Abstract
Aims
Right ventricular hypertrophy (RVH) is a common finding in Anderson–Fabry disease (AFD), but the prognostic role of right ventricular (RV) involvement has never been assessed. The aim of our study was to evaluate the prognostic significance of RVH and RV systolic function in AFD.
Methods and results
Forty‐five AFD patients (56% male patients) with extensive baseline evaluation, including assessment of RVH and RV systolic function, were followed‐up for an average of 51.2 ± 11.4 months. RV systolic function was assessed by standard and tissue Doppler echocardiography. Cardiovascular events were defined as new‐onset atrial fibrillation (AF), sustained ventricular arrhythmias, heart failure, or pacemaker/implantable cardioverter defibrillator implantation; renal events were defined as progression to dialysis and/or renal transplantation or significant worsening of glomerular filtration rate; and cerebrovascular events were defined as transient ischaemic attack or stroke.
Fourteen patients (31.1%) presented RVH, while RV systolic function was normal in all cases. During the follow‐up period, 13 patients (28.8%, 11 male) experienced 18 major events, including two deaths. Cardiovascular events occurred in eight patients (17.7%). The most common event was pacemaker/implantable cardioverter defibrillator implantation (six patients, 13.3%), followed by AF (three cases, 6.6%). Only one case of worsening New York Heart Association class (from II to III and IV) was observed. Ischaemic stroke occurred in three cases (6.6%). Renal events were recorded in three patients (6.6%). At univariate analysis, several variables were associated with the occurrence of events, including RVH (HR: 7.09, 95% CI: 2.17 to 23.14, P = 0.001) and indexes of RV systolic function (tricuspid annular plane systolic excursion HR: 0.77, 95% CI: 0.62 to 0.96, P = 0.02; and RV tissue Doppler systolic velocity HR: 0.76, 95% CI: 0.61 to 0.93, P = 0.01). At multivariate analysis, proteinuria (HR:8.3, 95% CI: 2.88 to 23.87, P < 0.001) and left ventricular mass index (HR: 1.02, 95% CI: 1.00 to 1.03, P = 0.03) emerged as the only independent predictors of outcome.
Conclusions
RVH and RV systolic function show significant association with clinical events in AFD, but only proteinuria and left ventricular mass index emerged as independent predictors of outcome. Our findings suggest that RV involvement does not influence prognosis in AFD and confirm that renal involvement and left ventricular hypertrophy are the main determinant of major cardiac and non‐cardiac events.
Keywords: Anderson–Fabry disease, RVH, RV systolic function, Prognosis
Introduction
Anderson–Fabry disease (AFD) is an X‐linked genetic lysosomal storage disease characterized by the progressive intracellular accumulation of neutral glycosphingolipids in different organs, including the heart.1 Cardiac involvement significantly contributes to morbidity and mortality in AFD patients, and left ventricular hypertrophy (LVH) is the main feature of overt cardiac involvement.1
The deposition of glycosphingolipids has been demonstrated in all cardiac cells2 , 3 leading to thickening of cardiac walls, including right ventricle (RV). However, few data concerning the clinical implications of RV involvement in AFD are currently available,4, 5, 6 and in particular, the prognostic implications of RV involvement have not been investigated so far.
We recently reported that right ventricular hypertrophy (RVH) parallels structural left ventricular changes and is correlated with LVH and overall disease severity, while RV systolic function is usually preserved, even in the presence of severe RVH.7
The prognostic value of RVH and RV systolic function has been clearly defined in other cardiomyopathies, including hypertrophic cardiomyopathy (HCM)8 and cardiac amyloidosis.9
In particular, RVH and RV systolic dysfunction are quite common in all types of cardiac amyloidosis, and systolic dysfunction represents a negative prognostic marker in this setting9 , 10 , 11 , 12
In the present study, we sought to investigate whether RVH and RV systolic function are associated with adverse prognosis in AFD patients.
Methods
Study population
We studied 45 genetically confirmed AFD patients belonging to 20 different families, referred to our Center for Rare Diseases, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy between September 2014 and December 2014.
All patients underwent an accurate clinical evaluation and standard 12‐lead electrocardiogram (ECG). Standard blood and urine laboratory analyses were also performed. Furthermore, serum N terminal pro brain type natriuretic peptide (NT‐proBNP) and Lyso GB3 (globotriaosylsphingosine) levels were obtained using an electrochemiluminescence method (Roche Diagnostics, Mannheim, Germany) and liquid chromatography/multiple reaction monitoring mass spectrometry method (Centogene, Rostock, Germany), respectively.
The Mainz severity score index, an accepted marker of global disease severity, was calculated as previously described.13
The FAbry STabilization indEX (Fastex), recently validated in a large population,14 was also calculated to assess stability or progression of the disease. The median interval between the two multidisciplinary evaluations used for Fastex calculation was 51.2 ± 11.4 months.
A Fastex score > 20% was considered indicative of clinical progression as previously reported.14
The study was in compliance with the Declaration of Helsinki. The research protocol was approved by the local Ethics committee, and informed written consent was obtained from all patients to participate in the study.
Electrocardiogram
An accurate analysis of standard ECG was performed, and the following ECG parameters were obtained: PR interval, QRS duration, QT interval, presence of LVH, and abnormalities in ST‐segment and/or T wave changes. LVH at ECG was defined according to Sokolov–Lyon criteria (S wave depth in V1 + tallest R wave height in V5‐V6 > 35 mm). ST‐segment/T wave abnormalities were considered as follows: ST‐segment depression/elevation ≥ 0.1 mV below or above the baseline at the J‐point in at least two leads, except V1‐V2‐V3 where it was considered only when ≥0.2 mV; T waves abnormalities considered were inversion (when the negative T wave amplitude was ≥0.1 mV), giant negative (when amplitude was ≥1.0 mV), and giant positive (defined as symmetrical positive T waves ≥1 mV).15
Echocardiography
All patients underwent an accurate complete M‐mode and B‐mode colour Doppler echocardiographic examination using a Toshiba Artida ultrasound system (TA‐700; Toshiba, Tokyo, Japan) equipped with a 3.5 MHz probe, according to American Society of Echocardiography guidelines.16 , 17 , 18
Echocardiographic data were acquired and analysed by two expert independent operators (FG and RL). Data were acquired with patients lying down in the left lateral supine position, at rest. Measurements were averaged over three heartbeats in sinus rhythm and five heartbeats in atrial fibrillation. Echocardiographic M‐mode and bidimensional parameters evaluated were LV end‐diastolic diameter, LV end‐systolic diameter, septal wall thickness, and LV posterior wall thickness. The greatest wall thickness measured at any site in the LV wall was regarded as the maximal wall thickness. LVH was defined according to LV mass index (LVMi): 95 g/m2 for women and 115 g/m2 for men. Chamber volumes were measured from the apical four‐chamber and two‐chamber views in all patients to obtain left atrium, right atrium, and LV end‐systolic and end‐diastolic volumes. LV ejection fraction (LVEF) was estimated using the biplane Simpson method, and it was considered normal if >55%.
