Abstract
Aims
Hypertension is the leading cause for the development of heart failure (HF). Here, we aimed to identify cardiomyocyte stretch‐induced circulating biomarkers for predicting hypertension‐associated HF.
Methods and results
Circulating levels of 149 proteins were measured by proximity extension assay at baseline examination in 4742 individuals from the Malmö Diet and Cancer study. Protein levels were compared with stretch‐activated gene expression changes in cultured neonatal rat ventricular myocytes (NRVMs) in response to 1–48 h of mechanical stretch. We also studied the association between protein levels and hypertension and HF incidence using respectively binary logistic and Cox regressions. Levels of 35 proteins were differentially expressed after Bonferroni correction in incident HF vs. control (P < 3.4E−4). Growth differentiation factor‐15 (GDF‐15), interleukin‐6 (IL‐6), IL‐1 receptor type 1, and urokinase plasminogen activator surface receptor had corresponding mRNA levels up‐regulated by stretch in NRVMs at all time points (P < 0.05). These four proteins were individually associated with increased risk of HF after age and sex adjustment [hazard ratio (HR) per standard deviation: 1.19 ≤ HR ≤ 1.49, P ≤ 4.90E−3]. GDF‐15 and IL‐6 were associated with HF independently of each other (1.22 ≤ HR ≤ 1.33, P ≤ 0.001). In subjects with hypertension, these associations remained significant after further adjustment for N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels (1.23 ≤ HR ≤ 1.45, P ≤ 0.001). A higher fasting value of a GDF‐15, IL‐6 score aggregate was associated with increased risk of hypertensive HF after adjustment for all traditional risk factors for HF and NT‐proBNP (HR = 1.31, P = 2.19E−4).
Conclusions
Cardiomyocyte mRNA levels of GDF‐15 and IL‐6 are consistently up‐regulated by stretch, and their circulating protein levels predict HF in hypertensive subjects independently of NT‐proBNP during long‐term follow‐up. Our results encourage further studies on lower blood pressure goals in hypertensive subjects with high GDF‐15 and IL‐6, and interventions targeted at stretch‐induced cardiomyocyte expressed biomarkers.
Keywords: Cardiomyocyte, Heart failure, Hypertension, Prospective cohort, Protein panel
Introduction
Heart failure (HF) is characterized by inadequate cardiac function for maintaining and supporting an individual's physiological requirement and results in frequent hospitalizations, impaired quality of life, and high mortality. 1 Lifetime risk to develop HF ranges from 20% to 45% depending on sex and race. 2 HF is a heterogeneous syndrome with different pathomechanisms, including neurohormonal activation, inflammation, myocardial stretch, matrix remodelling, and cardiomyocyte injury. 3 However, hypertension is the single most important modifiable risk factor for HF. 4
Although pro‐B‐type natriuretic peptide (BNP) level is a recognized biomarker for HF risk prediction, 5 , 6 additional plasma biomarkers for HF incidence are emerging (for review, see Ibrahim and Januzzi 7 ), but it is uncertain if they specifically reflect cardiomyocyte dysfunction or injury. The phenotypic feature of mechanically stretched cardiomyocytes in vitro mimics that of pressure overload‐induced cardiac hypertrophy in vivo. 8 It thus provides a well‐defined experimental model to study HF related to hypertension (i.e. hypertensive HF).
Here, we examined if plasma protein biomarkers that can be proven to be induced in cardiomyocytes by cardiovascular stress predict HF outcome in a general population. To do so, we combined gene expression profiling data from an in vitro cardiomyocyte stretch model with measurements in a prospective population‐based cohort of a panel of circulating proteins known or suggested to be involved in cardiovascular disease, cell proliferation, differentiation, or death. Given the focus on stretch‐activated cardiomyocyte biomarkers, we also stratified our analyses for presence vs. absence of hypertension at baseline examination.
Materials and methods
Study participants and data collection
The Malmö Diet and Cancer–Cardiovascular Cohort (MDC‐CC) is a prospective population‐based cohort designed to study the epidemiology of carotid artery disease collected from 1991 through 1994. 9 At baseline, all the MDC‐CC participants underwent medical history, physical examination, and laboratory and lifestyle assessment. Of the 5405 participants who came fasted, plasma samples were available in 4742 subjects for analysis of a panel of proteins.
Systolic blood pressure (SBP) and diastolic blood pressure were measured using a mercury‐column sphygmomanometer after 10 min of rest in the supine position. Data on current smoking and use of antihypertensive treatment were ascertained from a baseline questionnaire. 10 Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in metres. Diabetes mellitus (DM) at baseline was defined as a fasting whole blood glucose > 6.0 mmol/L or self‐report of a physician diagnosis or use of diabetes medication. Hypertension at baseline was defined as SBP ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or being on antihypertensive treatment.
All participants provided written informed consent, and the study was approved by the Ethics Committee at Lund University.
Follow‐up and endpoint retrieval
Heart failure was defined as the first incident hospitalization with HF as main diagnosis, and incident cases of HF were included irrespective of co‐morbid diagnoses. The endpoints were retrieved through record linkage of the personal identification number of each Swedish citizen with Swedish local or national registries as described previously 11 until 31 December 2016.
