Abstract
Aims
The purpose of this study is to identify echocardiography predictors of clinical response and reverse left ventricular (LV) remodelling in patients with functional mitral regurgitation (FMR) treated with MitraClip.
Method and results
We retrospectively analysed 86 high surgical risk patients with severe FMR; of those, 58 were implanted a MitraClip, and 28 received medical treatment and served as controls. At baseline and at 1‐year follow‐up, we performed clinical and echocardiography evaluation to assess global longitudinal strain (GLS) and myocardial work [global work index (GWI), global constructive work (GCW), global wasted work (GWW), global work efficiency (GWE)]. Mitral regurgitation was significantly reduced after MitraClip implantation (3.7 ± 0.4 vs. 1.7 ± 0.8, P < 0.001), and the procedure was associated with improvement in brain natriuretic peptide levels (980 ± 1027 vs. 420 ± 338 pg/mL, P < 0.001), New York Heart Association class status (3.2 ± 0.55 vs. 2.0 ± 0.6, P < 0.001), 6‐min walking test (233 ± 154 vs. 286 ± 114 m, P = 0.01) at follow‐up and reduction of left ventricle end‐systolic (LVESV) and left ventricle end‐diastolic volumes (LVEDV) (152 ± 68 vs. 136 ± 43 mL, P = 0.004 & 219 ± 74 vs. 193 ± 66 mL, P = 0.001, respectively). MitraClip procedure was associated with improvement of LV performance and significant increase of GWI (607 ± 282 vs. 650 ± 260 mmHg%, P = 0.045) and GCW (854 ± 288 vs. 949 ± 325 mmHg%, P < 0.001). Baseline ejection fraction (EF), GLS, GWI, GCW, and effective regurgitant orifice area were the variables that were associated with reduction of LVEDV 1 year after intervention (P < 0.05 for all) and baseline GCW of the LV was the only variable associated with reduction of LVESV (P = 0.002). Receiver operating characteristic curve analysis identified that a GLS cut‐off value of −8.65% (AUC 0.815, P = 0.007) was associated with a 20% reduction of the LVEDV with a sensitivity and specificity of 72% and 70%, respectively, and that a GCW cut‐off value of 846 mmHg% (AUC 0.759, P = 0.007) was associated with a 10% reduction of LVESV with sensitivity and specificity 79% and 74%, respectively.
Conclusions
Mitral valve repair with MitraClip has positive clinical and echocardiographic impact in patients with FMR 1 year after implantation. Preserved GLS and GCW values appear to be associated with LV reverse remodelling post intervention.
Keywords: MitraClip, Functional mitral regurgitation, Cardiac mechanics
Introduction
Transcatheter edge‐to‐edge mitral valve repair with MitraClip (Abbot Vascular, Santa Clara, CA, USA) implantation is an alternative method 1 , 2 , 3 , 4 , 5 for treating patients with severe mitral regurgitation (MR) who are considered to be inoperable or at high surgical risk. Even though EVEREST I and II 6 , 7 trials included mostly patients with primary MR, 65% of the patients treated worldwide had FMR and multiple registries and publications 8 , 9 , 10 , 11 have indicated the effectiveness of this method so far. Recently, two large randomized trials, COAPT and MITRA‐FR 12 , 13 , 14 have shown controversial results, ranging from dramatic improvement in clinical outcomes in the former study, to no significant difference compared with medical therapy alone in the latter study. Thus, there is an imperative need to identify which patients will show improvement (‘responders') and which will receive no significant benefit from this invasive and costly treatment. 15 Global longitudinal strain (GLS), as assessed by two‐dimensional speckle tracking imaging is an accurate echocardiographic marker to assess myocardial dysfunction. 16 , 17 , 18 GLS has been reported to have a prognostic value in heart failure (HF) and valvular heart disease patients. 19 , 20 , 21 Peak atrial longitudinal strain (PALS) is also a sensitive method to assess impaired left atrial (LA) function and fibrosis of the LA wall in MR. 22 , 23 , 24 Recently, the construction of LV longitudinal strain‐pressure curves using speckle tracking echocardiography has been proposed as a novel method for the assessment of the myocardial work efficiency of the LV in HF patients. 25 , 26 , 27 , 28 However, the effects of MitraClip implantation on myocardial work efficiency have not been fully investigated.
The aim of our study was to investigate the changes of GLS and myocardial work using speckle tracking imaging after MitraClip implantation in patients with FMR and to identify echocardiographic predictors of clinical improvement and/or reverse LV remodelling. For this reason, we conducted a clinical and echocardiographic study of patients with FMR treated with MitraClip at baseline and 1 year after implantation, and we compared the changes of their characteristics with the respective changes of these characteristics in FMR patients who received only optimal medical treatment for the same period.
Methods
Study population
We retrospectively analysed 86 (aged 71 ± 10 years), high surgical risk patients (logistic EuroSCORE 23.9 ± 17.6%), with moderate‐to‐severe and severe FMR [EROA 28.9 ± 13.9 mm2, regurgitant volume (RV) 42.9 ± 17.3 mL] and reduced LV contractility (EF 30.9 ± 8.1%, GLS −8.6 ± 3.8%). All patients experienced symptoms of HF [NYHA class 3.1 ± 0.6, quality of life (QoL) questionnaire 44 ± 18%], had raised levels of brain natriuretic peptide (BNP) (median 614 pg/mL), and were on optimal medical treatment. Of those, 58 underwent consecutive MitraClip implantation procedures (device group), and 28 who were matched to the device group (2:1 ratio) according to age, sex, and MR grade, were treated medically and served as controls (control group). All patients had thorough transthoracic and transesophageal echo examination for quantification of MR and evaluation of suitability criteria for MitraClip implantation (posterior mitral valve leaflet > 7 mm, MeanPG < 4 mmHg, MVA > 3 cm2, lack of calcification at the tips of the leaflets and degenerative MV disease). Patients with moderate‐to‐severe and severe functional MR (based on criteria defined by European Society of Cardiology guidelines) 29 were included. For those treated with MitraClip, success was defined as remaining MR ≤2+ (moderate). Informed consent was obtained from all participants and the study was approved by the local ethics committee.