A comprehensive assessment of RV geometry and systolic function was obtained from apical four‐chamber, RV‐focused apical four‐chamber, and modified apical four‐chamber and subcostal views.17 Two‐dimensional linear and two‐dimensional‐guided M‐mode measurements of RV free wall thickness were performed from the subcostal view at end‐diastole, and RVH was defined as RV free wall thickness >5 mm.18 Tricuspid annular plane systolic excursion (TAPSE) was recorded, and a value <17 mm was considered suggestive of RV systolic dysfunction. RV fractional area change (RV FAC) was assessed to provide an estimate of global RV systolic function. RV FAC < 35% was considered indicative of RV systolic dysfunction.17
Pulsed wave Doppler recordings of mitral and tricuspid inflow velocities were obtained. Systolic (septal Sa and lateral Sa), early diastolic (Ea), and late diastolic (Aa) tissue Doppler velocities were measured using pulsed spectral mode to record mitral annular velocities at septal and lateral corners. Normal septal Sa was considered >8.5 cm/s, and normal lateral Sa was considered >10 cm/s.18 Pulsed spectral Doppler was also applied to the RV free wall at the tricuspid annular level to obtain RV Sa wave as an adjunctive measure of RV longitudinal systolic function: an RV Sa velocity < 9.5 cm/s measured on the free wall was considered suggestive of RV systolic dysfunction.16 Myocardial performance index was obtained by colour tissue Doppler recordings by the sum of the isovolumetric contraction and relaxation time divided by the ejection time and a value > 0.54 was considered abnormal.16
Follow‐up and events
All patients underwent clinical evaluation at 1‐year intervals, unless differently indicated because of the changes in clinical conditions. The primary clinical endpoint of the study was the occurrence of any major cardiac and non‐cardiac event, including death.
Cardiac events were considered heart failure (HF), pacemaker (PM)/ICD implantation or new‐onset atrial fibrillation (AF), and sustained ventricular arrhythmias. HF was defined as hospitalization for symptoms/signs of HF or worsening of New York Heart Association (NYHA) class.
Cerebrovascular events were defined as ischaemic stroke or transient ischaemic attack.
Renal events were defined as progression to renal dialysis or renal transplantation or significant worsening of glomerular filtration rate (defined as decline of >3 mL/min/m2 per year).
Fastex index indicated a clinical progression in 21 cases (46.6%) and clinical stability in 24 cases (53.3%).
Statistics
Data were analysed with the SPSS statistical software version 21.1 (SPSS Inc., Florence, Italy). Continuous variables are presented as mean ± SD, and categorical variables as numbers and percentages.
The association of variables with the primary endpoint of major adverse clinical events (MACEs) was assessed by univariable Cox regression. Multivariable Cox regression analyses were then performed to identify variables independently associated with MACE.
To this scope, because in the study there were too many variables that showed a significant association with MACE and there was a limited number of MACE during follow‐up, in order to limit overfitting of the regression models, we initially performed separate multivariable analyses for three categories of variables, i.e. clinical, ECG, and echocardiographic variables, in order to identify those independently predictive of MACE for each category.
For these analyses, a forward multivariable Cox regression was applied, progressively including variables in the models with decreasing P value at univariable analysis and removing variables with P > 0.1. This allowed to limiting the number of variables included at each step in the models derived for each of the three categories of variables. Only variables with P value < 0.1 at univariable analysis were tested in multivariable analyses.
Then, a final global multivariable Cox regression analysis was performed, including in the model only the variables that, for each of the three categories, remained significantly associated (P < 0.05) with MACE in each of the three multivariable regression models.
Data are presented as mean (SD) or number (percentage). A P value < 0.05 was always required for statistical significance.
Results
Patient's characteristics
Main demographic, clinical, ECG, and echocardiographic characteristics of patients are reported in Table 1. The population consisted of 45 patients, 25 of whom (55.5%) were men; mean age was 52 ± 16 years. Twelve patients (26.6%) had a late‐onset type of the disease because of N215S mutation (cardiac variant). Thirty‐two patients (71.1%) were on enzyme replacement therapy (ERT), 24 (53.3%) with agalsidase‐alpha 0.2 mg/kg of body weight every 2 weeks, and 7 (15.5%) with agalsidase‐beta 1.0 mg/kg of body weight every 2 weeks. One patient (2.2%) was in treatment with migalastat.
Table 1.
