Abstract
Aims
Exhaled breath acetone (EBA) has been described as a new biomarker of heart failure (HF) diagnosis. EBA concentration increases according to severity of HF and is associated with poor prognosis, especially in acute decompensated HF. However, there are no data on chronic HF patients. The aim is to evaluate the role of EBA for predicting cardiac and overall mortality in chronic HF patients.
Methods and results
In GENIUS‐HF cohort, chronic patients were enrolled between August 2012 and December 2014. All patients had left ventricular ejection fraction ≤ 50%, and the diagnosis was established according to Framingham criteria. After consent, patients were submitted to clinical evaluation and exhaled breath collection. EBA identification and quantitative determination were done by spectrophotometry. The clinical characteristics associated with acetone were identified. All participants were followed for 18 months to assess cardiac and overall mortality. Around 700 participants were enrolled in the current analysis. Patients were 55.4 ± 12.2 years old, 67.6% male patients, and 81% New York Heart Association I/II with left ventricular ejection fraction of 32 ± 8.6%. EBA median concentration was 0.6 (0.3–1.2) ug/L. Acetone levels increased with the number of symptoms of HF and were associated with right HF signs/symptoms and liver biochemical changes. EBA at highest quartile (EBA > 1.2ug/L) was associated with a significantly worse prognosis (log rank test, P < 0.001). Cox proportional multivariable regression model revealed that EBA > 1.20ug/L was an independent predictor of cardiac (P = 0.011) and overall (P = 0.010) mortality in our population.
Conclusions
This study shows that EBA levels reflect clinical HF features, especially right HF signs/symptoms. EBA is an independent predictor of cardiac and overall mortality in chronic HF patients.
Keywords: Heart failure, Exhaled breath acetone, Biomarker, Prognosis
Introduction
The prevalence of heart failure (HF) has been rising all over the world. 1 Despite major improvements in the treatment of HF in the last decades, HF is still one of the main causes of hospitalization in both the United States and Brazil, and it is associated with high mortality. 2 , 3 In clinical practice, identifying patients at higher risk or groups of patients that may benefit from specific interventions is of paramount importance. In this sense, it is thus of great interest to identify new biomarkers of severity and prognosis. Natriuretic peptides are the most commonly used, but, recently, new non‐invasive biomarkers have been described. However, predicting the course of this highly variable syndrome remains challenging.
Exhaled breath acetone (EBA) has been shown to be a biomarker of HF diagnosis and severity. 4 , 5 It was also tested as a biomarker of prognosis. A previous study done by our group suggested that high levels of acetone could be associated with high risk of 12‐month mortality and heart transplantation. However, not only the sample size of the cohort was small but also most included patients had acute decompensated HF (ADHF) [78% New York Heart Association (NYHA) III/IV and 66% haemodynamic profile hot/wet or cold/wet]. 6
To the best of our knowledge, there are no data on the role of exhaled acetone as a biomarker of long‐term prognosis in patients with chronic HF with stable symptoms. Therefore, we sought to study the role of exhaled acetone in identifying specific patterns of clinical presentation and, most importantly, in predicting prognosis in a prospective, single‐centre cohort of patients with chronic HF.
Methods
Patients
Patients with HF diagnosis of different aetiologies referred to the outpatient clinic at the Heart Institute, University of Sao Paulo were invited to participate in the GENIUS‐HF cohort. The rationale and design of this study have been previously published. 7 To be included in this study, patients should have chronic HF with left ventricular ejection fraction ≤ 50% and stable symptoms in the last 3 months prior to baseline evaluation. The diagnosis of HF was established according to two major or one major and two minor Framingham criteria. Patients with dementia or any other mental or psychiatric disorders that would limit the collection of information were excluded. The enrolment was carried out from August 2012 to December 2014.