Clinical data
The following data were collected and recorded from all patients: age, sex, logistic EuroSCORE, NYHA class, 6‐min walk test (6MWT), BNP levels, QoL questionnaire (EQ‐5D‐5 L, EuroQol Group), blood pressure, heart rate, cardiac resynchronization therapy (CRT) device implantation, presence of dilated or ischemic cardiomyopathy, atrial fibrillation, and the compliance at HF medication (ACE inhibitors, B blockers, diuretics, and aldosterone antagonists).
Transthoracic echocardiography data
Transthoracic echocardiography exams were performed with GE Vivid I and GE E95 machines (GE Vingmed Ultrasound, Horten, Norway) at baseline and 1 year follow‐up. All images were recorded and reviewed at EchoPac workstation v.201 and v.203 (GE Vingmed Ultrasound, Horten, Norway) by three experienced echocardiographers (K.P, M.C, and I.I). Echo protocol included standard views of LV [long axis view, short axis view, apical 4 chamber (4C), 2 chamber (2C), and apical long axis view], LA volume, quantification of MR [colour Doppler, proximal isovelocity surface area (PISA), regurgitant volume, and E wave from mitral valve inflow and continuous wave Doppler]. Cut‐off values for severity of MR were set at 20 mm2 for EROA and 30 mL for Regurgitant Volume according to the guidelines of the European Association of Cardiovascular Imaging. 29 For PISA method, Nyquist limit was reduced below 40 cm/s, PISA radius was calculated at mid‐systole, and EROA was estimated using a standard formula. Regurgitant volume was calculated by multiplying EROA with the velocity time integral of the regurgitant jet. LVEF and LV volumes (LVEDV and LVESV) were calculated by biplane Simpson's method, while LA volume was calculated by biplane method of discs. In patients with atrial fibrillation, the mean value of measurements in five consecutive cardiac cycles was used for analysis.
Deformation imaging data
To assess LV and LA strain, speckle tracking echocardiography was used. Apical images of the LV (4C, 2C, and apical long axis) were analysed at Echopac workstation (GE Vingmed Ultrasound, Horten, Norway) and provided data about the strain of all segments of the LV creating the Bull's eye summary. Bull's eye analysis gives information about segmental strain along with the GLS of the LV at the same time. In this system, two basal and one apical point of the LV in all apical views have to be pointed out in order to generate tracking of the myocardium. Once the aortic valve closure is set, the system automatically follows the myocardial wall motion at systole and diastole. The percentage of lengthening and shortening of the segments represents the longitudinal strain and the combination of all 17 LV segments that represent the GLS.
LA strain is measured with zoomed view of LA, marking all segments by tracing the endocardium and calculating the PALS that corresponds to the rapid filling phase (reservoir) of the LA. 21 , 22 , 23
Left ventricular myocardial work is a novel echo marker that is based on LVGLS. It reflects the stroke work of the LV and is estimated by using the brachial artery systolic pressure and the GLS derived by speckle tracking method. Myocardial work is measured from pressure‐strain loops areas that are constructed from the LV pressure curves combined with strain (GLS). A step by step approach to calculate myocardial work and its components [global work index (GWI), global constructed work (GCW), global wasted work (GWW), and global work efficiency (GWE)] is estimation of the GLS of the LV, followed by timing the valvular events (mitral valve and aortic valve opening and closure—MVO, AVO, MVC, and AVC), and finally entering the brachial blood pressure of the patient. The myocardial work is calculated from mitral valve closure to mitral valve opening; the constructive work is the work performed during shortening in systole, adding the negative work during lengthening in isovolumetric relaxation; the wasted work is the negative work during lengthening in systole adding work during shortening in isovolumetric relaxation; and global work efficiency is the constructive work divided by the sum of constructive and wasted work. All these data are calculated automatically when you complete the steps that are required for this method.
Follow‐up
All patients underwent clinical, laboratory, and echocardiographic follow‐up 1 year after the screening. Echo exam was performed with conventional transthoracic echo and clinical examination included blood pressure, heart rate, weight, 6‐min walk test (6MWT), NYHA class, and QoL questionnaire. Laboratory exams included creatinine, BNP levels, troponin, haemoglobin, potassium, sodium, and liver function.
Statistical analysis
Continuous variables are presented as mean ± SD when normally distributed and as median and interquartile range otherwise. Categorical variables are expressed as percentages of the population. Continuous variables were tested by the Kolmogorov–Smirnov test to assess the normality of distribution. Variables with a non‐normal distribution were analysed after transformation into ranks. Categorical data were analysed using the χ 2 test.
Independent t‐test or Wilcoxon signed‐rank test were used for comparisons among groups. ANOVA (general linear model) for repeated measurements was applied (i) for measurements of the examined markers at baseline, 1 year after treatment used as a within‐subject factor and (ii) for the effects of treatment (MitraClip vs. medical treatment), as a between‐subject factors. The F and P values of the interaction between time of measurement of the examined markers and the examined covariates were calculated. The F and P values of the comparison between treatments were also calculated. The Greenhouse–Geisser correction was used when the sphericity assumption, as assessed by Mauchly's test, was not met. Post hoc comparisons were performed with Bonferroni's correction. Correlation between continuous variables was performed using Pearson's or Spearman's correlation coefficient. Logistic regression analysis was performed to assess echocardiography predictors of the terciles of LV diastolic and systolic volume reduction at follow‐up. The multivariable logistic regression model included age, sex, and baseline effective regurgitant orifice of MR and LV volumes. The odds ratio and 95% confidence intervals (CIs) are presented for the covariates included in the univariable or multivariable logistic regression analysis. Receiver operator curve analysis (ROC) curve analysis was performed to determine the cut‐off values for baseline echocardiography markers that predict the remodelling of the left ventricle in terms of LVEDV and LVESV reduction. The area under the curve and the respective 95% CI of each marker are reported. All tests were two‐sided, and a significance level of 5% was used. Statistical analysis was performed using the SPSS 22.0 statistical software package (SPSS Inc, Illinois, USA).
