Patient self‐reported dyspnoea (7‐point Likert scale) was also obtained verbally by a study coordinator at 24 and 96 h (or when the study regimen was discontinued if prior to 96 h) using a set script. Patient‐reported or physician reported adverse events were noted if they occurred. Study procedure timing is outlined in Supporting Information, Figure SS1 .
Biomarker collection and analyses
Plasma biomarkers
Blood samples were obtained for analysis of plasma N‐terminal pro b‐type natriuretic peptide (NT‐proBNP), plasma renin activity (PRA), cystatin C, and copeptin. Cystatin C and PRA were outsourced to Quest Diagnostics. Copeptin was outsourced to Quantigen (Fisher, IL) and assayed using a commercially available immunoassay (Brahms Copeptin Immunoassay, Thermofisher Scientific, Middleton, VA).
Urinary biomarker
Urine samples were collected for analysis of urinary neutrophil gelatinase‐associated lipocalin (uNGAL). Urine NGAL measurements were performed using a commercially available enzyme‐linked immunosorbent assay (Bioporto, Gentofte, Denmark) as published previously. 18 , 19 , 20 The intra‐assay and inter‐assay coefficients of variability are 5.6% and 6.4%, respectively. The lower limit of detection of the assay is 6.5 pg/mL.
Study endpoints
The primary efficacy endpoint was median urine output at 24 h post randomization (continuous variables were initially calculated as mean values but reported as median because of non‐normal distribution). Secondary efficacy endpoints were median urine output and change in serum sodium, assessed at 8, 48, and up to 96 h post randomization. Proportion of patients requiring escalation of study drug dose or the addition of metolazone, and change in self‐rated dyspnoea (Likert scale) at 24 and 96 h, were also compared. The primary safety endpoint was the mean change in serum creatinine (Scr) at 24 h post randomization. Other safety endpoints included change in estimated GFR (estimated from Scr using the Modification of Diet in Renal Disease 4 variable equation) at 24, 48, and up to 96 h post randomization, incidence of acute increases in Scr ≥ 26.5 μmol/L (0.3 mg/dL), and in‐hospital mortality. In addition to the clinical endpoints, changes in PRA, copeptin, NT‐proBNP, cystatin C, and urinary NGAL concentrations were also assessed. Urinary NGAL was standardized to urinary creatinine concentrations.
Statistical analysis
Initial target enrolment was for 50 patients (25 in each group), which provided 93% power to detect a difference of 50% in urine output at 24 h between treatments, α = 0.05. The power was calculated based on the magnitude of difference in urine output seen in the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure 13 (24‐h mean urine output for tolvaptan was approximately 4100 ± 2100 mL vs. 2300 ± 1100 mL compared with placebo when added to standard therapy) and in the Udelson et al. 15 study (24‐h mean increase in urine output with tolvaptan monotherapy 2600 ± 1500 mL vs. 900 ± 850 mL with furosemide). In August 2017, because of slow enrolment (Food and Drug Administration labelling change excluding hepatic disease and reduction in hospital census), the target sample was reduced to 33 subjects, providing 80% power assuming the same mean difference as before.
Analyses were per protocol (included data only when study subjects remained on study protocol therapy as there was substantial attrition after 48 h). Descriptive statistics were computed for each treatment group. For all comparative analyses between the two treatment groups, chi‐square test (or Fisher's exact test) for categorical variables and Wilcoxon–Mann–Whitney test for continuous variables were performed, respectively. All statistical comparisons were conducted using SAS (version 9.4).
Results
Study population and study regimens
During the study period, 242 patients were screened, and 209 met exclusion criteria (see Supporting Information, Figure S2 ). A total of 33 subjects were randomized, 18 to tolvaptan and 15 to continuous infusion furosemide. Subjects randomized to tolvaptan were more often female patients and more likely to have HF with reduced ejection fraction. Groups were similar with regard to age, comorbid conditions, and home loop diuretic dose. Comparison of baseline characteristics is presented in Table 1 .