Laboratory measurements
All laboratory assays were performed on overnight‐fasted blood samples obtained at the time of the baseline examination. Analysis of plasma high‐density lipoprotein cholesterol (HDL‐C) was performed according to standard procedures at the Department of Clinical Chemistry, Skåne University Hospital in Malmö. The levels of low‐density lipoprotein cholesterol (LDL‐C) were calculated according to the Friedewald formula. N‐terminal pro‐BNP (NT‐proBNP) levels were determined using the Dimension RxL automated NT‐proBNP method (Siemens Diagnostics, Nürnberg, Germany). 12
Plasma levels of 149 proteins were measured using Olink Proseek Multiplex proximity extension assay (PEA) at the Clinical Biomarkers Facility, Science for Life Laboratory, Uppsala, Sweden. PEA uses two highly specific oligonucleotide labelled antibodies per protein, which allows the formation of a PCR reporter sequence when both antibodies are bound to the target protein's surface. This sequence is then quantified by real‐time quantitative PCR. 13 Data are expressed as normalized protein expression arbitrary units.
Gene expression profiling of stretch‐regulated genes in cardiomyocytes
We have reported previously the cardiomyocyte gene expression profiling results that showed statistically differential expression of 205, 579, 737, 621, and 1542 genes in response to 1, 4, 12, 24, and 48 h of mechanical stretching, respectively 14 (GEO accession number GSE107551).
Briefly, primary cultures of neonatal rat ventricular myocytes were prepared from 2‐ to 4‐day‐old Sprague–Dawley rats, plated at a density of 2 × 105/cm2 on flexible bottomed collagen I‐coated 6‐well elastomere plates (BioFlex, Flexcell International Corporation, Hillsborough, NC, USA) and cultured overnight as described previously. 15 Cyclic mechanical stretch for 15 min to 48 h was performed in vitro using a Flexercell Strain Unit FX‐3000 apparatus (Flexercell Int. Corp., McKeesport, PA, USA). The amplitude of stretch varied between 10% and 25% at 0.5 Hz frequency. The vacuum varied in 2 s cycles at a level sufficient to promote cyclic stretch of the cardiomyocytes at the point of maximal distension of the culture surface. The effect of “ageing” was avoided by starting the stretch stepwise in the experimental groups and finished simultaneously to avoid of the cells in culture. The experiments were carried out in two separate sets, each having their own control: (i) 1, 4, and 12 h and (ii) 24 and 48 h of stretch. Afterwards, the cells were quickly frozen with nitrogen oxide and stored at −70°C. Total RNA was isolated, and gene expression profiling was performed using the GeneChip Rat Expression Set 230_2.0 Array (Affymetrix, Santa Clara, CA, USA). 14 The Affymetrix CEL files were imported into genespringtm GX 12.6 software (Agilent Technologies), and Robust Multichip Average normalization was performed. Genes were defined as differentially expressed if the fold change was at least 1.5‐fold and statistically significant (P < 0.05, one‐way ANOVA and Benjamini and Hochberg false discovery rate) (Rysä, Tokola, Ruskoaho).
Statistical analyses
spss (version 25.0) was used for all statistical analyses. Because of non‐normality, fasting plasma concentration of all 149 proteins and NT‐proBNP were transformed with the natural logarithm. For all statistical analyses, except for Student's t‐test, the studied proteins were scaled to multiples of one standard deviation (SD) and centred on zero prior analysis. Participants with prevalent HF were excluded as well as those with missing measurement of NT‐proBNP, leaving 4469 individuals for statistical analyses.
Student's t‐tests and Pearson χ 2 tests were used to compare respectively the continuous and dichotomous variables at baseline examination. Student's t‐tests were also used to compare plasma levels of the 149 proteins between incident HF and participants who remained free from HF during follow‐up. Logistic regression models adjusted for age and sex were used to analyse the associations between baseline levels of growth differentiation factor‐15 (GDF‐15), interleukin‐6 (IL‐6), IL‐1 receptor like 1 (referred to as soluble ST2 and abbreviated as ST2), urokinase plasminogen activator surface receptor (uPAR), and hypertension at baseline examination. The associations between GDF‐15, IL‐6, ST2, uPAR, and HF incidence were investigated using multivariable Cox proportional hazards models. Hazard ratios (HRs) were expressed per SD increment of protein level. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, and all four proteins. Model 3 was adjusted for age, sex, NT‐proBNP levels, BMI, SBP, current smoking, prevalent DM, and fasting plasma levels of LDL‐C and HDL‐C. Partial Spearman correlation was used to test for association between SBP and plasma levels of GDF‐15 and IL‐6 adjusting for age and sex. The associations between GDF‐15, IL‐6, and HF incidence stratifying for hypertension were investigated using multivariable Cox proportional hazards models. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, and NT‐proBNP levels; and Model 3 was further adjusted for GDF‐15 and IL‐6. Finally, Model 4 was adjusted for age, sex, NT‐proBNP levels, BMI, SBP, current smoking, prevalent DM, and fasting plasma levels of LDL‐C and HDL‐C.