Intra‐observer and inter‐observer variability
Twenty‐five patients were selected and measured by two cardiologists (experienced in echocardiography and strain imaging), blinded to each other's results. Intra‐observer variability was performed by one of them repeating measurements of the same images at different times and inter‐observer variability was performed by the other cardiologist repeating again measurements from the same images. Both variabilities were calculated by intraclass correlation coefficient (ICC) and the standard error of measurement. Both GCW and GLS demonstrated excellent intra‐observer and inter‐observer variability with ICC values more than 0.95 (GCW intra‐observer variability ICC: 0.953, 95% CI: 0.860–0.984, GCW inter‐observer variability ICC: 0.967, 95% CI: 0.889–0.987, GLS intra‐observer variability ICC: 0.965, 95% CI: 0.937–0.988 and GLS inter‐observer variability ICC: 0.991, 95% CI: 0.980–0.996).
Results
The overall population included 86 high surgical risk patients with ischemic or dilated cardiomyopathy, severely dilated LV (LVEDV 226 ± 78 mL) with reduced contractility (EF 30.9 ± 8.1%, GLS −8.6 ± 3.8%), severely dilated LA (LA volume 142 ± 84 mL), and concomitant moderate‐to‐severe and severe functional MR (MR grade 3.6 ± 0.5, EROA 28.9 ± 13.9 mm2, regurgitant volume 42.9 ± 17.3 mL). All patients suffered from symptomatic HF (NYHA class 3.1 ± 0.6, 6MWT 255 ± 139 m) and were followed‐up for 11.8 ± 2.3 months. Fifty‐eight patients received the MitraClip device (Abbot Vascular, Santa Clara, CA, USA), and 28 patients were treated with optimal medical treatment alone. There were no statistically significant differences in the baseline clinical and echocardiographic characteristics between the study groups (Tables 1 and 2 ). All patients continued on optimal medical treatment for HF (B blockers, ACE inhibitors, furosemide, and eplerenone), and CRT implantation was prerequisite if needed for a patient to be enrolled into the study. The procedural success reported was 97.3% and at 12‐month follow‐up 81.4% of the patients treated with MitraClip remained at MR ≤2+.
Table 1.
Baseline demographic and clinical characteristics of the study population
| Demographic and clinical data | Overall population | Device group | Control group |
|---|---|---|---|
| Number of patients | 86 | 58 | 28 |
| Age (years) * | 71 ± 10 | 72 ± 10 | 71 ± 11 |
| Male sex, N (%) * | 67 (76.1%) | 42 (72.4%) | 25(86.2%) |
| NYHA class * | 3.1 ± 0.6 | 3.2 ± 0.5 | 3.0 ± 0.5 |
| Dilated cardiomyopathy * (%) | 31 | 32.8 | 29.2 |
| CRT implantation * | 34.9 | 34.5 | 37.5 |
| Atrial fibrillation * (%) | 45 | 49.1 | 37.5 |
| 6MWT * (m) | 255 ± 139 | 240 ± 144 | 288 ± 124 |
| QoL questionnaire * (%) | 44 ± 18 | 43 ± 17 | 47 ± 19 |
| BNP * (median, pg/mL) | 614 | 627 | 454 |
| Logistic EuroSCORE * (%) | 23 ± 17 | 23 ± 15 | 26 ± 21 |
| ACE inhibitors * (%) | 89.2 | 93.1 | 83.3 |
| B blockers * (%) | 96.4 | 96.6 | 100 |
| Diuretics * (%) | 100 | 100 | 100 |
| Aldosterone inhibitors* (%) | 92.8 | 91.4 | 95.8 |
ACE, angiotensive converting enzyme; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy; NYHA, New York Heart Association; QoL, quality of life; 6MWT, 6‐min walking distance.
Non‐statistically significant differences between the two subgroups at baseline for all markers (P > 0.05).
Table 2.
Echocardiographic data of two subgroups at baseline and 12 months after treatment
| Echocardiographic data | Device group | Control group | F, P value * | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | P value | Baseline | Follow‐up | P value | ||
| MR EF (%) | 3.7 ± 0.4 | 1.7 ± 0.8 | <0.001 | 3.4 ± 0.5 | 3.35 ± 0.98 | 0.694 | 58, <0.001 |
| 31.9 ± 8.4 | 33.2 ± 9.8 | 0.178 | 32.8 ± 6.4 | 33 ± 6.5 | 0.893 | 0.43, 0.51 | |
| LVEDV (mL) | 219 ± 74 | 193 ± 66 | 0.001 | 214 ± 62 | 224 ± 52 | 0.442 | 6.69, 0.01 |
| LVESV (mL) | 152 ± 68 | 136 ± 43 | 0.004 | 144 ± 48 | 148 ± 44 | 0.611 | 12.4, 0.001 |
| LA volume (mL) | 140 ± 69 | 119 ± 59 | <0.001 | 125 ± 64 | 132 ± 56 | 0.343 | 12.9, 0.001 |
| GLS (%) | ‐8.6 ± 3.7 | ‐8.6 ± 3.7 | 0.922 | −9.8 ± 3.5 | −9.9 ± 3.9 | 0.893 | 0.002, 0.96 |
| PALS (%) | 7.6 ± 4.6 | 7.3 ± 4.6 | 0.678 | 7.4 ± 4.2 | 7.9 ± 4.2 | 0.646 | 0.18, 0.67 |
| GWI (mmHg%) | 607 ± 282 | 650 ± 260 | 0.045 | 686 ± 338 | 618 ± 315 | 0.287 | 3.8, 0.04 |
| GCW (mmHg%) | 854 ± 288 | 949 ± 325 | <0.001 | 850 ± 305 | 833 ± 366 | 0.795 | 3.42, 0.04 |
| GWW (mmHg%) | 138 ± 65 | 131 ± 75 | 0.623 | 152 ± 105 | 191 ± 120 | 0.025 | 4.0, 0.04 |
| GWE (%) | 80 ± 10 | 82 ± 8 | 0.147 | 81 ± 11 | 76 ± 14 | 0.032 | 6.0, 0.02 |
EF, ejection fraction; EROA, effective regurgitant orifice area; GCW, global constructed work; GLS, global longitudinal strain; GWE, global work efficiency; GWI, global work index; GWW, global wasted work; LA, left atrial; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; MR, mitral regurgitation; PALS, peak atrial longitudinal strain.
F, P values refer to the interaction term of mitral clip vs. optimal medical treatment for differences of echocardiographic data between the baseline and follow‐up, P values by post hoc analysis with Bonferroni correction.