TABLE 1.
Patient characteristics at baseline
| Tolvaptan (N = 18) | Furosemide (N = 15) | P value | |
|---|---|---|---|
| Age, years | 53 ± 11.7 | 59 ± 8.9 | 0.142 |
| Male, % | 11 (61.1) | 14 (93.3) | 0.046 |
| Heart failure characteristics | |||
| Ischaemic aetiology, % | 5 (29.4) | 3 (21.4) | 0.698 |
| LVEF | 24 ± 7.2 | 33 ± 14.3 | 0.093 |
| LVEF≥40%, N (%) | 1 (5.6) | 7 (46.7) | 0.012 |
| Comorbid conditions | |||
| Coronary artery disease | 2 (11.1) | 4 (28.6) | 0.365 |
| Hypertension | 10 (55.6) | 8 (53.3) | 0.898 |
| Dyslipidemia | 4 (22.2) | 1 (6.7) | 0.346 |
| Atrial arrhythmia | 6 (33.3) | 6 (42.9) | 0.581 |
| Diabetes mellitus | 9 (50.0) | 8 (53.3) | 0.849 |
| Chronic kidney disease | 3 (16.7) | 3 (21.4) | 1.000 |
| Home medications | |||
| Loop diuretic | 15 (83.3) | 12 (80.0) | 1.000 |
| Home oral loop dose (Furosemide equivalents) | 93 ± 63.5 | 109 ± 77.5 | 0.693 |
| Thiazide diuretic | 3 (16.7) | 2 (13.3) | 1.000 |
| Beta‐blocker | 11 (61.1) | 9 (60.0) | 0.948 |
| ACE inhibitor | 9 (50.0) | 5 (33.3) | 0.335 |
| ARB | 2 (11.1) | 2 (13.3) | 1.000 |
| MRA | 6 (33.3) | 4 (26.7) | 0.722 |
| Digoxin | 1 (5.6) | 1 (6.7) | 1.000 |
| Antiplatelet | 10 (55.6) | 7 (50.0) | 0.755 |
| Anticoagulant | 5 (27.8) | 4 (26.7) | 1.000 |
| Calcium channel blocker | 0 (0) | 0 (0) | N/A |
| Statin | 7 (38.9) | 6 (40.0) | 0.948 |
| Other | |||
| SBP, mmHg | 102 (94, 114) | 108 (92, 142) | 0.426 |
| DBP, mmHg | 73 (68, 77) | 75 (65, 91) | 0.600 |
| HR, bpm | 83 (77, 95) | 82(71, 101) | 1.000 |
| Serum potassium, mmol/L | 4.1 ± 0.5 | 4.2 ± 0.5 | 0.636 |
| Serum albumin, g/L | 33 ± 4 | 30 ± 6 | 0.137 |
| White blood cell count, 109/L | 7.6 ± 2.9 | 9.3 ± 3.5 | 0.124 |
| SCr, μmol/L | 101.7 (77.8, 133.5) | 76.9 (67.2, 107.9) | 0.093 |
| BUN, mmol/L | 9.3 (5.0, 15.0) | 7.8(7.1, 12.1) | 0.928 |
| NT‐proBNP, ng/L | 5,190 (3,834, 9,917) | 8,080 (4,488, 12,645) | 0.143 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; DBP, diastolic blood pressure; HR, heart rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SBP, systolic blood pressure; SCr, serum creatinine
Mean ± SD, median (IQR), or N (%).
All subjects remained per protocol to the 24‐h post‐randomization assessments. One subject in each group deviated from study protocol by 48 h. By 72 and 96 h, respectively, only eight and seven subjects randomized to tolvaptan remained per protocol, whereas 12 and 11 subjects randomized to continuous infusion furosemide remained per protocol. Attrition from study protocol was due to clinical decisions of the primary treating physician (clinical resolution or diuretic switch to bumetanide). Dose escalation occurred in four subjects in each group after the first 24 h. The median daily dose throughout for tolvaptan and furosemide was 30 and 120 mg, respectively. Metolazone was used in four subjects in the tolvaptan group compared with one in the furosemide group.