A GDF‐15, IL‐6 score was created in the participants with hypertension at baseline by summing the standardized values of the respective protein levels. Kaplan–Meier survival curve was used to describe the rate of primary HF in quartiles of baseline levels of the GDF‐15, IL‐6 score. Multivariable Cox proportional hazards models were adopted to examine the association between the GDF‐15, IL‐6 score and incident HF. Model 1 was adjusted for age, sex, and NT‐proBNP levels. Model 2 was further adjusted for BMI, SBP, current smoking, prevalent DM, and fasting plasma levels of LDL‐C and HDL‐C. Participants without complete data on covariates were excluded from the analysis. HRs were expressed per SD increment of the GDF‐15, IL‐6 score, or per increasing quartiles of the score (quartiles 2 through 4 compared with quartile 1).
Receiver operating characteristic (ROC) curves were constructed to assess the predictive ability of different models.
Bonferroni correction was used to determine significance for the multiple tests performed to compare plasma levels of the 149 proteins in incident HF vs. HF‐free participants. For all other tests, a two‐sided P value of <0.05 was considered statistically significant.
Results
Population characteristics
The characteristics of the study participants at baseline examination are listed in Table 1. Among 4469 participants free from HF at baseline examination, 284 developed new‐onset HF during a median follow‐up time of 23.4 years (25–75% inter‐quartile range 20.3–24.3). Almost all traditional risk factors for HF were significantly more disadvantageous in individuals with incident HF compared with controls.
Table 1.
Population characteristics
| Controls (n = 4185) | Incident HF (n = 284) | P a | |
|---|---|---|---|
| Age (years) | 57.17 ± 5.94 | 61.07 ± 4.84 | 7.34E−27 |
| Sex, n (% women) | 2572 (61.5) | 134 (47.2) | 1.91E−06 |
| BMI (kg/m2) | 25.56 ± 3.82 | 27.67 ± 4.77 | 1.20E−18 |
| Fasting LDL‐C (mmol/L) | 4.16 ± 0.98 | 4.20 ± 0.99 | 0.49 |
| Fasting HDL‐C (mmol/L) | 1.40 ± 0.37 | 1.30 ± 0.35 | 1.26E−05 |
| SBP (mmHg) | 140.28 ± 18.60 | 149.01 ± 20.01 | 3.25E−14 |
| DBP (mmHg) | 86.46 ± 9.22 | 89.82 ± 9.56 | 3.42E−09 |
| NT‐proBNP (pg/mL) | 93.18 ± 139.63 | 201.98 ± 576.71 | 5.28E−19 |
| Diabetes prevalence, n (%) | 286 (6.8) | 51 (18.0) | 6.41E−12 |
| Antihypertensive medication use, n (%) | 627 (15.0) | 113 (39.8) | 1.38E−27 |
| Smoker, n (%) | 1086 (26.0) | 81 (28.6) | 0.328 |
Values are displayed as M ± SD or frequency in per cent.
BMI, body mass index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; LDL‐C, low‐density lipoprotein cholesterol; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SBP, systolic blood pressure.
P values were obtained from Student's t‐tests for the continuous variables or from Pearson χ 2 tests for the binary variables.
Plasma levels of a protein panel and heart failure
Overnight‐fasted plasma levels of 149 proteins at baseline examination were compared between incident HF and participants who remained free from HF during follow‐up (Table S1). A total of 35 protein biomarkers were significantly different after Bonferroni correction for multiple testing (P < 3.4E−4), of which all were elevated in incidence HF vs. controls (Table S1).
Gene expression levels in stretched cardiomyocytes
Our recent gene expression profiling study of neonatal rat ventricular myocytes characterized the transcriptional profiles triggered by 1–48 h of mechanical stretch. 14 Out of the proteins with elevated baseline levels in HF incidence, 13 were also dysregulated at gene expression level in stretched cardiomyocytes ( Table S2), of which four (GDF‐15, IL‐6, ST2, and uPAR) were statistically significantly up‐regulated at all the time points examined (1, 4, 12, 24, and 48 h) (P < 0.05) (Figure 1A,B).
Figure 1.