During this period, 700 participants, 18 to 80 years old, were enrolled in the study. The Institutional Ethics Committee (CAPPesq−Comissão de Ética para Análise de Projetos de Pesquisa) approved the study (SDC 2368/03/162). After enrolment, five patients withdrew the informed consent and were excluded. Once written informed consent was obtained, patients were submitted to a cardiologist appointment, blood and exhaled breath collection, and followed for 18 months. During cardiologist appointment, demographic data and clinical information, including HF symptoms and signs, were obtained.
Breath collection and acetone analysis
Breath collection was performed using a portable non‐invasive device previously described. 4 , 8 Patients received a standard meal, composed of coffee and milk, bread and cheese, and a fruit, at least 45–60 min before sampling in order to avoid fasting‐induced ketone body increase. Each volunteer blew 7.6 L of breath by non‐forced exhalations through a wet scrubber, which contained 5.0 mL of icy distilled water and was immersed in an ice water bath. The generation of small bubbles in cold water by a diffuser increased gas–water contact and enhanced the efficiency of soluble compounds extraction. Breath samples were stored in a freezer at −80°C until acetone analysis could be done. The defrosted aqueous extracts were analysed by spectrophotometry after reaction with salicyladehyde. The quantification of acetone was made using a calibration curve built with liquid standard mixtures. This method has been previously reported. 9 , 10 All chemical analyses were performed at the Institute of Chemistry, University of Sao Paulo.
Outcomes
The follow‐up was assessed for 18 months by telephone contact and review of medical records every 6 months to retrieve information about the vital status of patients. Cardiac and all‐cause mortality were the main outcomes analysed in this study. We also analysed hospitalizations for HF.
Statistical analysis
Categorical variables are expressed as absolute (n) and relative frequency (%). Continuous variables are expressed by mean ± standard deviation or median (interquartile range, p25th–p75th) according to its normality distribution (Kolmogorov–Smirnov test). Baseline characteristics were compared using one‐way ANOVA followed by ANOVA post‐hoc test Dunnet for continuous variables and chi‐square test for categorical variables. Univariable survival curves (Kaplan–Meier) were computed to assess the influence of acetone quartiles in the survival rate. We also used univariable and multivariable Cox proportional regression model to predict mortality in 18 months. The SPSS version 20.0 (Software Statistical Package for the Social Science) was used for statistical analysis.
Sample size
In a previous study 6 that included patients with chronic and ADHF, 4 deaths out of 30 chronic HF patients with stable symptoms (NYHA I/II and haemodynamic profile A) were observed in 12 months. Three of them occurred in patients with EBA higher than the median concentration of acetone in that population of chronic HF patients (1.22 ug/L) and one with EBA lower than that. Using these data as prior, a study of at least 334 chronic HF patients to test the hypothesis that EBA could be a predictor of mortality, would have 95%power at a significance level of 5%.
Results
Baseline characteristics
Between August 2012 and December 2014, 695 patients were enrolled in the study. Table 1 depicts the baseline characteristics of the sample of the study. Most patients were male patients (67%), with hypertensive or ischaemic aetiology (48%), NYHA class I/II (81%) with mean left ventricular ejection fraction of 32.0 ± 8.6%, and median (interquartile range) B‐type natriuretic peptide (BNP) levels of 149 (54–355) pg/mL. The great majority was taking guideline‐directed medical therapy: betablockers, 96.8%; angiotensin converter enzyme inhibitor or angiotensin receptor blocker, 91.4%; and spironolactone, 66.4% of patients. Twenty percent of patients were taking oral hypoglycemic drugs, but none of them were taking sodium‐glucose cotransporter 2 inhibitors (SGLT‐2i). (Table 1 ).
Table 1.