Urine output, net fluid balance, and vital statistics
Prior to randomization, median urine output and net fluid balance were numerically greater in the furosemide group compared with tolvaptan group but did not approach statistical significance due to large variability. There were no significant differences in median urine output or net fluid balance between groups at 24 h or any time point up to 96 h, either cumulatively or for each 24‐h interval. There were no significant differences in median systolic or diastolic blood pressure or heart rate between the groups at any time point. Self‐rated dyspnoea score or proportion of subjects with at least moderate improvement was not different between groups at 24 or at 96 h or study drug termination (data not shown). Urine output and fluid balance comparisons are summarized in Figures 1 and 2 . Vitals are summarized in Supporting Information, Table S1 .
Figure 1.
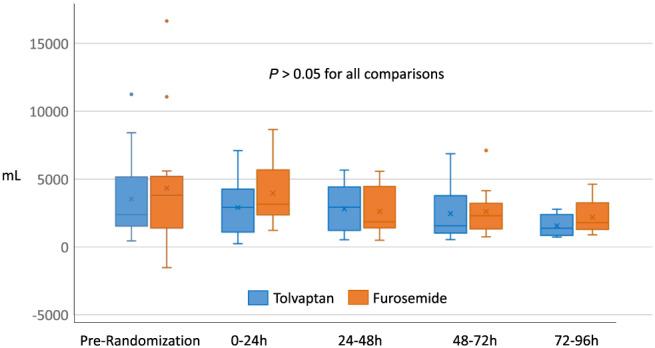
Median daily urine output.
Figure 2.
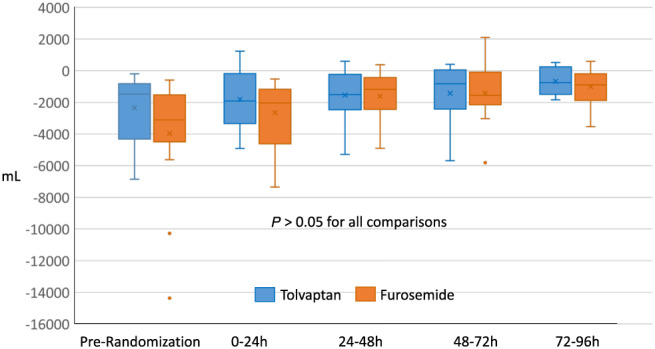
Median daily net fluid balance.
Renal function, electrolytes, and biomarkers
Renal function at time of randomization, as indicated by Scr concentration and estimated GFR, was numerically but not statistically better in the furosemide group. Scr concentrations decreased modestly in the tolvaptan group, although with the small sample size and variability, the differences compared with furosemide were not statistically significant. Similarly, intergroup differences did not reach statistical significance in estimated GFR or blood urea nitrogen concentrations. Incidence of acute increases in Scr ≥ 26.5 μmol/L (0.3 mg/dL) was rare, regardless of whether or not the definition incorporated a 25% increase over baseline. Renal function endpoints are summarized in Table 2 and Supporting Information, Table S 2 .
TABLE 2.