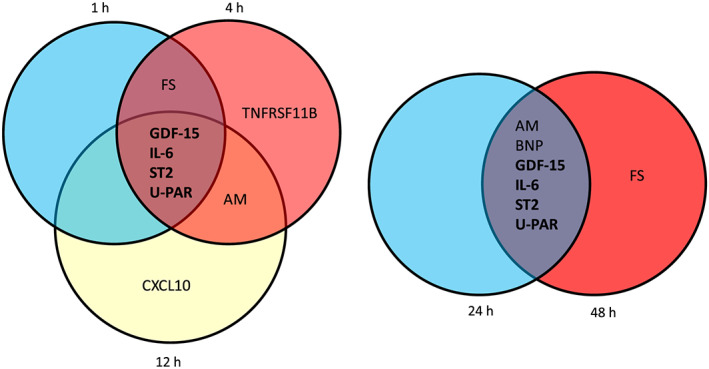
Circulating proteins up‐regulated in incidence of heart failure with corresponding stretch‐activated gene expression changes in cultured neonatal rat ventricular myocytes in response to 1, 4, 12, 24, or 48 h of cyclic mechanical stretch. Venn diagrams indicate the overlap of the genes (bold) that were significantly up‐regulated (>1.5‐fold) compared with non‐stretched cells in all time points. AM, adrenomedullin; BNP, B‐type natriuretic peptide; CCL20, C‐C motif chemokine ligand 20; CHI3L1, chitinase 3‐like 1; CXCL10, C‐X‐C motif chemokine ligand 10; CXCL13, C‐X‐C motif chemokine ligand 13; FABP4, fatty acid binding protein 4; FS, follistatin; GDF‐15, growth differentiation factor‐15; IL‐6, interleukin 6; TNFRSF11B, TNF receptor superfamily member 11b; ST2, interleukin 1 receptor type I; uPAR, urokinase plasminogen activator receptor.
Protein levels and hypertension
Among the 4469 study participants, 2810 were hypertensive at baseline examination. In a next step, we investigated if baseline plasma levels of GDF‐15, IL‐6, ST2, and uPAR were associated with hypertension. In models adjusted for age and sex, there was a statistically significant positive correlation between the three biomarkers GDF‐15, IL‐6 and ST2 and hypertension (P ≤ 2.2E−3) ( Table S3).
Protein levels and risk of heart failure
Next, we tested if plasma levels of GDF‐15, IL‐6, ST2, and uPAR are associated with HF incidence (Table 2). A higher fasting value of each of these four biomarkers was associated with increased risk of HF after age and sex adjustment (1.19 ≤ HR ≤ 1.49, P ≤ 4.90E−3) (Model 1, Table 2). The associations remained significant in a fully adjusted model for all traditional HF risk factors (1.17 ≤ HR ≤ 1.27, P ≤ 0.02) ( Table S4). The area under the ROC curve was 0.773 (95% CI, 0.746–0.800) for all the traditional risk factors and changed to 0.774 (95% CI, 0.747–0.801) when GDF‐15, IL‐6, ST2, and uPAR were added to the model.
Table 2.
Circulating protein levels and incidence of heart failure
| HF (n = 284/4185) | ||||
|---|---|---|---|---|
| Biomarkers | ||||
| GDF‐15 | IL‐6 | ST2 | uPAR | |
| Model 1 | ||||
| HR per SD (95% CI) | 1.49 (1.31–1.68) | 1.34 (1.22–1.48) | 1.19 (1.06–1.35) | 1.34 (1.18–1.51) |
| P | 3.80E−10 | 9.47E−10 | 4.90E−03 | 2.86E−06 |
| Model 2 | ||||
| HR per SD (95% CI) | 1.33 (1.12–1.58) | 1.22 (1.10–1.37) | 1.02 (0.89–1.17) | 1.03 (0.88–1.22) |
| P | 0.001 | 3.72E−04 | 0.737 | 0.692 |
Values are hazard ratios (95% confidence intervals) for incidence of heart failure per SD unit increase of protein from Cox proportional hazards models. Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, and all four biomarkers together.
CI, confidence interval; GDF‐15, growth differentiation factor‐15; HF, heart failure; IL‐16, interleukin‐16; uPAR, urokinase plasminogen activator receptor.
We also studied if the four proteins were associated with future HF independently of each other by incorporating all four biomarkers in the same Cox proportional hazards model. Higher fasting levels of both GDF‐15 and IL‐6 were independently associated with increased risk of HF after adjustment for age and sex (HR = 1.33, CI = 1.12–1.58, P = 0.001 and HR = 1.22, CI = 1.10–1.37, P = 3.72E−4, respectively) (Model 2, Table 2). However, ST2 and uPAR were no longer significantly associated with HF incidence and were thus not further investigated.
Growth differentiation factor‐15 and interleukin‐6 levels in hypertensive heart failure
There was a positive correlation between SBP and plasma levels of GDF‐15 and IL‐6 (r = 0.07, P < 0.001 and r = 0.11, P < 0.001, respectively). In a next step, we tested if plasma levels of GDF‐15 and IL‐6 were associated with the risk of HF in normotensive (1659 individuals, of which 46 developed new‐onset HF) and in hypertensive individuals (2810 individuals, of which 238 developed new‐onset HF). None of the proteins were associated with the incidence of HF in normotensive individuals. However, higher plasma levels of GDF‐15 and IL‐6 were individually associated with increased risk of HF in hypertensive individuals after adjusting for age and sex (P ≤ 4.7E−9) (Model 1, Table 3). The relationships remained statistically significant after further adjustment for fasting plasma NT‐proBNP levels, (P ≤ 6.3E−8) (Model 2, Table 3) as well as further adjustment for BMI, SBP, current smoking, prevalent DM, and fasting plasma levels of LDL‐C and HDL‐C (HR = 1.27, CI = 1.10–1.47, P = 0.001 and HR = 1.21, CI = 1.06–1.38, P = 0.004, respectively). Furthermore, GDF‐15 and IL‐6 were associated with HF in hypertensive participants independently of each other in a model adjusted for age, sex, and NT‐proBNP levels (HR = 1.32, CI = 1.14–1.53, P = 2.3E−4 and HR = 1.23, CI = 1.09–1.39, P = 0.001, respectively) (Model 3, Table 3).