Clinical data in MitraClip vs. controls
1 year after transcatheter edge‐to‐edge repair, there was a significant reduction of BNP levels (980 ± 1027 pg/mL vs. 420 ± 338 pg/mL, P < 0.001), improvement of NYHA class (3.20 ± 0.55 to 2.0 ± 0.6, P < 0.001, Figure 1 ), and increase of 6 min walking distance (233 ± 154 m to 286 ± 114 m, P = 0.01). On the other hand, patients treated with optimal medical treatment demonstrated a non‐significant increase in BNP levels (528 ± 608 pg/mL vs. 568 ± 695 pg/mL, P = 0.478) and in addition to that NYHA class status (2.6 ± 0.5 vs. 2.6 ± 0.6, P = 0.576, Figure 1 ) and 6MWD (293 ± 127 m vs. 305 ± 126 m, P = 0.451) remained stable.
Figure 1.
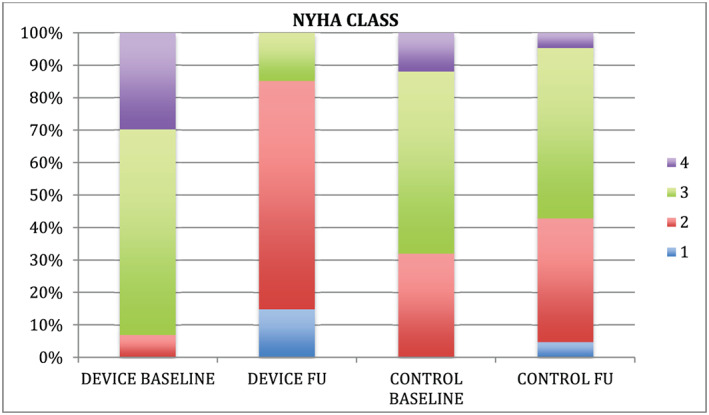
Comparison of NYHA class improvement after a 12‐month period between the two subgroups.
Conventional echocardiographic data in MitraClip vs. controls
As far as standard echocardiographic measurements are concerned, 1 year after MitraClip implantation MR grade was significantly reduced (P < 0.001, Figure 2 , Table 2 ), and LVEDV, LVESV, and LA volumes were also significantly reduced (P = 0.001, P = 0.004, and P < 0.001, Table 2 ). On the other hand, patients treated with optimal medical treatment did not show any significant change in their MR grade (P = 0.694, Figure 2 , Table 2 ), LVEDV, LVESV, or LA volumes (P = 0.442, P = 0.611, and P = 0.343, respectively, Table 2 ).
Figure 2.
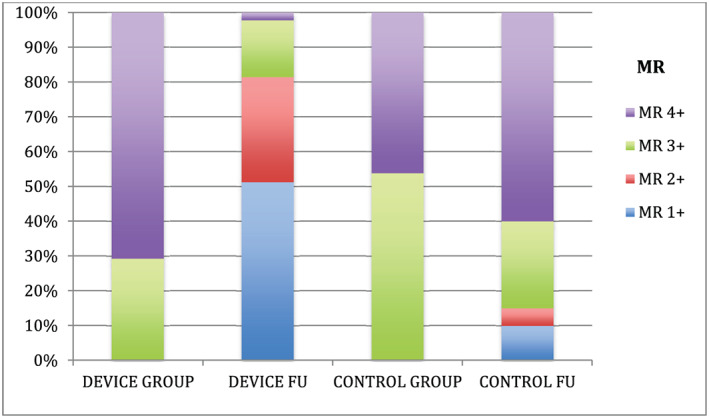
Comparison of MR at baseline and 12‐month follow‐up between device and control group.
Deformation imaging data in MitraClip vs. controls
Both groups did not demonstrate any changes of GLS (P = 0.922 for device group and P = 0.893 for control group, Table 2 ) and LA strain (P = 0.678 and P = 0.646 for device and control group, respectively, Table 2 ) after 12 months of follow‐up. On the other hand, novel echocardiographic markers such as myocardial work were proven to be more sensitive in recognizing differences between these subgroups. In particular, in device group GWI and GCW demonstrated statistically significant increase (P = 0.045 and P < 0.001, respectively, Table 2 ), whereas patients in the control group did not preserve the LV work after 12 months of treatment. In particular, GWE was significantly reduced (P = 0.032, Table 2 ), GWW was significantly increased (P = 0.025, Table 2 ), and GCW and GWI remained unchanged (P = 0.795 and P = 0.287, respectively, Table 2) in the control group.
Predictors of clinical response and LV remodelling 1 year after MitraClip procedure
In the device group, both baseline GLS and baseline GCW were significantly associated with the percentage reduction of the LVEDV 1 year after MitraClip implantation (b = −0.460, P = 0.01 & b = 0.528, P = 0.004, respectively), but only baseline GCW was significantly associated with the percentage reduction of the LVESV at follow‐up (b = 0.455, P = 0.003). This response was not demonstrated at the control group, and baseline GLS and GCW were not associated with reduction of the LVEDV (b = −0.072, P = 0.594 & b = 0.405, P = 0.320, respectively). A direct comparison of the baseline clinical and echocardiographic data of two subgroups according to percentage reduction of the LVEDV<20% (upper tercile) demonstrated that the baseline LV EF, GLS, GWI, GCW, and EROA but not baseline LVEDV were more abnormal in those with LVEDV reduction <20% compared with the patients with LVEDV change >20% (Table 3 ). By logistic regression analysis, baseline GLS was found to be associated with 20% reduction of the baseline LVEDV (upper tercile of the study cohort) either in univariate analysis (odds ratio = 0.249, 95% CI 0.627–0.970, P = 0.026) or in multivariate linear regression analysis adjusted for age, sex, baseline LVEDV, BNP levels, and baseline EROA (odds ratio = 0.342, 95% CI 0.519–0.974, P = 0.03). A receiver operating characteristic curve (ROC curve) identified a cut‐off value for GLS of −8.65% (AUC 0.815, 95% CI: 0.647–0.983; P = 0.007) to predict 20% reduction of LVEDV, with a sensitivity and specificity of 72% and 70%, respectively (Figure 3 ).