Demographic, clinical, and echocardiographic features of the Fabry disease cohort
| Total | Men (n = 25) | Women (n = 20) | |
|---|---|---|---|
| Age | 52 ± 16 | 51.4 ± 13.6 | 51.8 ± 18.7 |
| BMI, kg/m2 | 24.9 ± 3.7 | 23.6 ± 3.1 | 26.6 ± 3.9 |
| Hypertension | 16 (35.5%) | 11 (44%) | 5 (25%) |
| GFR mL/min/1.73 m2 | 87.6 ± 31.1 | 81.9 ± 36.3 | 94.7 ± 21.7 |
| Cardiac variant | 12 (26.6%) | 8 (32%) | 4 (20%) |
| Cardiac variant with LVH | 8 (17.7%) | 7 (28%) | 1 (5%) |
| Systolic BP, mmHg | 121.4 ± 14 | 124.2 ± 13.7 | 118 ± 13.8 |
| Diastolic BP, mmHg | 76.7 ± 9 | 77 ± 8.6 | 76.5 ± 9.8 |
| Proteinuria 24 h, g/L | 0.25 ± 0.39 | 0.25 ± 0.26 | 0.24 ± 0.52 |
| MSSI | 21 ± 14.7 | 28.2 ± 15.1 | 12 ± 7.8 |
| Echocardiography | |||
| RVH | 14 (31.1) | 12 (48%) | 2 (10%) |
| RVWT, mm | 4.4 ± 2 | 5.1 ± 2.2 | 3.4 ± 1.1 |
| TAPSE, mm | 21.7 ± 3.2 | 21.3 ± 3.4 | 22.2 ± 2.8 |
| RV FAC, % | 47.9 ± 6.4 | 45.9 ± 6.4 | 50.3 ± 5.8 |
| RV Sa, cm/s | 13.2 ± 2.2 | 12.6 ± 2.2 | 13.8 ± 1.9 |
| RA, mL | 44.5 ± 20 | 48.6 ± 20.9 | 30.9 ± 13.2 |
| LVEF, % | 62.6 ± 6.1 | 60.3 ± 6.3 | 65.5 ± 4.6 |
| LVWT, mm | 13.2 ± 5 | 15.8 ± 5.2 | 10.1 ± 2.1 |
| LVMi, g/m2 | 135.2 ± 78 | 171.6 ± 85.9 | 89.8 ± 29.4 |
| LVH | 22 (48.8%) | 15 (60%) | 7 (35%) |
| LAVi, ml/m2 | 36 ± 13.2 | 38.8 ± 14 | 30 ± 4.3 |
| E/e′ | 8.3 ± 4.2 | 9.6 ± 5 | 6.7 ± 2 |
| Septal Sa, cm/s | 7.6 ± 1.9 | 6.9 ± 1.8 | 8.6 ± 1.4 |
| Lateral Sa, cm/s | 8.3 ± 2 | 7.9 ± 2 | 8.8 ± 1.9 |
| Medications | |||
| ERT | 32 (71.1%) | 22 (88%) | 10 (50%) |
| Agalsidase alfa | 24 (53.3%) | 16 (64%) | 8 (40%) |
| Agalsidase 𝜷 | 7 (15.5%) | 5 (20%) | 2 (10%) |
| Migalastat | 1 (2.2%) | 1 (4%) | 0 |
| ACE I/sartans | 16 (35.5%) | 10 (40%) | 6 (30%) |
| 𝜷 blockers | 8 (17.7%) | 7 (28%) | 1 (5%) |
| Ca2+ antagonists | 4 (8.8%) | 2 (8%) | 2 (10%) |
| Antiplatelet drugs | 16 (35.5%) | 11 (44%) | 5 (25%) |
| Anticoagulant | 2 (4.4%) | 1 (4%) | 1 (5%) |
| Statins | 4 (8.8%) | 4 (16%) | 0 |
BMI, body mass index; ERT, enzyme replacement therapy; GFR, glomerular filtration rate calculated with the modification of diet in renal disease study (MDRD) equation; MSSI, Mainz severity score index; lateral Sa, lateral tissue Doppler systolic velocity; LAVi, left atrium volume index; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMi, left ventricular mass index; LVWT left ventricular wall thickness; RA, right atrium volume; RV FAC, right ventricle fractional area change; RV Sa, right ventricle tissue Doppler systolic velocity; RVH, right ventricular hypertrophy; RVWT, right ventricular wall thickness; Septal Sa, septal tissue Doppler systolic velocity; TAPSE, tricuspid anular plane systolic excursion.
Eight patients had previously experienced a major event before enrolment in the study: three patients (6.6%) had history of ischaemic strokes, three patients of previous atrial fibrillations (one paroxysmal and two persistent), and three (6.6%) of kidney transplant (one of them had also experienced atrial fibrillation). Sixteen patients (35.5%) were treated for arterial hypertension. The analysis of ECG variables showed first degree atrio‐ventricular block in 5 patients (11.1%), prolonged QRS duration in 11 patients (24.4%), LVH in 25 patients (55.5%), and ST‐segment T wave abnormalities in 20 cases (44.4%). Short PR interval was present in three patients (6.6%).
Echocardiography showed LVH in 22 patients (48.8%), 7 female patients. In 15 (33.3%) patients (3 female patients and 12 male patients), LVH was severe (defined as LVMi > 122 g/m2 in female patients and >149 g/m2 in male patients). Only four patients (8.8%) had an LVEF < 55%; all of them, however, had LVEF ranging between 48 and 53%. RVH was documented in 14 patients (31.1%). TAPSE and RV FAC values were normal in all patients. RV Sa velocity was normal in all patients but one.
Left atrial volume was within the normal range (left atrium volume index < 34 mL/m2) in 35 patients (77.7%). The majority of patients had grades I (20 patients, 44.4%) and II (three patients, 6.6%) diastolic dysfunction, while no patients had grade III diastolic dysfunction.
Follow‐up
Over an average follow‐up period of 51.2 ± 11.4 months, we recorded 18 major clinical events in 13 patients (28.8%, 10 male patients). Clinical, ECG, and echocardiographic characteristics of the patients who experienced events are shown in Table 2.
Table 2.