Table 3.
Circulating protein levels and incidence of heart failure stratifying for baseline hypertension
| Normotensive HF (n = 46/1613) | Hypertensive HF (n = 238/2572) | |||
|---|---|---|---|---|
| HR per SD (95% CI) | P | HR per SD (95% CI) | P | |
| Model 1 | ||||
| GDF‐15 | 1.32 (0.95–1.83) | 0.100 | 1.49 (1.31–1.71) | 4.71E−09 |
| IL‐6 | 1.15 (0.89–1.47) | 0.278 | 1.39 (1.25–1.55) | 1.44E−09 |
| Model 2 | ||||
| GDF‐15 | 1.31 (0.95–1.81) | 0.095 | 1.45 (1.27–1.66) | 4.36E−08 |
| IL‐6 | 1.14 (0.88–1.48) | 0.309 | 1.35 (1.21–1.50) | 6.30E−08 |
| Model 3 | ||||
| GDF‐15 | 1.28 (0.91–1.80) | 0.162 | 1.32 (1.14–1.53) | 2.31E−04 |
| IL‐6 | 1.07 (0.79–1.43) | 0.675 | 1.23 (1.09–1.39) | 0.001 |
Values are hazard ratios (95% confidence intervals) for incidence of heart failure per SD unit increase of protein from Cox proportional hazards models. Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, and fasting plasma levels of N‐terminal pro‐B‐type natriuretic peptide. Model 3 is further adjusted for GDF‐15 and IL‐6.
CI, confidence interval; GDF‐15, growth differentiation factor‐15; HF, heart failure; IL‐16, interleukin‐1.
Protein score and hypertensive heart failure
We aggregated levels of GDF‐15 and IL‐6 into a score by summing the standardized values of the respective protein levels in the 2810 participants with hypertension at baseline examination. The cumulative incidence rate of HF according to quartiles of baseline fasting plasma concentration of the GDF‐15, IL‐6 score was higher for subjects in the upper quartiles (Figure 2). A higher value of the biomarker score was associated with increased risk of HF in individuals with hypertension after adjusting for age, sex, and fasting plasma levels of NT‐proBNP (Model 1) (HR = 1.51, CI = 1.33–1.71, P = 1.03E−10) (Table 4). The association remained significant after further adjustment for BMI, SBP, current smoking, prevalent DM, and fasting plasma levels of LDL‐C and HDL‐C at baseline (Model 2), with a multivariable adjusted HR of 1.31 (95% CI, 1.14–1.51, P = 2.19E−4) per one SD increment of the GDF‐15, IL‐6 score for risk of future HF (Tables 4 and S5). There was a linear relationship between the GDF‐15, IL‐6 score and the risk of incident HF in both Models 1 and 2 (P for trend respectively equal to 5.23E−9 and 0.001), and the top vs. bottom quartile of the protein score in the fully adjusted model was associated with an HR of 2.26 (95% CI, 1.36–3.77) (Table 4). In the hypertensive subpopulation, the area under the ROC curve was 0.751 (95% CI, 0.720–0.782) for all the traditional risk factors and changed to 0.754 (95% CI, 0.723–0.784) when the GDF‐15, IL‐6 score was added to the model.
Figure 2.
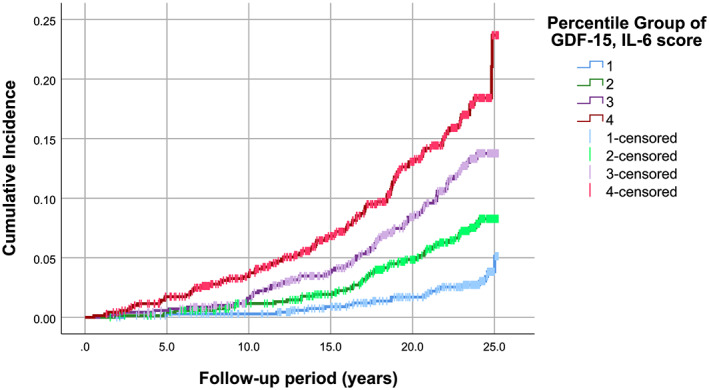
Cumulative incidence rate of heart failure events according to quartiles of a multiple‐marker score with GDF‐15 and IL‐6 aggregated levels in hypertensive individuals (n = 2810). GDF‐15, growth differentiation factor‐15; IL‐6, interleukin 6.
Table 4.