Renal endpoints comparison
| Tolvaptan | Furosemide | P value | |
|---|---|---|---|
| Serum creatinine (μmol/L) | |||
| Baseline (n = 18 vs. 15) | 101.7 (77.8, 133.5) | 76.9 (67.2, 107.9) | 0.093 |
| 24‐h change (n = 18 vs. 15) | −7.1 (−12.4, −3.5) | −0.9 (−16.8, 13.3) | 0.416 |
| 48‐h change (n = 16 vs. 15) | −15.9 (−27.4, 7.1) | −1.8 (−5.3, 12.4) | 0.093 |
| 72‐h change (n = 8 vs. 13) | −17.7 (−33.6, 15.9) | 2.7 (0, 19.5) | 0.232 |
| 96‐h change (n = 7 vs. 11) | −15.0 (−32.7, 25.6) | 9.7 (−9.7, 19.5) | 0.205 |
| Blood urea nitrogen (mmol/L) | |||
| Baseline (n = 18 vs. 15) | 9.3 (5.0, 15.0) | 7.9 (7.1, 12.1) | 0.928 |
| 24‐h change (n = 18 vs. 15) | −0.7 (−1.1, 0.4) | −0.4 (−2.5, 0.4) | 0.649 |
| 48‐h change (n = 16 vs. 15) | −1.2 (−3.6, 0.5) | −0.4 (−3.2, 1.1) | 0.513 |
| 72‐h change (n = 8 vs. 13) | −2.1 (−5.4, 2.1) | 0.0 (−1.4, 2.1) | 0.363 |
| 96‐h change (n = 7 vs. 11) | −1.1 (−5.0, 3.2) | −1.1 (−2.5, 3.6) | 0.555 |
| Estimated glomerular filtration rate (mL/min/m2) | |||
| Baseline (n = 18 vs. 15) | 65.8 (36.0, 98.2) | 90.0 (66.0, 107.0) | 0.143 |
| 24‐h change (n = 18 vs. 15) | 5.75 (2.00, 8.00) | 0.80 (−11.20, 13.00) | 0.187 |
| 48‐h change (n = 16 vs. 15) | 10.50 (−7.15, 13.00) | 1.00 (−9.00, 4.00) | 0.105 |
| 72‐h change (n = 8 vs. 13) | 9.00 (−17.80, 18.00) | −1.00 (−16.00, 0.00) | 0.310 |
| 96‐h change (n = 7 vs. 11) | 7.00 (−8.00, 15.00) | −3.00 (−19.00, 10.00) | 0.173 |
Median (IQR: 25% and 75% quantile).
Serum sodium and potassium concentrations were not different between groups at the time of randomization. As expected, serum sodium increased in the tolvaptan group, while decreasing in the furosemide group, and the intergroup differences in median change being statistically significant at 48, 72, and 96 h. Changes in serum potassium and magnesium were comparable between the tolvaptan and furosemide groups. Comparison of electrolyte effects are summarized in Supporting Information, Table S 3 .
As expected, copeptin plasma concentrations increased in the tolvaptan group. Cystatin C decreased in the tolvaptan group, with median change in concentrations being significantly different than furosemide at 24 h, but the difference was lost at 96 h. There was high variability in other plasma and urinary biomarkers measured. As a consequence, there were no detectable differences between tolvaptan and furosemide groups in NT‐proBNP, PRA, or urinary NGAL:Scr concentrations at any time point. Summary of biomarker changes is provided in Table 3 .
TABLE 3.