Clinical and echocardiographic characteristics of patients with events
| Family | Age/gender | a‐GAL‐A activity on leukocytes nmol/mg/h | a‐GAL‐A activity on leukocytes % | GLA mutation | GAL A protein effect | NYHA | GFR (MDRD, mL/min/1.73 m2) | Proteinuria (g/L/24 h) | LVWT (mm) | LVMi (g/m2) | RVWT (mm) | Fastex score | CV events | Non‐CV events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 M | 4.04 | 9,52 | c.548G > C | p. Gly183Ala | II | 36.6 | 0.69 | 26 | 426.39 | 10 | 55 | PM | ‐ |
| 1 | 55/M | 0.95 | 2,23 | c.548G > C | p. Gly183Ala | II | 50.7 | 1.02 | 20 | 195 | 7 | 60 | AF | ‐ |
| 2 | 43/F | n.a. | c.758 T > C | p.Ile253Thr | III | 68.9 | 2.39 | 13 | 93 | 2.5 | 100 | ‐ | eGFR decline, Stroke | |
| 2 | 68/M | 0.8 | 1.98 | c.758T > C | p.Ile253Thr | III | 92 | 0.57 | 17 | 185.7 | 4 | 100 | ‐ | Stroke |
| 3 | 52/M | 2.08 | 4.90 | c.639 + 1G > A | splicing alteration | II | 21 | 18 | 239.6 | 6 | 0 | ‐ | eGFR decline (dialysis) | |
| 4 | 69/F | n.a. | c.644A > G | p.(ans215Ser) | III | 77 | 0.59 | 13 | 122.6 | 6 | 50 | ‐ | Death | |
| 5 | 66/F | n.a. | c.680G > A | p.(arg227Gln) | II | 92.3 | 0.13 | 13 | 141.8 | 6 | 75 | PM, AF | ‐ | |
| 6 | 70/M | 7.72 | 18.16 | c.644A > G | p.(ans215Ser) | II | 66.6 | 0.09 | 23 | 268.3 | 7.5 | 85 | PM, AF | ‐ |
| 7 | 78/M | 2,0 | 4,70 | c.644A > G | p.(ans215Ser) | II | 58.6 | 0.07 | 28 | 301.9 | 7.2 | 65 | PM | ‐ |
| 8 | 50/M | 3,25 | 7,66 | c.747C > A | p.(Ans249Lys) | II‐III | 98.2 | 0.44 | 25 | 281.7 | 7.7 | 100 | Worsening NHYA | ‐ |
| 9 | 51/M | 2.45 | 5,78 | c.547 + 1G > T | Splicing alteration | II | 24.2 | 18 | 220.5 | 5 | 55 | PM | Kidney TX | |
| 10 | 50/M | 6,55 | 15,41 | c.644° > G | p.(ans215Ser) | I | 62 | 0.7 | 12 | 95.8 | 3.1 | 95 | PM | ‐ |
| 5 | 39/M | 0.63 | 1,49 | c.680G > A | p.(arg227Gln) | I | 182 | 0.15 | 12 | 104.1 | 2.5 | 35 | ‐ | Death, Stroke |
AF, atrial fibrillation; GFR, glomerular filtration rate calculated with The Modification of Diet in Renal Disease Study (MDRD) equation; LVMi left ventricular mass index; LVWT, left ventriclular wall thickness; PM, pacemaker implantation; RVWT, right ventricular wall thickness.
The Fastex score was calculated using the available online tool (www.fastex.online). The normal range for a‐GAL‐A activity is 20 to 65 nmol/mg/hr. a‐GAL‐A activity was not measured in female patients. We report proteinuria dosage only for patients with GFR ≥ 30 mL/min/m2 according to MDRD method.
Events included two deaths (4.4%), both not apparently related to AFD (one patient with severe neurologic impairment died from sepsis and one died from non‐Hodgkin lymphoma).
Cardiovascular events occurred in eight patients (17.7%). The most common event was PM implantation (six patients, 13.3%; Figure 1 ); the second most common event was AF (three cases, 6.6%). We reported only one case of worsening NYHA class (from II to III and IV). The patient with NYHA progression was affected by severe angina in the absence of obstructive coronary artery disease, occurring for minimal effort and associated to documented transient LV systolic dysfunction. No patients were hospitalized for HF, and no sustained ventricular arrhythmia occurred. Ischaemic stroke occurred in three cases (6.6%). Renal events were observed in three patients (6.6%): one patient underwent renal transplantation, and two patients presented significant worsening of glomerular filtration rate with one case of progression to renal dialysis. Among the eight patients who had cardiovascular events, 7 (87.5%) experienced a cardiovascular event as their only major clinical event during the follow‐up period. No life‐threatening ventricular arrhythmias were recorder on yearly ECG Holter monitoring. Among the 13 patients presenting events, 12 (92.3%) presented a Fastex > 20%, reflecting disease progression. On the contrary, only nine patients (20%) with no clinical events presented a Fastex > 20%.
Figure 1.
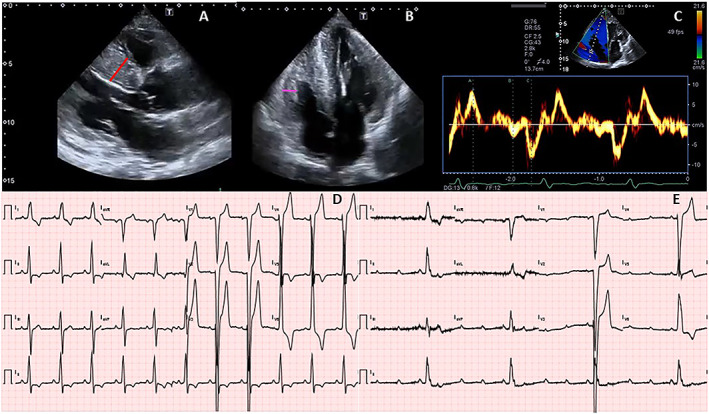
Example of a patient experiencing a major event (complete heart block requiring pacemaker implantation). (A) Parasternal long‐axis view; left ventricular hypertrophy is evident, with a maximun septal thickness of 26 mm (red line). (B) Four‐chamber view; biventricular hypertrophy is clear, with a right ventricular free wall thickness of 10 mm (purple line). (V) Tissue Doppler interrogation of the right ventricle, with normal S values (9.5 cm/s). (D) Twelve‐lead electrocardiogram (ECG), performed the same day of the echocardiogram. The ECG shows sinus rythm with heart rate 65 bpm, normal PR interval (187 ms), delayed intraventricular conduction, and LVH with repolarization abnormalities. (E) Twelve‐lead ECG recorded when the patient presented to the emergency room for dizziness, 30 months after the initial evaluation, showing complete heart block with heart rate of 24 bpm.
Table 3 shows the results of univariable and separate multivariable survival Cox regression analyses for clinical, ECG, and echocardiographic variables. Although several variables were associated with the primary endpoint, among clinical variables, only Mainz severity score index score and proteinuria emerged as independent predictors. Among ECG and echocardiography variables, the presence and degree of RVH as well as all the indices of RV systolic function were associated with events; however, only QRS duration and LVMi showed an independent association with the primary endpoint within each category of variables considered for analysis.