Incidence of heart failure in hypertensive individuals in relation to baseline levels of a score for GDF‐15 and IL‐6 aggregated levels
|
Hypertensive HF (n = 238/2572) Model 1 a |
Hypertensive HF (n = 237/2565) Model 2 b |
|
|---|---|---|
| Overall | ||
| HR c per SD | 1.51 (1.33–1.71) | 1.31 (1.14–1.51) |
| P | 1.03E−10 | 2.19E−04 |
| Quartiles | ||
| HR d Q1 | 1.0 (reference) | 1.0 (reference) |
| HR d Q2 | 1.85 (1.10–3.10) | 1.62 (0.96–2.73) |
| HR d Q3 | 2.76 (1.68–4.51) | 2.10 (1.27–3.47) |
| HR d Q4 | 3.61 (2.22–5.87) | 2.26 (1.36–3.77) |
| P for trend e | 5.23E−09 | 0.001 |
HF, heart failure; HR, hazard ratio.
Model 1 was adjusted for: age, sex and plasma concentration of N‐terminal pro‐B‐type natriuretic peptide at baseline.
Model 2 was adjusted for: age, sex, body mass index, systolic blood pressure, current smoking, prevalent diabetes mellitus, and fasting plasma levels of low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and N‐terminal pro‐B‐type natriuretic peptide at baseline. In Model 2, individuals without incomplete data on covariates were excluded from the analysis.
HRs are expressed per 1‐SD increment of the growth differentiation factor‐15 (GDF‐15), interleukin‐16 (IL‐6) score. 95% confidence intervals of the HRs are reported in parentheses.
HRs are expressed as categories of the GDF‐15, IL‐6 score quartiles using quartile 1 as reference. 95% confidence intervals of the HRs are reported in parentheses.
P for trend across quartiles of the GDF‐15, IL‐6 score in a linear regression model.
Discussion
We here demonstrate that GDF‐15 and Il‐6, which are induced in cardiomyocytes in response to mechanical overload, were associated with increased incidence of HF during long‐term follow‐up in a community‐based population. We also established that in participants developing hypertensive HF, GDF‐15 and IL‐6 provided prognostic information independently of the established biomarker NT‐proBNP both as single biomarkers and as a combined score.
We identified several proteins with elevated plasma levels in apparently healthy participants who developed HF later in life as compared with individuals remaining HF free. Four of these biomarkers displayed a cardiomyocyte‐specific constant up‐regulation in response to mechanical stretch, GDF‐15, IL‐6, uPAR (inflammation markers), and ST2 (myocyte stress and fibrosis marker). Regulation of GDF‐15 by stretch was in concordance with a previous study. 16 The fact that the four biomarkers of up‐regulation already started after 1 h of stretch in cardiomyocytes and was maintained under 48 h indicates an early and consistent cellular response to pressure overload. Interestingly, an increase in plasma levels of GDF‐15, IL‐6, and ST2 was measured in human plasma of hypertensive subjects. Furthermore, circulating GDF‐15, IL‐6, uPAR, and ST2 were individually associated with new‐onset HF. It has previously been reported that elevated plasma levels of uPAR were associated with an increased risk of HF in the MDC‐CC, 17 and we here confirmed this finding using another type of laboratory measurement (PEA vs. ELISA). Previous studies have also shown that levels of circulating ST2 were a predictor of incident HF in prospective cohorts of middle‐aged 18 and elderly participants. 19 When we combined the four biomarkers in the same regression model in an attempt to identify different pathophysiological pathways for HF, only GDF‐15 and IL‐6 remained significantly associated with HF incidence. We thus focused this study on these two biomarkers.
Growth differentiation factor is a stress‐responsive member of the transforming growth factor‐β cytokine superfamily and is expressed and secreted in response to inflammation. 20 In middle‐aged Framingham Heart Study participants, plasma levels of GDF‐15 predicted incident HF in both single‐marker and multiple‐marker analyses that accounted for traditional risk factors and BNP in the multimarker model. 18 , 21 We here repeat the finding of GDF‐15 being predictive of HF independently of NT‐proBNP in another prospective cohort of middle‐aged participants. Furthermore, we extend the results by showing an association between GDF‐15 and incident HF independently of NT‐proBNP in participants with overt hypertension, and by having a much longer follow‐up time to event (median 23.4 vs. 14.3 years).
Interleukin‐6 is a soluble cytokine with pleiotropic effects in inflammation and has been shown to be involved in the development of HF. Despite no association between circulating IL‐6 levels and incident HF in offspring from the Framingham Heart Study, 21 IL‐6 was found to be associated with an increased risk for congestive HF independently of traditional risk factors in two different cohorts of elderly with short follow‐up time (mean 3.6–5.2 years). 22 , 23 We here broaden these observations to encompass middle‐aged subjects with long time to event. Furthermore, we established an association between IL‐6 and incident hypertensive HF, which was independent of NT‐proBNP.
Growth differentiation factor‐15 and IL‐6 remained independently associated with hypertensive HF when combined in the same model, which indicates that they reflect different pathways of HF pathophysiology. Furthermore, the GDF‐15 and IL‐6 aggregated score predicted hypertensive HF independently of the traditional risk factors for HF and NT‐proBNP, which suggests that measurement of GDF‐15 and IL‐6 might add complementary information with respect to prediction of HF in subjects with hypertension.