Biomarker comparisons
| Tolvaptan | Furosemide | P value | ||
|---|---|---|---|---|
| NT‐proBNP (ng/L) | ||||
| Baseline (n = 18 vs. 15) | 5,190 (3,834, 9,917) | 8,080 (4,488, 12,645) | 0.143 | |
| % change 0–24 h (n = 18 vs. 15) | 2.28 (−17.74, 14.91) | −15.25 (−53.52, 9.56) | 0.187 | |
| % change 0–96 h (n = 7 vs. 11) | 15.97 (−45.86, 39.50) | −26.32 (−80.52, 1.12) | 0.469 | |
| Cystatin C (mg/L) | ||||
| Baseline (n = 18 vs. 15) | 1.16 (0.91, 1.37) | 1.03 (0.95, 1.32) | 0.691 | |
| % change 0–24 h (n = 18 vs. 15) | −8.56 (−13.68, −5.51) | 3.57 (−6.19, 10.99) | 0.038 | |
| % change 0–96 h (n = 7 vs. 10) | 2.38 (−14.13, 17.91) | 1.77 (−7.07, 9.94) | 1.000 | |
| Plasma renin activity (ng/mL) | ||||
| Baseline (n = 18 vs. 15) | 15.25 (1.00, 32.33) | 16.88 (6.06, 39.12) | 0.986 | |
| % change 0–24 h (n = 18 vs. 15) | −23.31 (−34.79, 29.23) | −16.31 (−40.75, 71.43) | 0.396 | |
| % change 0–96 h (n = 7 vs. 10) | −49.22 (−62.96, 12.66) | 1.68 (−39.60, 152.38) | 0.354 | |
| Copeptin A (ng/mL) | ||||
| Baseline (n = 17 vs. 15) | 56.50 (19.40, 65.00) | 25.40 (17.30, 54.70) | 0.162 | |
| % change 0–24 h (n = 17 vs. 15) | 29.30 (−14.96, 55.68) | −9.33 (−24.06, −0.65) | 0.059 | |
| % change 0–96 h (n = 6 vs. 11) | 35.58 (11.45, 90.15) | −14.32 (−39.31, 4.13) | 0.119 | |
| Urinary NGAL:Cr (ng/mL/g) | ||||
| Baseline (n = 18 vs. 15) | 28.31 (16.58, 135.56) | 40.26 (32.29, 73.68) | 0.600 | |
| % change 0–24 h (n = 17 vs. 15) | −20.50 (−40.52, −3.74) | −10.49 (−45.12, 50.34) | 0.910 | |
| % change 0–48 h (n = 17 vs. 14) | −7.42 (−36.65, 73.95) | −14.20 (−48.34, 51.89) | 0.463 | |
| % change 0–72 h (n = 8 vs. 13) | 49.37 (−7.24, 123.81) | −24.94 (−35.56, 34.22) | 0.089 | |
Median (IQR: 25% and 75% quantile).
Discussion
Loop diuretics remain a cornerstone in the management of congestive HF. However, their limitations in efficacy and safety are well recognized. This randomized pilot study provides initial evidence that monotherapy with the vasopressin receptor antagonist tolvaptan may be an effective alternative diuretic strategy for select patients in acute HF, although this study was unable to demonstrate superior efficacy compared with a well‐established regimen of intravenous furosemide. 16 This study was unique in design, as it evaluated tolvaptan as monotherapy in patients with HF that required hospitalization for decongestive therapy. Other studies with tolvaptan in HF have been limited to application as monotherapy in outpatients with chronic stable HF or as an adjunctive therapy to loop diuretics in the hospital setting.
Several prospective, randomized, controlled trials have clearly demonstrated the efficacy of tolvaptan for augmenting diuresis when administered in combination with furosemide for the management of congestive HF symptoms. 12 , 13 , 21 , 22 , 23 , 24 Even the most recent trials of tolvaptan in acute HF, considered negative trials because of unclear benefits on dyspnoea symptoms, demonstrated greater early fluid and weight loss with the addition of tolvaptan to loop diuretics. 25 , 26 , 27
However, very limited data exist with regard to the utility of tolvaptan as monotherapy. The potential for tolvaptan to be used as an alternative to furosemide was considered in a study by Udelson et al. 15 Eighty‐three patients with stable New York Heart Association functional class II–III systolic HF and evidence of congestive symptoms were randomized to tolvaptan 30 mg, furosemide 80 mg, or the combination for 7 days. Tolvaptan monotherapy was associated with a significantly greater daily urine output (approximately 1700 mL/24 h greater than furosemide monotherapy) and decrease in body weight compared with both furosemide or the combination. It differed from the current study as the patients were compensated chronic systolic HF with reduced ejection fraction, and the comparator was furosemide administered orally. Jujo et al. conducted a study of 60 patients with acute HF comparing low doses of tolvaptan 7.5 mg once daily to furosemide 40 mg IV once daily for 5 days. 28 Tolvaptan provided comparable daily negative fluid balance. The current study and that by Jujo et al. suggest tolvaptan administered orally may provide at least comparable diuresis with intravenous furosemide.