Baseline characteristics of heart failure patients
| Variable | Total (n = 695) |
|---|---|
| Age (years) | 55.4 ± 12.2 |
| Gender (Male) n (%) | 470 (67.6) |
| Race n (%) | |
| Asian | 7 (1.0) |
| Black | 107 (15.4) |
| Mixed | 345 (49.6) |
| White | 236 (34.0) |
| Hypertension n (%) | 448 (64.5) |
| Diabetes n (%) | 205 (29.5) |
| Dyslipidemia n (%) | 462 (66.5) |
| Smoking n (%) | 64 (9.2) |
| Chronic renal failure n (%) | 188 (27.0) |
| Dialytic renal failure n (%) | 8 (1.2) |
| Heart failure class n (%) | |
| NYHA I | 130 (18.7) |
| NYHA II | 433 (62.3) |
| NYHA III | 127 (18.3) |
| NYHA IV | 5 (0.7) |
| Left ventricular ejection fraction (%) | 32.0 ± 8.6 |
| Left ventricular diastolic diameter (mm) | 64.1 ± 8.3 |
| RV dysfunction (%) | 221 (31.8) |
| Mild | 112 (16.1) |
| Moderate/severe | 109 (15.7) |
| Heart failure aetiology n (%) | |
| Hypertensive | 181 (26.0) |
| Ischaemic | 152 (21.9) |
| Chagasic | 118 (17.0) |
| Idiopathic | 108 (19.6) |
| Other | 136 (8.6) |
| Weight (kg) | 75.8 ± 19.1 |
| Body mass index (BMI) | 27.9 ± 6.0 |
| Respiratory rate (irm) | 17.1 ± 1.5 |
| Heart rate (bpm) | 71.2 ± 14.3 |
| Systolic blood pressure (mmHg) | 123.5 ± 23.8 |
| Diastolic blood pressure (mmHg) | 76.2 ± 14.6 |
| Dyspnoea n (%) | 599 (86.2) |
| Orthopnoea n (%) | 263 (37.8) |
| Paroxysmal nocturnal dyspnoea n (%) | 150 (21.6) |
| Jugular venous distension n (%) | 250 (36.0) |
| Pulmonary rales n (%) | 52 (7.5) |
| Peripheral oedema n (%) | 127 (18.3) |
| Third heart sound n (%) | 50 (7.2) |
| Hepatojugular reflux n (%) | 71 (10.2) |
| Capillary filling time (3–5 s) n (%) | 19 (2.7) |
| Ascites n (%) | 18 (2.6) |
| Hepatomegaly n (%) | 79 (11.4) |
| Creatinine (mg/dL) | 1.27 ± 0.77 |
| Blood urea nitrogen (mg/dL) | 49.3 ± 23.7 |
| Glomerular filtration rate (mL/min) | 68.2 ± 22.7 |
| Sodium (mg/dL) | 139.3 ± 2.72 |
| Potassium (mg/dL) | 4.8 ± 0.6 |
| Haemoglobin (mg/dL) | 13.9 ± 1.7 |
| Haematocrit (%) | 43.0 ± 5.2 |
| Blood glucose fasting (mg/dL) | 113.6 ± 51.2 |
| Glycated haemoglobin | 6.3 ± 0.7 |
| High sensitive I troponin (ng/dL) | 0.040 ± 0.062 |
| Serum albumin (g/dL) | 4.0 ± 0.4 |
| Alanine aminotransferase (mg/dL) | 32.3 ± 16.8 |
| Aspartate aminotransferase (mg/dL) | 28.5 ± 14.6 |
| Gamma glutamyl transpeptidase (mg/dL) | 110.2 ± 187.1 |
| Alkaline phosphatase (mg/dL) | 95.4 ± 57.4 |
| Total Bilirubin (mg/dL) | 0.64 ± 0.44 |
| Exhaled breath acetone (ug/dL) | 0.6 (0.3–1.2) |
| Brain natriuretic peptide (pg/mL) | 149 (54–355) |
| Medication in use | |
| Betablocker n (%) | 673 (96.8) |
| ACEI n (%) | 427 (61.4) |
| ARB n (%) | 207 (30.0) |
| ACEI or ARB n (%) | 633 (91.1) |
| Spironolactone n (%) | 448 (66.4) |
| Diuretic n (%) | 631 (90.8) |
| Loop diuretic n (%) | 504 (72.5) |
| Thiazide diuretic n (%) | 135 (19.4) |
| Hydralazine n (%) | 116 (16.7) |
| Nitrate n (%) | 98 (14.1) |
| Digoxin n (%) | 165 (23.7) |
| Lipid lowering drugs n (%) | 332 (47.8) |
| Oral anticoagulants n (%) | 141 (20.3) |
| Oral hypoglycaemic drugs n (%) | 139 (20.0) |
| Insulin n (%) | 55 (7.9) |
ACEI, angiotensin converter enzyme inhibitor; ARB, angiotensin receptor blocker; NYHA, New York Heart Association; RV, right ventricle.