Regarding potential therapeutic implications, patients with symptomatic HF treated for 12 months with valsartan, an angiotensin II receptor antagonist, had reduced BNP but not GDF‐15 levels as compared with the placebo group. 24 This indicates that GDF‐15 and BNP have different underlying regulatory mechanisms in HF. However, based on the pathobiology of GDF‐15 and the role of systemic inflammation in hypertension, anti‐inflammatory, anti‐oxidant, or anti‐aging therapies may be more beneficial to individuals with hypertension and increased GDF‐15 levels. 20 In patients with previous myocardial infarction, treatment with canakinumab, an IL‐1β inhibitor, reduced circulating levels of IL‐6 in a dose‐dependent manner, 25 and there was a statistically significant trend over the three tested doses for a reduction in the risk of HF. 26 This further raises the possibility that anti‐inflammatory therapy could also be protective against HF development in hypertensive patients, especially in patients with high levels of GDF‐15 and IL‐6.
This study has several limitations that deserve to be mentioned. Although we investigated a large number of proteins on the basis of existing evidence for associations with cardiovascular disease, cell proliferation, differentiation, or death, we might be missing some relevant proteins with our targeted approach. Furthermore, protein levels were assessed by the multiplex antibody‐based PEA, and confirmation with other immunoassays would be beneficial. In the stretch model, measurements were only performed at the transcriptional level, although it would have been informative to also determine protein levels. In addition, almost two‐thirds of the study participants were hypertensive at baseline examination, and we might thus be underpowered to study normotensive HF incidence. Finally, although we demonstrated experimentally that GDF‐15 and IL‐6 are modulated by mechanical stretch in cardiomyocytes, we cannot exclude that in response to hypertension, other cell types and organs contribute to an elevation of plasma GDF‐15 and IL‐6 levels especially because those biomarkers are not cardiomyocyte specific. For example, under pathological conditions, GDF‐15 can be produced by both cardiovascular and noncardiovascular cell types, 20 and circulating levels of IL‐6 may be modulated by physical exercise and stress. 27
In conclusion, by using a proteomic platform, we identified 35 circulating protein biomarkers that were associated with incident HF, and four of these were persistently dysregulated by mechanical stretch in cardiomyocytes. As a combined score, GDF‐15 and IL‐6 were associated with long‐term incident HF in subjects with hypertension after accounting for potential clinical cofounders and NT‐proBNP. Thus, plasma levels of GDF‐15 and IL‐6 could be used as biomarkers specific for early cardiomyocytes dysfunction or injury due to hypertension and thus to monitor the pathophysiological process ultimately susceptible to lead to HF.
Conflict of interest
The authors declare that they have no competing interests.
Funding
C.F. was supported by the Albert Påhlsson Research Foundation, the Crafoord Research Foundation, the Ernhold Lundström Research Foundation, the Royal Physiographic Society in Lund, and the Åke Wiberg Foundation. O.M. was supported by the Knut and Alice Wallenberg Foundation, the Göran Gustafsson Foundation, the Swedish Heart and Lung Foundation, the Swedish Research Council, the Novo Nordisk Foundation, Region Skåne, and Skåne University Hospital. M.O.M. was supported by the European Research Council (consolidator grant no. 649021), the Swedish Research Council, the Swedish Heart and Lung Foundation, the Region Skåne, the Swedish Diabetes Foundation, the Novo Nordisk Foundation, the Albert Påhlsson Foundation, and the Linneus Foundation for the Lund University Diabetes Center. C.F., O.M., J.N., and M.O.M. additionally acknowledge support from the Swedish Foundation for Strategic Research for IRC15‐0067. H.R. was supported by the Academy of Finland (grant 266661), Sigrid Jusélius Foundation, and Finnish Foundation for Cardiovascular Research. J.R. was supported by the Academy of Finland (grant 276747) and Finnish Foundation for Cardiovascular Research.
Supporting information
Table S1. Plasma protein levels at baseline examination in participants that developed heart failure (HF) under follow‐up and in those that remained free from HF during follow‐up time. Bold denotes proteins induced by stretch in cardiomyocytes at all time points. See olink proteomics website for the protein biomarkers full name (https://www.olink.com/products/complete‐protein‐biomarkers‐list/).
Table S2. Circulating proteins upregulated in incidence of heart failure with corresponding gene expression changes in cultured neonatal rat ventricular myocytes (NRVM)s in response to 1, 4, 12, 24 or 48 hours of cyclic mechanical stretch. Bold denotes proteins induced by stretch in cardiomyocytes at all time points.
Table S3. Circulating protein levels in hypertension
Table S4. Circulating protein levels and incidence of heart failure
Table S5. Incidence of heart failure in hypertensive individuals in relation to baseline levels of a score for GDF‐15 and IL‐6 aggregated levels in a fully adjusted model.