Although we hypothesized that tolvaptan would provide superior diuresis, the current study was unable to detect any difference in total urine output or net fluid balance. The inability to detect a significant increase in diuresis in the current study was surprising but may have been a result of selection of a less severe acute HF population than was evaluated in our retrospective study. Patients generally had mild hyponatremia, and we excluded those requiring vasopressors and inotropes upon presentation, and those with cardiohepatic disease. In addition, the diuretic response to the continuous infusion furosemide was robust, which suggests a more diuretic resistant population was not selected in hindsight.
Safety
Although rigorous longitudinal outcome data are lacking, conventional decongestion with loop diuretics has been associated with physiologic effects that could have detrimental consequences when accounting for the pathophysiology of HF. This includes development or exacerbation of electrolyte disturbances such as hyponatremia and hypokalemia and acute worsening of kidney function (also known as cardiorenal syndrome type 1). These adverse consequences of loop diuretics are all independently associated with worse cardiovascular outcomes and may complicate acute management decisions as well. Aquaresis with tolvaptan represents a potentially advantageous approach to the management of volume overload in HF. In previous randomized studies, tolvaptan has been associated with a favourable safety profile, devoid of significant effects on serum electrolytes and incidence of acutely worsening renal function.
The safety of tolvaptan as a diuretic is similarly reflected in our study results. In contrast to conventional diuretics, exacerbation of hyponatremia are unlikely with tolvaptan, as serum sodium tended to increase in that treatment group. The study was unable to replicate the renoprotective effects demonstrated in the study by Jujo et al.; however, cystatin C transiently improved the tolvaptan group (whether the effect would have been sustained out to 96 h if study attrition had not occurred is unknown). The biomarker data related to neurohormonal activation (i.e. PRA and NTproBNP) or tubular injury (i.e. uNGAL) were inconclusive.
Limitations
The results of this study should be interpreted with limitations of a pilot study in mind and should be considered hypothesis generating until confirmed in larger studies. The sample size was small, and many subjects were discontinued from the study protocol after 48 h, so the results beyond 48 h must be interpreted with caution. The open‐label pragmatic design for titration of therapy also contributed to the frequency of patients being discontinued from the study protocol and potentially introduces bias in the subjective endpoint of dyspnoea rating. The doses evaluated do not allow for determination of comparative effects at higher doses of both drugs, which is common with furosemide after the reporting of the Diuretic Optimization Strategies Evaluation trial. 17 There was high inter‐subject variability in the biomarkers, so there was limited power to detect subtle but possibly clinically relevant differences. The incidence of acute worsening renal function was low in our study but could have been because patients were removed from study protocol treatment if they did not respond. Finally, the requirement for hyponatremia reduces overall generalizability of the results to all patients presenting with acute HF, however, as discussed in the introduction, these patients may represent a subpopulation that may benefit from alternatives to loop diuretics. Despite the limitations, the results support the aquaretic efficacy of vasopressin receptor antagonist monotherapy in HF, which has also been proposed by others. 29
Conclusions
Diuresis with an oral tolvaptan‐based diuretic regimen was similar, but not superior, to an intravenous furosemide‐based diuretic regimen for acute HF in patients with hyponatremia.
Conflict of interest
Dr Ng has served as a consultant for Amgen in a capacity unrelated to the study focus. No other investigators report any potential conflicts of interest with the current study.
Funding
The study was funded by an investigator sponsored study grant from Otsuka America Pharmaceuticals, Inc.