Table 3.
Univariable and separate multivariable survival Cox regression analyses for clinical, electrocardiogram, and echocardiographic variables related to the occurrence of major events
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Clinical variables | ||||||
| Age | 1.02 | 0.99–1.06 | ||||
| Sex | 3.27 | 0.89–11.89 | 0.07 | |||
| BMI | 0.96 | 0.82–1.12 | ||||
| MSSI | 1.07 | 1.03–1.11 | <0.001 | 1.08 | 1.01–1.15 | 0.01 |
| GFR MDRD (mL/min/1.73 m2) | 0.97 | 0.95–0.99 | 0.008 | |||
| Proteinuria (g/L) | 4.47 | 2.11–9.45 | <0.001 | 5.6 | 1.46–21.8 | 0.01 |
| NYHA class | 2.86 | 1.52–5.40 | 0.001 | |||
| Hypertension | 5.34 | 1.63–17.45 | 0.006 | |||
| ECG parameters | ||||||
| QRS (ms) | 1.03 | 1.01–1.05 | 0.001 | 1.03 | 1.00–1.05 | 0.006 |
| PR (ms) | 1.02 | 1.00–1.03 | 0.03 | |||
| Repolarization abnormalities | 3.38 | 1.04–11.00 | 0.04 | |||
| LVH | 3.51 | 1.36–9.06 | 0.009 | |||
| cQT (ms) | 1.01 | 0.99–1.03 | ||||
| HR | 0.95 | 0.91–1.00 | 0.08 | |||
| Echocardiographic parameters | ||||||
| RVWT (mm) | 1.40 | 1.12–1.76 | 0.003 | |||
| RVH | 7.09 | 2.17–23.14 | 0.001 | |||
| RV Sa (cm/s) | 0.76 | 0.61–0.93 | 0.01 | |||
| TAPSE (mm) | 0.77 | 0.62–0.96 | 0.02 | |||
| RV E/A ratio | 0.13 | 0.01–1.43 | 0.09 | |||
| LVWT (mm) | 1.20 | 1.10–1.31 | <0.001 | |||
| LVMi (g/m2) | 1.01 | 1.00–1.01 | <0.001 | 1.01 | 1.00–1.03 | 0.03 |
| LAVi (mL/m2) | 1.02 | 1.00–1.05 | 0.04 | |||
| LVEF (%) | 0.85 | 0.78–0.92 | <0.001 | |||
| LV E/A ratio | 0.27 | 0.07–0.94 | 0.04 | |||
| E/e′ | 1.12 | 1.03–1.22 | 0.009 | |||
| Septal Sa (cm/s) | 0.59 | 0.43–0.82 | 0.002 | |||
| Lateral Sa (cm/s) | 0.66 | 0.49–0.89 | 0.006 | |||
BMI, Body mass index; HR, heart rate; LVH, ECG signs of left ventricle hypertrophy according to Sokolow and Lyon criterion; lateral Sa, lateral systolic tissue Doppler velocity; LAVi, left atrium volume index; LVEF, left ventricle ejection fraction; LVMi, left ventricular mass index; LVWT, left ventricular wall thickness; MSSI, Mainz severity score index; RV Sa, right ventricle systolic tissue Doppler velocity; RVH, right ventricle hypertrophy defined as right ventricle wall thickness > 5 mm; RVWT, right ventricular wall thickness; Septal Sa, septal systolic tissue Doppler velocity; TAPSE, tricuspid annular plane systolic excursion.
Table 4 summarizes the results of multivariable Cox regression analysis, including only the variables found independently correlated with the primary endpoint at separate multivariable analyses of each category. As shown, only proteinuria (HR: 8.3, 95% CI: 2.88 to 23.87, P < 0.001) and LVMi (HR: 1.02, 95% CI: 1.00 to 1.03, P = 0.03) emerged as significant risk factors in the stepwise selection of covariates for the multivariate model. The Kaplan–Meier curves demonstrated a significant difference in the event‐free survival rates between patients with and without LVH (P = 0.018, Figure 2 A) and patients with and without high proteinuria levels (P = 0.001, Figure 2 B).
Table 4.
Multivariable Cox regression analysis results
| Multivariate analysis | |||
|---|---|---|---|
| Hazard ratio | 95% CI | P | |
| MSSI | 1.01 | 0.94–1.08 | ‐ |
| Proteinuria (g/L) | 8.30 | 2.88–23.87 | <0.001 |
| LVMi | 1.02 | 1.00–1.03 | 0.03 |
| QRS (ms) | 1.00 | 0.98–1.03 | ‐ |
LVMi, left ventricular mass index; MSSI, Mainz severity score index.
Figure 2.
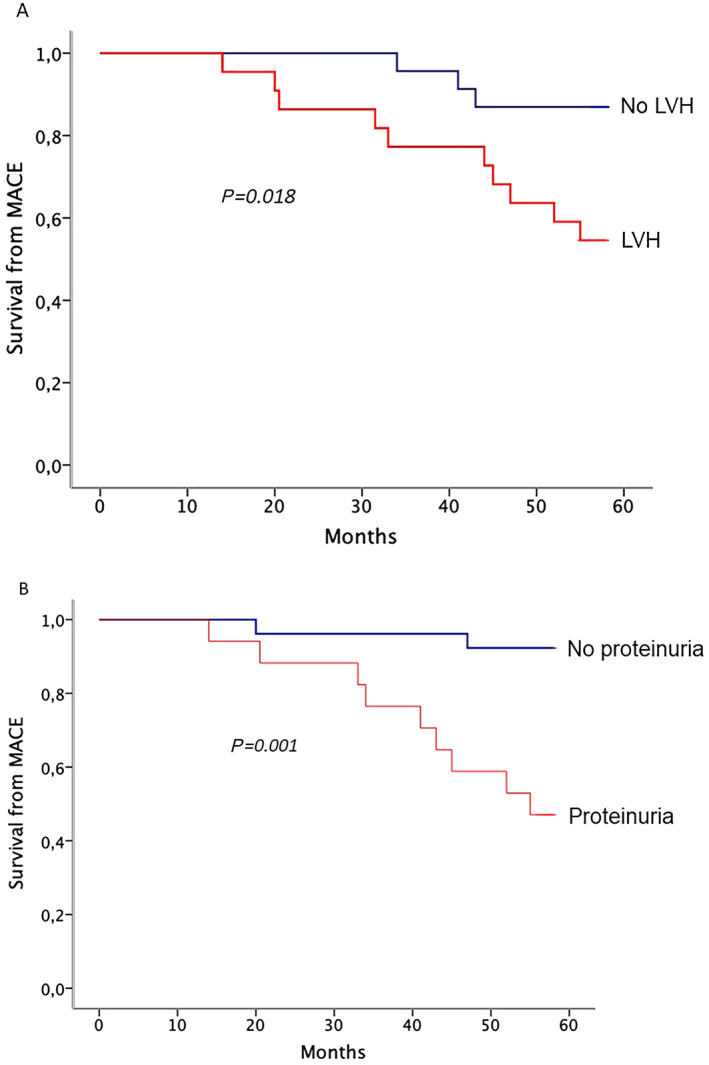
(A) Kaplan–Meier event‐free survival curves for occurrence of major adverse clinical events in patients with (red) and without (blue) left ventricular hypertrophy. (B) Kaplan–Meier event‐free survival curves for occurrence of major adverse clinical events in patients with (red) and without (blue) high proteinuria levels.