Table 3.
Baseline echocardiography and clinical characteristics of MitraClip patients according to reduction of the LVEDV (ΔLVEDV) at 1 year follow‐up
| Baseline clinical and echocardiography data | ΔLVEDV | ΔLVEDV | P value |
|---|---|---|---|
| >20% | <20% | ||
| Log EuroSCORE (%) | 26.7 ± 15.7 | 23.6 ± 16.4 | 0.564 |
| BNP (pg/mL) | 1133 ± 1350 | 893 ± 769 | 0.534 |
| NYHA Class | 3.2 ± 0.6 | 3.1 ± 0.5 | 0.385 |
| 6MWD* (m) | 192 ± 150 | 278 ± 153 | 0.1 |
| Dilated cardiomyopathy (%) | 40 ± 50 | 33 ± 48 | 0.668 |
| GLS (%) | −10.4 ± 4.3 | −7.4 ± 2.8 | 0.01 |
| LVEDV (mL) | 249 ± 90 | 226 ± 57 | 0.389 |
| LVESV (mL) | 166 ± 89 | 163 ± 52 | 0.904 |
| EF (%) | 34 ± 10 | 28 ± 7 | 0.03 |
| EROA (mm2) | 38 ± 22 | 27 ± 10 | 0.02 |
| GWI (mmHg%) | 778 ± 382 | 522 ± 188 | 0.006 |
| GCW (mmHg%) | 982 ± 389 | 691 ± 201 | 0.003 |
| GWW (mmHg%) | 114 ± 47 | 136 ± 75 | 0.526 |
| GWE (%) | 82 ± 7 | 78 ± 11 | 0.386 |
ΔLVEDV is the reduction of 20% of LVEDV (upper tercile of the study cohort).
EF, ejection fraction; EROA, effective regurgitant orifice area; GCW, global constructed work; GLS, global longitudinal strain; GWE, global work efficiency; GWI, global work index; GWW, global wasted work; LA, left atrial; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; MR, mitral regurgitation; PALS, peak atrial longitudinal strain.
Figure 3.
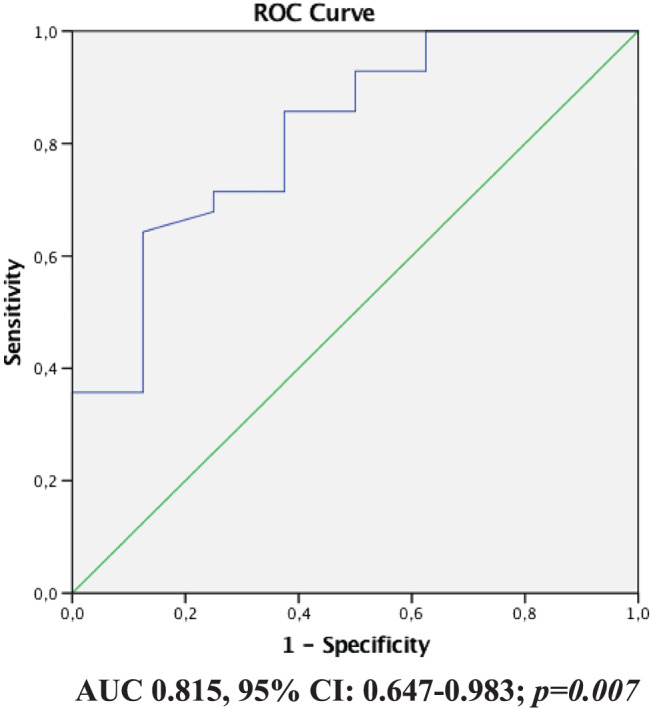
ROC curve of GLS for reduction of the LVEDV. A cut‐off value for GLS of −8.65% (AUC 0.815, 95% CI: 0.647–0.983; P = 0.007) was associated with a 20% reduction of LVEDV, with a sensitivity and specificity of 72% and 70%, respectively.
Among all examined variables (Table 4 ), baseline GCW was the only marker associated with reverse remodelling, either referring to the absolute or the percentage difference of the LVESV (b = 0.420, P = 0.023 & b = 0.444, P = 0.004, respectively). By logistic regression analysis, baseline GCW was found to be associated with 10% reduction of the baseline LVESV (upper tercile of the study cohort) either in univariate (odds ratio = 1.004, 95% CI 1.001–1.007, P = 0.01) or in multivariable logistic regression analysis adjusted for age, sex, baseline LVESV, BNP levels and baseline EROA (odds ratio = 0.012, 95% CI 1.002–1.023, P = 0.02). Furthermore, ROC curve analysis identified a cut‐off value for baseline GCW of 846 mmHg% (AUC 0.759, 95% CI: 0.588–0.930; P = 0.007) to be associated with 10% reduction of the LVESV with sensitivity and specificity 79% and 74%, respectively (Figure 4 ).
Table 4.
Baseline echocardiography and clinical characteristics of MitraClip patients according to reverse remodelling (reduction of 10% of LVESV)
| Baseline clinical and echocardiography data | ΔLVESV >10% | ΔLVESV <10% | P value |
|---|---|---|---|
| Log EuroSCORE (%) | 21.3 ± 13.4 | 27 ± 17.6 | 0.273 |
| BNP (pg/mL) | 1115 ± 1324 | 853 ± 738 | 0.471 |
| NYHA Class | 3.1 ± 0.7 | 3.2 ± 0.5 | 0.339 |
| 6MWD (m) | 232 ± 143 | 257 ± 147 | 0.599 |
| Dilated cardiomyopathy (%) | 35 ± 49 | 31 ± 47 | 0.783 |
| GLS (%) | −9.2 ± 4.6 | −8.0 ± 2.9 | 0.369 |
| LVEDV (mL) | 232 ± 72 | 209 ± 77 | 0.1 |
| LVESV (mL) | 164 ± 68 | 140 ± 66 | 0.12 |
| EF (%) | 32 ± 10 | 31 ± 8 | 0.750 |
| EROA (mm2) | 35 ± 21 | 27 ± 12 | 0.132 |
| GWI (mmHg%) | 706 ± 392 | 568 ± 187 | 0.07 |
| GCW (mmHg%) | 1011 ± 343 | 762 ± 206 | 0.002 |
| GWW (mmHg%) | 131 ± 71 | 123 ± 62 | 0.797 |
| GWE (%) | 79 ± 10 | 81 ± 10 | 0.656 |
ΔLVESV is the reduction of 10% of LVESV (upper tercile of the study cohort).