Supporting information
Figure S1. Study Procedure Time Points
DC = discontinuation of protocol; NT‐proBNP = N‐terminal pro‐b‐type natriuretic peptide; PRA = plasma renin activity; uNGAL = urinary neutrophil gelatinase‐associated lipocalin
Figure S2. Study Population CONSORT diagram
Table S1. Vital Statistics Comparison
Table S2. Incidence of Acute Increases in Serum Creatinine
Table S3. Electrolyte Changes Comparison
Acknowledgements
The study was supported by an investigator sponsored study grant from Otsuka America Pharmaceutical, Inc. Investigators would like to thank Jorge Caro, Osbaldo Rodriguez, and Myriam Moreno for their help in study coordination and data collection. We would also like to thank the cardiology fellows, pharmacy residents (Jocelyn Chaing, Michelle Lew, Esther Oh), and cardiac intensive care unit nurses of LAC+USC Medical Center for their help in the conduct of the study. Finally, thanks to Michael Bennett, Ph.D. and John Jefferies, M.D., at the Cincinnati Children's Hospital Medical Center for conducting the urinary NGAL assays.
Ng, T. M. H. , Grazette, L. P. , Fong, M. W. , Yoon, A. J. , Lou, M. , Kuo, A. , Upadhyay, R. Y. , Han, E. E. , Mehra, A. , and Elkayam, U. (2020) Tolvaptan vs. furosemide‐based diuretic regimens in patients hospitalized for heart failure with hyponatremia (AQUA‐AHF). ESC Heart Failure, 7: 1927–1934. 10.1002/ehf2.12783.
References
- 1. Asadollahi K, Beeching N, Gill G. Hyponatraemia as a risk factor for hospital mortality. QJM 2006; 99: 877–880. [DOI] [PubMed] [Google Scholar]
- 2. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009; 122: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital‐associated hyponatremia on selected outcomes. Arch Intern Med 2010; 170: 294–302. [DOI] [PubMed] [Google Scholar]
- 4. Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, Colby C, Shorr AF. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin 2008; 24: 1601–1608. [DOI] [PubMed] [Google Scholar]
- 5. Gill G, Huda B, Boyd A, Skagen K, Wile D, Watson I, van Heyningen C. Characteristics and mortality of severe hyponatraemia—a hospital‐based study. Clin Endocrinol (Oxf) 2006; 65: 246–249. [DOI] [PubMed] [Google Scholar]
- 6. Klein L, O'Connor CM, Leimberger JD, Gattis‐Stough W, Pina IL, Felker GM, Adams KF Jr, Califf RM, Gheorghiade M. Lower serum sodium is associated with increased short‐term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) study. Circulation 2005; 111: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 7. Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry. Eur Heart J 2007; 28: 980–988. [DOI] [PubMed] [Google Scholar]
- 8. Rossi J, Bayram M, Udelson JE, Lloyd‐Jones D, Adams KF, Oconnor CM, Stough WG, Ouyang J, Shin DD, Orlandi C, Gheorghiade M. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card Care 2007; 9: 82–86. [DOI] [PubMed] [Google Scholar]
- 9. Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Pina IL, Fonarow GC, DeMarco T, Pauly DF, Rogers J, DiSalvo TG, Butler J, Hare JM, Francis GS, Stough WG, O'Connor CM. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med 2007; 167: 1998–2005. [DOI] [PubMed] [Google Scholar]
- 10. Rusinaru D, Buiciuc O, Leborgne L, Slama M, Massy Z, Tribouilloy C. Relation of serum sodium level to long‐term outcome after a first hospitalization for heart failure with preserved ejection fraction. Am J Cardiol 2009; 103: 405–410. [DOI] [PubMed] [Google Scholar]
- 11. Ng TM, Cao DX, Patel KA, Wong YM, Prasad M, Lou M, Elkayam U. Association of hyponatremia to diuretic response and incidence of increased serum creatinine levels in hospitalized patients with acute decompensated heart failure. Cardiology 2014; 128: 333–342. [DOI] [PubMed] [Google Scholar]
- 12. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007; 297: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 13. Gheorghiade M, Gattis WA, O'Connor CM, Adams KF Jr, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 2004; 291: 1963–1971. [DOI] [PubMed] [Google Scholar]