Fernandez, C. , Rysä, J. , Ström, K. , Nilsson, J. , Engström, G. , Orho‐Melander, M. , Ruskoaho, H. , and Melander, O. (2020) Circulating protein biomarkers predict incident hypertensive heart failure independently of N‐terminal pro‐B‐type natriuretic peptide levels. ESC Heart Failure, 7: 1891–1899. 10.1002/ehf2.12757.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members , Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Huffman MD, Berry JD, Ning HY, Dyer AR, Garside DB, Cai X, Daviglus ML, Lloyd‐Jones DM. Lifetime risk for heart failure among White and Black Americans. J Am Coll Cardiol 2013; 61: 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braunwald E. Heart failure. JACC Heart Fail 2013; 1: 1–20. [DOI] [PubMed] [Google Scholar]
- 4. Kazzam E, Ghurbana BA, Obineche EN, Nicholls MG. Hypertension—still an important cause of heart failure? J Hum Hypertens 2005; 19: 267–275. [DOI] [PubMed] [Google Scholar]
- 5. Smith JG, Newton‐Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, Platonov PG, Hedblad B, Engström G, Wang TJ, Melander O. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol 2010; 56: 1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. New Engl J Med 2004; 350: 655–663. [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim NE, Januzzi JL. Established and emerging roles of biomarkers in heart failure. Circ Res 2018; 123: 614–629. [DOI] [PubMed] [Google Scholar]
- 8. Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 1997; 59: 551–571. [DOI] [PubMed] [Google Scholar]
- 9. Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima‐media thickness. J Intern Med 2005; 257: 430–437. [DOI] [PubMed] [Google Scholar]
- 10. Hellstrand S, Sonestedt E, Ericson U, Gullberg B, Wirfalt E, Hedblad B, Orho‐Melander M. Intake levels of dietary long‐chain PUFAs modify the association between genetic variation in FADS and LDL‐C. J Lipid Res 2012; 53: 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enhorning S, Hedblad B, Nilsson PM, Engstrom G, Melander O. Copeptin is an independent predictor of diabetic heart disease and death. Am Heart J 2015; 169: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Serio F, Ruggieri V, Varraso L, De Sario R, Mastrorilli A, Pansini N. Analytical evaluation of the Dade Behring Dimension RxL automated N‐terminal proBNP (NT‐proBNP) method and comparison with the Roche Elecsys 2010. Clin Chem Lab Med 2005; 43: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 13. Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, Eriksson A, Dickens ER, Ohlsson S, Edfeldt G, Andersson AC. Homogenous 96‐plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014; 9: e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rysa J, Tokola H, Ruskoaho H. Mechanical stretch induced transcriptomic profiles in cardiac myocytes. Sci Rep‐Uk 2018; 8: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pikkarainen S, Tokola H, Majalahti‐Palviainen T, Kerkela R, Hautala N, Bhalla SS, Charron F, Nemer M, Vuolteenaho O, Ruskoaho H. GATA‐4 is a nuclear mediator of mechanical stretch‐activated hypertrophic program. J Biol Chem 2003; 278: 23807–23816. [DOI] [PubMed] [Google Scholar]
- 16. Frank D, Kuhn C, Brors B, Hanselmann C, Ludde M, Katus HA, Frey N. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch‐specific gene program. Hypertension 2008; 51: 309–318. [DOI] [PubMed] [Google Scholar]
- 17. Borne Y, Persson M, Melander O, Smith JG, Engstrom G. Increased plasma level of soluble urokinase plasminogen activator receptor is associated with incidence of heart failure but not atrial fibrillation. Eur J Heart Fail 2014; 16: 377–383. [DOI] [PubMed] [Google Scholar]
- 18. Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012; 126: 1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parikh RH, Seliger SL, Christenson R, Gottdiener JS, Psaty BM, deFilippi CR. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community‐dwelling population. J Am Heart Assoc 2016; 5: e003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem 2017; 63: 140–151. [DOI] [PubMed] [Google Scholar]
- 21. Ho JE, Lyass A, Courchesne P, Chen G, Liu C, Yin X, Hwang SJ, Massaro JM, Larson MG, Levy D. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc 2018; 7: e008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 2003; 108: 2317–2322. [DOI] [PubMed] [Google Scholar]
- 23. Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB, Framingham Heart Study . Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction—the Framingham Heart Study. Circulation 2003; 107: 1486–1491. [DOI] [PubMed] [Google Scholar]
- 24. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth‐differentiation factor‐15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation 2010; 122: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 26. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti‐inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019; 139: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 27. Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta 2015; 443: 71–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Plasma protein levels at baseline examination in participants that developed heart failure (HF) under follow‐up and in those that remained free from HF during follow‐up time. Bold denotes proteins induced by stretch in cardiomyocytes at all time points. See olink proteomics website for the protein biomarkers full name (https://www.olink.com/products/complete‐protein‐biomarkers‐list/).
Table S2. Circulating proteins upregulated in incidence of heart failure with corresponding gene expression changes in cultured neonatal rat ventricular myocytes (NRVM)s in response to 1, 4, 12, 24 or 48 hours of cyclic mechanical stretch. Bold denotes proteins induced by stretch in cardiomyocytes at all time points.
Table S3. Circulating protein levels in hypertension
Table S4. Circulating protein levels and incidence of heart failure
Table S5. Incidence of heart failure in hypertensive individuals in relation to baseline levels of a score for GDF‐15 and IL‐6 aggregated levels in a fully adjusted model.