Discussion
In the present study we explored for the first time the possible association of RVH and RV systolic function with major clinical events in patients with AFD.
Our data show that both RVH and RV systolic function were associated with clinical outcome in AFD at univariate analysis, together with several other clinical, electrocardiographic, and echocardiographic variables. However, only proteinuria and LVMi emerged as independent predictors of outcome in our patients.
We previously found that RV involvement is common in AFD and is associated with LVH and global disease severity. However, RV systolic function is usually preserved in AFD patients, at variance with patients affected by cardiac amyloidosis, who exhibit similar levels of LVH and RVH.
Indeed, in our population, all indices of RV systolic function were within the normal range even when severe RVH was present,7 which is in contrast with the subclinical longitudinal systolic dysfunction of the LV that is usually found even in the absence of LVH.19
We hypothesize that accumulation of Gb3 in longitudinal myocardial fibres, which are more prevalent in the left ventricle, accounts for the early abnormalities detectable by tissue Doppler, while the different fibre arrangement and orientation in the RV free wall could explain the preserved RV systolic function even in the presence of significant RVH.
Our findings are in line with most echocardiographic studies assessing cardiac involvement in AFD, although a high prevalence of impaired RV systolic function was reported in a study.4 Of note, in a recent cardiac magnetic resonance study, Niemann et al.20 confirmed that the degree of RV involvement in AFD is related to the cardiomyopathy stage, showing no evidence of fibrosis. The authors also showed that ERT has no direct impact on the RV, with morphological and functional RV parameters remaining unchanged both in patients treated with ERT as well as in untreated patients during 2.3‐year follow‐up.20
The lack of correlation between RVH and systolic function with prognosis is in contrast with the findings recently reported in cardiac amyloidosis, in which RV systolic function was found to be a strong prognostic determinant10 with lower TAPSE values being associated with worse clinical outcome. In cardiac AL amyloidosis patients, Ghio et al.11 showed that TAPSE < 17 mm identifies a subgroup with poor prognosis. In this study, despite similar RV wall thickness, patients with lower TAPSE had thicker LV walls, suggesting that LV involvement itself, rather than RV amyloid infiltration, is an important determinant of RV systolic dysfunction.11 Similarly, Bodez et al. showed that TAPSE < 14 mm independently predicted major cardiac events in different types of cardiac amyloidosis.12 The independent predictors of low TAPSE were N terminal pro brain type natriuretic peptide, estimated LV filling pressures, LVEF, and pulmonary pressures but not RV late gadolinium enhancement. Cappelli et al.9 found that in cardiac AL amyloidosis, RV longitudinal systolic dysfunction is a negative prognostic marker, and interventricular septum thickness was the only independent predictor of RV longitudinal function. The authors speculated that ventricular interdependence rather than disease extension or RV afterload changes could have a prominent role in inducing the deterioration of RV systolic function.9 RVH occurs irrespective of systolic pulmonary artery pressure also in HCM, and the presence of RVH was found to independently predict the occurrence of cardiovascular events in these patients. Interestingly, even in HCM, the extent of RVH seems to significantly correlate with LVMi, and the role of ventricular interdependence has been advocated as well8 , 21 , 22
In our study, all AFD patients with RVH also had LVH, and RVH was linearly associated with LVH, confirming that RVH parallels the degree of left ventricular involvement. However, RV systolic function was preserved in all patients, thus suggesting that the mechanisms of ventricular interdependence does not play a major role in AFD; even patients with the thickest septum (>25 mm), indeed, presented normal indices of RV systolic function.
On the other hand, an afterload increase is unlikely to be responsible for RVH in AFD, as severe diastolic dysfunction is rarely observed, and pulmonary pressures is usually within the normal range until progression to dilated cardiomyopathy, which was absent in our population.
Our findings suggest that RVH recognizes different mechanisms in AFD as compared with other forms of LVH such as cardiac amyloidosis and HCM, likely related to intramyocardial storage with no or little role for afterload increase and/or ventricular interdependence. In such a scenario, it is reasonable that LVH is such a powerful predictor of clinical events, that RV involvement has no additional role in determining prognosis. However, lack of statistical association should not be interpreted as a proof of not being associated. The small sample size might not have been sufficient to detect an independent association of RV involvement with events, and our data need to be confirmed in larger, specifically designed, study.
The main finding of our work was that proteinuria emerged as the strongest independent predictor of adverse events, together with LVMi, confirming once again that cardiac and renal involvement drive prognosis in AFD. There is a strong literature showing a synergistic effect of renal and cardiac involvement in the outcome of Fabry patients. 24 , 25 , 26 , 27 , 28
Recently, Siegenthaler et al.23 found that cardio‐renal syndrome is associated with a high risk of complications (composite outcome of renal replacement therapy, hospitalization for HF, new onset AF, PM/implantable cardioverter defibrillator implantation, stroke/transient ischaemic attack, and death) in a prospective multicenter cohort of 104 AFD patients. The authors suggest that cardiac and renal involvement may reflect an advanced Gb3 deposition in vital organs; moreover, pathophysiological mechanisms beyond Gb3 deposition may enhance the vicious circle of cardio‐renal syndrome in AFD, being very difficult to differentiate between the effects of AFD itself and the effects of inter‐organ crosstalk.