- 14. Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D, Krasa HB, Maggioni A, Ouyang J, Swedberg K, Zannad F, Zimmer C, Udelson JE, Everest I. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail 2013; 19: 390–397. [DOI] [PubMed] [Google Scholar]
- 15. Udelson JE, Bilsker M, Hauptman PJ, Sequeira R, Thomas I, O'Brien T, Zimmer C, Orlandi C, Konstam MA. A multicenter, randomized, double‐blind, placebo‐controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. J Card Fail 2011; 17: 973–981. [DOI] [PubMed] [Google Scholar]
- 16. Ng TM, Hshieh S, Chan CY, Elkayam U. Clinical experience with low‐dose continuous infusion of furosemide in acute heart failure: assessment of efficacy and safety. J Cardiovasc Pharmacol Ther 2012; 17: 373–381. [DOI] [PubMed] [Google Scholar]
- 17. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008; 3: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett MR, Piyaphanee N, Czech K, Mitsnefes M, Devarajan P. NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatr Nephrol 2012; 27: 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah A, Terrell T, Arnold P, Bennett MR, Basu RK. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant 2016; 31: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, Orlandi C. Vasopressin V2‐receptor blockade with tolvaptan in patients with chronic heart failure: results from a double‐blind, randomized trial. Circulation 2003; 107: 2690–2696. [DOI] [PubMed] [Google Scholar]
- 22. Udelson JE, Orlandi C, Ouyang J, Krasa H, Zimmer CA, Frivold G, Haught WH, Meymandi S, Macarie C, Raef D, Wedge P, Konstam MA, Gheorghiade M. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo‐controlled trial. J Am Coll Cardiol 2008; 52: 1540–1545. [DOI] [PubMed] [Google Scholar]
- 23. Costello‐Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C, Burnett JC Jr. Vasopressin‐2‐receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol 2006; 290: F273–F278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuzaki M, Hori M, Izumi T, Asanoi H, Tsutamoto T. Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Cardiovasc Drugs Ther 2011; 25: S19–S31. [DOI] [PubMed] [Google Scholar]
- 25. Konstam MA, Kiernan M, Chandler A, Dhingra R, Mody FV, Eisen H, Haught WH, Wagoner L, Gupta D, Patten R, Gordon P, Korr K, Fileccia R, Pressler SJ, Gregory D, Wedge P, Dowling D, Romeling M, Konstam JM, Massaro JM, Udelson JE, Secret of Chf Investigators C, Committee M . Short‐term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol 2017; 69: 1409–1419. [DOI] [PubMed] [Google Scholar]
- 26. Felker GM, Mentz RJ, Cole RT, Adams KF, Egnaczyk GF, Fiuzat M, Patel CB, Echols M, Khouri MG, Tauras JM, Gupta D, Monds P, Roberts R, O'Connor CM. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 1399–1406. [DOI] [PubMed] [Google Scholar]
- 27. Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, Fujii H, Kitai T, Nishioka T, Sugi K, Onishi Y, Noda M, Kagiyama N, Satoh Y, Yoshida K, Goldsmith SR. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail 2016; 22: 423–432. [DOI] [PubMed] [Google Scholar]
- 28. Jujo K, Saito K, Ishida I, Furuki Y, Kim A, Suzuki Y, Sekiguchi H, Yamaguchi J, Ogawa H, Hagiwara N. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail 2016; 3: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldsmith SR. Future study with tolvaptan in acute heart failure. J Am Coll Cardiol 2017; 69: 467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study Procedure Time Points
DC = discontinuation of protocol; NT‐proBNP = N‐terminal pro‐b‐type natriuretic peptide; PRA = plasma renin activity; uNGAL = urinary neutrophil gelatinase‐associated lipocalin
Figure S2. Study Population CONSORT diagram
Table S1. Vital Statistics Comparison
Table S2. Incidence of Acute Increases in Serum Creatinine
Table S3. Electrolyte Changes Comparison