EF, ejection fraction; EROA, effective regurgitant orifice area; GCW, global constructed work; GLS, global longitudinal strain; GWE, global work efficiency; GWI, global work index; GWW, global wasted work; LA, left atrial; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; MR, mitral regurgitation; PALS, peak atrial longitudinal strain.
Figure 4.
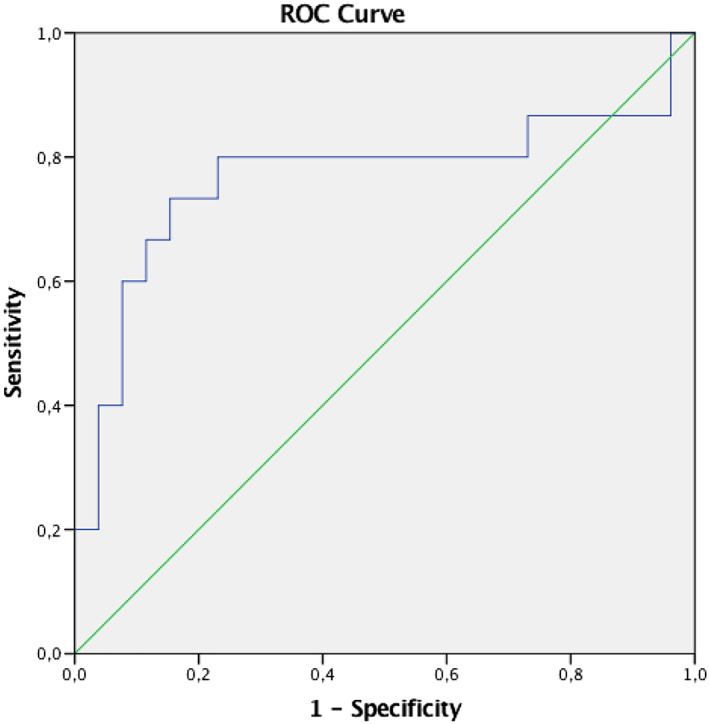
ROC curve of GCW for reverse LV remodelling. A cut‐off value for GCW of 846 mmHg% (AUC 0.759, 95% CI: 0.588–0.930; P= 0.007) was associated with a 10% reduction of LVESV, with a sensitivity and specificity of 79% and 74%, respectively.
Discussion
In our study, we compared the changes of clinical and echocardiographic characteristics between baseline and 1 year follow‐up examination in patients with functional MR who underwent MitraClip implantation vs. those who remained under optimal medical treatment for the same period. All 86 patients were on optimal medical treatment, had prior CRT and defibrillator implanted as indicated, and had symptoms of HF. The primary findings of the study were the following: (i) LV GLS predicts clinical and echocardiographic improvement in patients treated with MitraClip; (ii) markers of cardiac energetics improved in patients treated with MitraClip, whereas they deteriorated in patients treated conservatively at 1 year of follow‐up; (iii) GCW predicts reverse remodelling of the left ventricle, as assessed by reduction of LV end‐systolic volume, 1 year after MitraClip implantation.
In the current study, we demonstrated that LVGLS is an important predictor of LVEDV reduction following transcatheter edge‐to‐edge (E2E) repair with use of the MitraClip device. GLS is a more sensitive method to evaluate LV performance than ejection fraction, as it is unaffected from geometric assumptions. 16 , 17 , 18 , 19 Reduction of this parameter in patients with cardiomyopathy correlates with development of fibrosis and impairment of contractility. In our patients, GLS was associated with clinical improvement, as assessed by improved NYHA class and 6MWD, and correlated with BNP reduction at follow‐up. Further, a cut‐off value of less than or equal to −8.65% predicted 20% reduction of LVEDV after MitraClip implantation. However, GLS was not related with the respective reduction of the LVESV. Baseline GCW, a component of myocardial work, was on the other hand significantly associated with the reduction of the LVESV after MitraClip implantation. A cut‐off value of 846 mmHg% was associated with 10% reduction of the LVESV with sensitivity and specificity 79% and 74%, respectively. Global constructive work is load and pressure independent, in contrast to LV GLS and thus may be more sensitive in detecting the response of the LV after MitraClip implantation. Our findings suggest that LV GLS and GCW before MitraClip implantation may be valid markers to predict a positive LV remodelling, as assessed by reduction by LV end diastolic and systolic volumes, after MitraClip implantation, in patients with functional MR, significantly dilated LV with impaired contractility. Thus, these markers may facilitate patient selection indicating those patients with severe FMR who may not respond to a successful E2E repair because of extensive myocardial fibrosis and lack of myocardial reserve.
These findings are important in the era of evolving understanding of functional MR. 3 , 5 , 30 In patients with ischemic or dilated cardiomyopathy, prognosis, and rehospitalizations are determined by the degree of underlying LV dysfunction (traditionally evaluated by the ejection fraction). FMR has long been considered a ‘bystander' disease impacting on prognosis as well but nevertheless a ‘normal valve' malfunctioning because of the cardiomyopathy. 30 It is though becoming clear that in many of these patients, the valvulopathy becomes so significant that it actually is the main driver of adverse clinical events; the COAPT study 14 proved that treating significant FMR has a major impact on rehospitalizations and mortality, with a magnitude not seen with many of the traditional HF treatments. On the other hand, the MITRA‐FR study 12 showed that there are HF patients that fail to show any clinical benefit after correction of FMR with the MitraClip. These different outcomes have led to the hypothesis of proportionate vs. disproportionate MR for the degree of underlying LV dilatation and dysfunction. 13 , 31 Thus, we need markers of myocardial dysfunction that will provide insight on the severity and stage of LV dysfunction and at the same time markers indicating severity and impact of coexistent FMR on outcomes. Our study suggests that a preserved baseline GLS is a good a predictor of LVEDV reduction and a preserved baseline GCW an important predictor of LVESV reduction after successful MV repair with MitraClip. Additionally, both GLS and GCW were also associated with markers of clinical improvement (NYHA class, 6MWD, and BNP). These results are in line with previous publications showing that reverse LV remodelling (as assessed by LVEDV) was present in patients with preserved LV contractility. 6 However, other studies demonstrated that LV performance (assessed by EF and GLS) was not associated with reduction of the LVEDV and LVESV. 32 The differences between our study population and the previous ones 32 (69% ischemic cardiomyopathy with LVEDVi = 125 mL/m2 in our study vs. 35% ischemic cardiomyopathy with LVEDVi = 96 mL in the aforementioned study) might explain the different results.