Of note, renal disease is an independent predictor of LVH in AFD patients, and the presence of an end‐stage renal disease at the initiation of ERT is significantly associated with cardiovascular disease progression. LVH, on the other hand, has been confirmed to be a significant predictor of cardiovascular events in a recent large‐scale registry‐based long‐term study.24 Our findings confirm the role of renal and cardiac involvement in the prediction of cardiac and non‐cardiac events, emphasizing the importance of proteinuria and LVH in risk stratification of AFD patients.
It is noteworthy that most frequent cardiac events in our study consisted of clinically relevant brady‐arrythmias, requiring PM implantation, which mainly occurred in male patients with overt cardiomyopathy and receiving ERT. Thus, our data confirm that in patients with the most severe form of AFD, major events may still occur despite ERT and optimal conventional therapy.25
Limitations of the study
The main limitation of our study is the small sample size, a common issue in rare diseases, which carries some statistical implications. Our data show an association of RV involvement with clinical outcome, thus indicating that our sample size allowed detecting differences in RV hypertrophy and systolic function between patients with and without events. However, at the multivariate analysis, we did not find an independent association between RV variables and events: we cannot exclude that this result could be related to the small sample size. Moreover, we tested the prognostic significance of RV involvement to predict cardiac and non‐cardiac events. We are aware that a careful assessment of the endpoints should have been performed, but the low rate of each event, occurred in few patients, would have turned the statistic power too low to detect significant reliable associations.
A technical limitation was that we could not perform three‐dimensional echo evaluation of the RV, as this technique was not available at the time of baseline evaluation. Nevertheless, it is unlikely that significant abnormalities of RV function could have been detected by this technique, as multiparametric evaluation of RV systolic function revealed normal indices in all patients.
RV function was assessed indirectly by TAPSE and RV FAC; however, several data have shown an excellent correlation of these parameters with RV ejection fraction estimated with cardiac magnetic resonance.29 , 30 Furthermore, there is solid evidence that TAPSE is a strong independent predictor of prognosis in different clinical settings.31 , 32
Conclusions
In our study, both RVH and RV systolic function showed significant association with clinical events in AFD, but only proteinuria and LVMi emerged as independent predictors of outcome. These findings confirm that renal involvement and LVH drive prognosis in AFD, predicting major cardiac and non‐cardiac events. The presence and extension of RVH reflect the stage of cardiac involvement in AFD, but, at variance with other phenocopies, RVH and RV systolic function are not independently associated with outcome.
Conflict of interest
Francesca Graziani: honoraria for board meetings and travel support from Amicus Therapeutics, Sanofi‐Genzyme, and Shire. Antonia Camporeale: honoraria for presentations and board meetings from Amicus Therapeutics, Sanofi‐Genzyme, and Shire; and research grant from Amicus Therapeutics. Maurizio Pieroni: speaker and advisory board honoraria and travel support from Sanofi‐Genzyme, Amicus Therapeutics, and Shire. Rosa Lillo: honoraria for board meetings and travel support from Amicus Therapeutics and Shire.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Graziani, F. , Lillo, R. , Panaioli, E. , Pieroni, M. , Camporeale, A. , Verrecchia, E. , Sicignano, L. L. , Manna, R. , Lombardo, A. , Lanza, G. A. , and Crea, F. (2020) Prognostic significance of right ventricular hypertrophy and systolic function in Anderson–Fabry disease. ESC Heart Failure, 7: 1605–1614. 10.1002/ehf2.12712.
References
- 1. Linhart A, Elliott PM. The heart in Anderson‐Fabry disease and other lysosomal storage disorders. Heart 2007; 93: 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry's disease: ceramidetrihexosidase deficiency. N Engl J Med 1967; 276: 1163–1167. [DOI] [PubMed] [Google Scholar]
- 3. Sheppard MN, Cane P, Florio R, Kavantzas N, Close L, Shah J, Lee P, Elliott P. A detailed pathologic examination of heart tissue from three older patients with Anderson‐Fabry disease on enzyme replacement therapy. Cardiovasc Pathol 2010; 19: 293–301. [DOI] [PubMed] [Google Scholar]
- 4. Kampmann C, Baehner FA, Whybra C, Bajbouj M, Baron K, Knuf M, Wiethoff CM, Trübel H, Beck M. The right ventricle in Fabry disease. Acta Paediatric Int J Paediatric Supp 2005; 94: 15–18. [DOI] [PubMed] [Google Scholar]
- 5. Palecek T, Dostalova G, Kuchynka P, Karetova D, Bultas J, Elleder M, Linhart A. Right ventricular involvement in Fabry disease. J Am Soc Echocardiogr 2008; 21: 1265–1268. [DOI] [PubMed] [Google Scholar]
- 6. Wuest W, MacHann W, Breunig F, Weidemann F, Koestler H, Hahn D, Wanner C, Beer M. Right ventricular involvement in patients with Fabry disease and the effect of enzyme replacement therapy. RoFo Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgeb Verfahren 2011; 183: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 7. Graziani F, Laurito M, Pieroni M, Pennestrì F, Lanza GA, Coluccia V, Camporeale A, Pedicino D, Verrecchia E, Manna R, Crea F. Right ventricular hypertrophy, systolic function, and disease severity in Anderson‐Fabry disease: an echocardiographic study. J Am Soc Echocardiogr 2017; 30: 282–291. [DOI] [PubMed] [Google Scholar]
- 8. Nagata Y, Konno T, Fujino N, Hodatsu A, Nomura A, Hayashi K, Nakamura H, Kawashiri MA, Yamagishi M. Right ventricular hypertrophy is associated with cardiovascular events in hypertrophic cardiomyopathy: evidence from study with magnetic resonance imaging. Can J Cardiol 2015; 31: 702–708. [DOI] [PubMed] [Google Scholar]