In patients treated with E2E repair successfully, cardiac energetics were either stable (GWE) or improved (GWI and GCW). In contrast, in those patients treated with OMT alone, there was a worsening of echocardiography markers of cardiac energetics. These results provide an important insight as, to our knowledge, they have not been previously reported in this group of patients medically treated for functional MR during a long‐term follow‐up. A main concern after invasive correction of FMR has been the possibility of adversely impacting the LV with increase afterload as the systolic backflow of blood into the low pressure LA is corrected with E2E approximation. This however was not confirmed in our study as GCW improved in the functional MR patients after MitraClip implantation, whereas it deteriorated in the control group of patients who were treated medically at 1 year of follow‐up.
Edge‐to‐edge mitral valve repair with the MitraClip is an important therapy for HF patients with significant functional MR. However, identifying those patients that will have a significant clinical and echocardiographic improvement is critical. The combination of novel markers of LV dysfunction assessed by speckle tracking imaging and MR severity may facilitate patient selection for MitraClip therapy to detect those patients that will derive the most benefit, while avoiding unnecessary and costly procedures in those that will not be expected to improve.
The following study limitations should be acknowledged. This is a single centre observational study following patients referred for evaluation and treatment of functional MR. Myocardial work and its components (GWI, GCW, GWW, and GWE) can be estimated by GE machines only (GE Vingmed Ultrasound, Horten, Norway).
In conclusion, in the current study, we have shown that in patients with severely impaired LV function and moderate to severe MR, MitraClip implantation resulted in improved clinical and echocardiographic markers of cardiac performance compared with patients under optimal medical treatment and that the baseline GLS and constructive myocardial work, as assessed by speckle tracking imaging, are valid predictors of LV reverse remodelling 1 year after intervention.
Conflict of interest
None declared.
Papadopoulos, K. , Ikonomidis, I. , Chrissoheris, M. , Chalapas, A. , Kourkoveli, P. , Parissis, J. , and Spargias, K. (2020) MitraClip and left ventricular reverse remodelling: a strain imaging study. ESC Heart Failure, 7: 1409–1418. 10.1002/ehf2.12750.
Clinical Trials ID: NCT04218578
References
- 1. Lavall D, Mehrer M, Schirmer SH, Reil JC, Wagenpfeil S, Böhm M, Laufs U. Long‐term hemodynamic improvement after transcatheter mitral valve repair. J Am Soc Echocardiogr 2018; 31: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 2. Van Wijngaarden SE, Kamperidis V, Al‐Amri I, van der Kley F, Schalij MJ, Marsan NA, Bax JJ, Delgado V. Effects of transcatheter mitral valve repair with MitraClip on left ventricular and atrial hemodynamic load and myocardial wall stress. J Card Fail 2018; 24: 137–145. [DOI] [PubMed] [Google Scholar]
- 3. D'Ascenzo F, Moretti C, Marra WG, Montefusco A, Omede P, Taha S, Castagno D, Gaemperli O, Taramasso M, Frea S, Pidello S. Meta‐analysis of the usefulness of MitraClip in patients with functional mitral regurgitation. Am J Cardiol 2015; 116: 325–331. [DOI] [PubMed] [Google Scholar]
- 4. Chatzistergiou KT, Papanastasiou CA, Kokkinidis DG, Ziakas AG, Karvounis HI, Karamitsos TD. MitraClip device for patients with functional mitral valve regurgitation: A systematic review. Hellenic J Cardiol 2019. ‐Apr; 60: 101–107. [DOI] [PubMed] [Google Scholar]
- 5. Grayburn PA, Foster E, Sangli C, Weissmen NJ, Massaro J, Glower DG, Feldman T, Mauri L. Relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodelling after Mitraclip therapy. Circulation 2013; 128: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 6. Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D, EVEREST Investigators . Percutaneous Mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge to edge REpair STudy) cohort. J Am Coll Cardiol 2009; 54: 686–694. [DOI] [PubMed] [Google Scholar]
- 7. Feldman T, Foster E, Glower DD, Kar S, Rinaldl MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C. Percutaneous Repair or Surgery for Mitral Regurgitation. N Engl J Med 2011; 364: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 8. Attizzani GF, Ohno Y, Capodanno D, Cannata S, Dipasqua F, Imme S, Mangiafico S, Barbanti M, Ministeri M, Cageggi A, Pistritto AM. Extended use of percutaneous edge‐to‐edge mitral valve repair beyond EVEREST (Endovascular Valve Edge‐to‐Edge repair) criteria: a 30‐day and 12‐month clinical and echocardiographic outcomes from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry. JACC Cardiovasc Interv 2015; 8: 74–82. [DOI] [PubMed] [Google Scholar]
- 9. Nickenig G, Estevez‐Loureiro R, Franzen O, Tamburino C, Vanderheyden M, Lüscher TF, Moat N, Price S, Dall'Ara G, Winter R, Corti R, Grasso C, Snow TM, Jeger R, Blankenberg S, Settergren M, Tiroch K, Balzer J, Petronio AS, Büttner HJ, Ettori F, Sievert H, Fiorino MG, Claeys M, Ussia GP, Baumgartner H, Scandura S, Alamgir F, Keshavarzi F, Colombo A, Maisano F, Ebelt H, Aruta P, Lubos E, Plicht B, Schueler R, Pighi M, di Mario C, Transcatheter valve treatment sentinel registry investigators of the EURObservational research programme of the European Society of Cardiology . Percutaneous mitral valve edge‐to‐edge repair: in‐hospital results and 1‐year follow up of 628 patients of the 2011‐2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014; 64: 875–884. [DOI] [PubMed] [Google Scholar]
- 10. Taramasso M, Maisano F, Latib A, Denti P, Buzzatti N, Cioni M, la Canna G, Colombo A, Alfieri O. Clinical outcomes of MitraClip for the treatment of functional mitral regurgitation. EuroIntervention 2014; 10: 746–752. [DOI] [PubMed] [Google Scholar]
- 11. Giannini C, D'Ascenzo F, Fiorelli F, Spontoni P, Swaans MJ, Velazquez EJ, Armeni P, Adamo M, De Carlo M, Petronio AS. A meta‐analysis of MitraClip combined with medical therapy vs medical therapy alone for treatment of mitral regurgitation in heart failure patients. ESC Heart Fail 2018; 5: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Obadia JF, Messika‐Zeitoun D, LEurent G, Lung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 13. Pibarot P, Delgado V, Bax JJ. MITRA‐FR vs COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging 2019; 20: 620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, COAPT investigators . transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 15. Asch FM, Grayburn PA, Siegel RJ, Kar S, Lim DS, Zaroff JG, Mishell JM, Whisenant B, Mack MJ, Lindenfeld J, Abraham WT, Stone GW, Weissman NJ, COAPT Investigators . Echocardiographic outcomes after transcatheter leaflet approximation in patients with secondary mitral regurgitation: the COAPT trial. J Am Coll Cardiol 2019; 74: 2969–2979. [DOI] [PubMed] [Google Scholar]
- 16. Yingchocharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta‐analysis. J Am Soc Echocardiogr 2013; 26: 185–191. [DOI] [PubMed] [Google Scholar]
- 17. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfuction: a systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart 2014; 100: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 18. D'Elia N, Caselli S, Kosmala W, Lancelloti P, Morris D, Muraru D, Takeuchi M, van den Bosch A, van Grootel RW, Villarraga H, Marwick TH. Normal global longitudinal strain: an individual patient meta‐analysis. JACC Cardiovasc Imaging 2019. Aug 26.pii S1936‐878X(19)30733–8. [DOI] [PubMed] [Google Scholar]
- 19. Thomas JD, Kinno M. The prognostic role of global longitudinal strain in severe primary mitral regurgitation: moving past the proof‐of‐concept era. JACC Cardiovasc Imaging 2018; 11: 1245–1247. [DOI] [PubMed] [Google Scholar]
- 20. Citro R, Baldi C, Lancellotti P, Silverio A, Provenza G, Bellino M, di Muro MR, Mastrogiovanni G, de Rosa R, Galasso G, Bossone E, Giudice P, Piscione F. Global longitudinal strain predicts outcome after MitraClip implantantion for secondary mitral regurgitation. J Cardiovasc Med (Hagerstown) 2017; 18: 669–678. [DOI] [PubMed] [Google Scholar]
- 21. Cameli M, Mandoli GE, Nistor D, Lisi E, Massoni A, Crudele F, Stricagnoli M, Lunghetti S, Mondillo S. Left heart longitudinal deformation analysis in mitral regurgitation. Int J Cardiovasc Imaging 2018; 34: 1741–1751. [DOI] [PubMed] [Google Scholar]
- 22. Cameli M, Lisi M, Giacomin E, Caputo M, Navarri R, Malandrino A, Ballo P, Agricola E, Mondillo S. Chronic mitral regurgitation: left atrial deformation analysis by two‐dimensional speckle tracking echocardiography. Echocardiography 2011; 28: 327–334. [DOI] [PubMed] [Google Scholar]
- 23. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU, Industry representatives , Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee . Standardization of left atrial, right ventricular and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018; 19: 591–600. [DOI] [PubMed] [Google Scholar]
- 24. Cameli M, Incampo E, Modillo S. Left atrial deformation: useful index for early detection of cardiac damage in chronic mitral regurgitation. IJC Heart Vasc 2017; 17: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan J, Edwards NFA, Khandheria BK, Shiino K, Sabapathy S, Anderson B, Chamberlain R, Scalia GM. A new approach to assess myocardial work by non‐invasive left ventricular pressure‐strain relations in hypertension and dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2019; 20: 31–39. [DOI] [PubMed] [Google Scholar]
- 26. Manganaro R, Marchetta S, Dulgheru R, Ilardi F, Sugimoto T, Robinet S, Cimino S, Go YY, Bernard A, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, López‐Fernández T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, von Bardeleben R, Vinereanu D, Zamorano JL, Rosca M, Calin A, Moonen M, Magne J, Cosyns B, Galli E, Donal E, Carerj S, Zito C, Santoro C, Galderisi M, Badano LP, Lang RM, Oury C, Lancellotti P. Echocardiographic reference renges for normal non‐invasive myocardial work indices: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2019; 20: 582–590. [DOI] [PubMed] [Google Scholar]
- 27. Russell K, Eriksen M, Aaberge L, Wilhelmsen M, Skulstad H, Remme EW, Haugaa KH, Opdahl A, Fjeld JG, Gjesdal O, Edvardsen T. A novel clinical method for quantification of regional left ventricular pressure‐strain loop area: a non‐invasive index of myocardial work. Eur Heart J 2012; 33: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galli E, Leclercq C, Hubert A, Bernard A, Smiseth OA, Mabo P, Samset E, Hernandez A, Donal E. Role of myocardial constructive work in the identification of responders to CRT. Eur Heart J Cardiovasc Imaging 2018; 19: 1010–1018. [DOI] [PubMed] [Google Scholar]
- 29. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 30. Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis and therapeutic considerations. J Am Coll Cardiol 2015; 65: 1231–1248. [DOI] [PubMed] [Google Scholar]
- 31. Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional MitraL regurgitation: a new conceptual framework that reconciles the results of the MITRA‐FR and COAPT trials. JACC Cardiovasc Imaging 2019; 12: 353–362. [DOI] [PubMed] [Google Scholar]
- 32. Cimino S, Maestrini V, Cantisani D, Petronilli V, Filomena D, Mancone M, Sardella G, Fedele F, Lancellotti P, Agati L. 2D/3D echocardiographic determinants of left ventricular reverse remodeling after MitraClip implantation. Eur Heart J Cardiovasc Imaging 2019; 20: 558–564. [DOI] [PubMed] [Google Scholar]