- 9. Cappelli F, Porciani MC, Bergesio F, Perlini S, Attanà P, Pignone AM, Salinaro F, Musca F, Padeletti L, Perfetto F. Right ventricular function in AL amyloidosis: characteristics and prognostic implication. Eur Heart J Cardiovasc Imaging 2012; 13: 416–422. [DOI] [PubMed] [Google Scholar]
- 10. Bellavia D, Pellikka PA, Dispenzieri A, Scott CG, Al‐Zahrani GB, Grogan M, Pitrolo F, Oh JK, Miller FA Jr. Comparison of right ventricular longitudinal strain imaging, tricuspid annular plane systolic excursion, and cardiac biomarkers for early diagnosis of cardiac involvement and risk stratification in primary systematic (AL) amyloidosis: a 5‐year cohort stud. Eur Heart J Cardiovasc Imaging 2012; 13: 680–689. [DOI] [PubMed] [Google Scholar]
- 11. Ghio S, Perlini S, Palladini G, Marsan NA, Faggiano G, Vezzoli M, Klersy C, Campana C, Merlini G, Tavazzi L. Importance of the echocardiographic evaluation of right ventricular function in patients with AL amyloidosis. Eur J Heart Fail 2007; 9: 808–813. [DOI] [PubMed] [Google Scholar]
- 12. Bodez D, Ternacle J, Guellich A, Galat A, Lim P, Radu C, Guendouz S, Bergoend E, Couetil JP, Hittinger L, Dubois‐Randé JL, Plante‐Bordeneuve V, Deux JF, Mohty D, Damy T. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid 2016; 23: 158–167. [DOI] [PubMed] [Google Scholar]
- 13. Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A, Gal A, Beck M. The Mainz severity score index: a new instrument for quantifying the Anderson‐Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 2004; 65: 299–307. [DOI] [PubMed] [Google Scholar]
- 14. Mignani R, Pieruzzi F, Berri F, Burlina A, Chinea B, Gallieni M, Pieroni M, Salviati A, Spada M. FAbry STabilization indEX (FASTEX): an innovative tool for the assessment of clinical stabilization in Fabry disease. Clin Kidney J 2016; 9: 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biagini E, Pazzi C, Olivotto I, Musumeci B, Limongelli G, Boriani G, Pacileo G, Mastromarino V, Bacchi Reggiani ML, Lorenzini M, Lai F, Berardini A, Mingardi F, Rosmini S, Resciniti E, Borghi C, Autore C, Cecchi F, Rapezzi C. Usefulness of electrocardiographic patterns at presentation to predict long‐term risk of cardiac death in patients with hypertrophic cardiomyopathy. Am J Cardiol 2016; 118: 432–439. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–3914. [DOI] [PubMed] [Google Scholar]
- 17. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher CK, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 18. Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population‐based study. Eur J Echocardiogr 2010; 11: 51–56. [DOI] [PubMed] [Google Scholar]
- 19. Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 2003; 107: 1978–1984. [DOI] [PubMed] [Google Scholar]
- 20. Niemann M, Breunig F, Beer M, Herrmann S, Strotmann J, Hu K, Emmert A, Voelker W, Ertl G, Wanner C, Weidemann F. The right ventricle in Fabry disease: natural history and impact of enzyme replacement therapy. Heart 2010; 96: 1915–1919. [DOI] [PubMed] [Google Scholar]
- 21. Finocchiaro G, Knowles JW, Pavlovic A, Perez M, Magavern E, Sinagra G, Haddad F, Ashley EA. Prevalence and clinical correlates of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Am J Cardiol 2014; 113: 361–367. [DOI] [PubMed] [Google Scholar]
- 22. Severino S, Caso P, Cicala S, Galderisi M, De Simone L, D'Andrea A, D'Errico A, Mininni N. Involvement of right ventricle in left ventricular hypertrophic cardiomyopathy: analysis by pulsed Doppler tissue imaging. Eur J Echocardiogr 2000; 1: 281–288. [DOI] [PubMed] [Google Scholar]
- 23. Siegenthaler M, Huynh‐Do U, Krayenbuehl P, Pollock E, Widmer U, Debaix H, Olinger E, Frank M, Namdar M, Ruschitzka F, Nowak A. Impact of cardio‐renal syndrome on adverse outcomes in patients with Fabry disease in a long‐term follow‐up. Int J Cardiol 2017; 249: 261–267. [DOI] [PubMed] [Google Scholar]
- 24. Patel MR, Cecchi F, Cizmarik M, Kantola I, Linhart A, Nicholls K, Strotmann J, Tallaj J, Tran TC, West ML, Beitner‐Johnson D, Abiose A. Cardiovascular events in patients with Fabry disease: natural history data from the Fabry registry. J Am Coll Cardiol 2011; 57: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 25. Weidemann F, Niemann M, Störk S, Breunig F, Beer M, Sommer C, Herrmann S, Ertl G, Wanner C. Long‐term outcome of enzyme‐replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 2013; 274: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 27. Linhart A, Kampmann C, Zamorano JL, Sunder‐Plassmann G, Beck M, Mehta A, Elliott PM, European FOS Investigators . Cardiac manifestations of Anderson‐Fabry disease: results from the international Fabry outcome survey. Eur Heart J 2007; 28: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 28. Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR. Ten‐year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 2015; 52: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Focardi M, Cameli M, Carbone SF, Massoni A, De Vito R, Lisi M, Mondillo S. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 2015; 16: 47–52. [DOI] [PubMed] [Google Scholar]
- 30. Anavekar NS, Gerson D, Skali H, Kwong RY, Kent Yucel E, Solomon SD. Two‐dimensional assessment of right ventricular function: an echocardiographic‐MRI correlative study. Echocardiography 2007; 24: 452–456. [DOI] [PubMed] [Google Scholar]
- 31. Ghio S, Temporelli PL, Klersy C, Simioniuc A, Girardi B, Scelsi RA, Cicoira M, Tarro Genta F, Dini FL. Prognostic relevance of a non‐invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur J Heart Fail 2013; 15: 408–414. [DOI] [PubMed] [Google Scholar]
- 32. Aloia E, Cameli M, D'Ascenzi F, Sciaccaluga C, Mondillo S. TAPSE: an old but useful tool in different diseases. Int J Cardiol 2016; 225: 177–183. [DOI] [PubMed] [Google Scholar]