Continuous variables are expressed as mean ± SD or median and interquartile range [median (IQR)] and categorical variables as absolute number and percentage [n (%)].
The median concentration of EBA was 0.6(0.3–1.2) ug/dL. There was no difference between acetone levels in patients with [0.7 (0.4–1.2)] ug/L and without diabetes [0.6 (0.3–1.2)] ug/L. Patients were analysed according to the EBA quartile. Baseline characteristics of the sample in each EBA quartile are depicted in Table 2 . Patients (n = 164) in the upper EBA quartile (EBA > 1.2ug/L) tend to be older; to have lower weight and body mass index; lower left ventricular ejection fraction and systolic blood pressure; and higher heart rate when compared with inferior quartiles. The incidence of Chagas aetiology in the upper quartile was higher than in inferior ones.
Table 2.
Baseline characteristics according to exhaled breath acetone quartile
| Variable | Quartile 1 (n = 178) | Quartile 2 (n = 168) | Quartile 3 (n = 169) | Quartile 4 (164) | P |
|---|---|---|---|---|---|
| Age (years) | 53.2 ± 11.6 | 55.5 ± 11.8 | 56.7 ± 12.4 * | 56.3 ± 12.8 * | 0.033 |
| Gender (male) n (%) | 115 (64.6) | 107 (63.7) | 115 (68.0) | 123 (75.0) | 0.110 |
| Ethnicity n (%) | 0.225 | ||||
| Asian | 3 (1.7) | 3 (1.8) | 1 (0.6) | 0 (0) | |
| Black | 23 (12.9) | 20 (11.9) | 33 (19.5) | 30 (18.3) | |
| Mixed | 88 (49.4) | 87 (51.9) | 87 (51.5) | 73 (44.5) | |
| White | 64 (36.0) | 58 (34.5) | 48 (28.4) | 61 (9.0) | |
| Hypertension n (%) | 124 (69.7) | 101 (60.1) | 121 (71.6) | 90 (54.9) * | 0.003 |
| Diabetes n (%) | 42 (23.6) | 53 (31.5) | 58 (34.3) | 46 (28.0) | 0.146 |
| Dyslipidemia n (%) | 120 (67.4) | 133 (79.2) * | 103 (60.9) | 94 (57.3) * | < 0.001 |
| Smoking n (%) | 22 (12.4) | 7 (4.2) | 19 (11.2) | 16 (9.8) | 0.048 |
| Chronic renal failure n (%) | 33 (18.5) * | 48 (28.6) * | 45 (26.6) | 58 (35.4) * | 0.006 |
| HF class n (%) | 0.107 | ||||
| NYHA I | 39 (21.9) | 33 (19.6) | 30 (17.8) | 22 (13.4) | |
| NYHA II | 110 (61.8) | 104 (61.9) | 114 (67.5) | 101 (61.6) | |
| NYHA III | 28 (15.7) | 31 (18.5) | 25 (14.8) | 38 (23.2) | |
| NYHA IV | 1 (0.6) | 0 (0) | 0 (0) | 3 (0.4) | |
| Ejection fraction (%) | 33.3 ± 8.9 | 32.7 ± 8.2 | 31.8 ± 8.8 | 30.4 ± 8.6 * | 0.015 |
| LVDD (mm) | 63.6 ± 7.7 | 64.7 ± 7.9 | 63.8 ± 8.0 | 64.5 ± 9.5 | 0.521 |
| Mod/Severe RV dysfunction n(%) | 20 (11.2) | 22 (13.1) | 22 (13.0) | 43 (26.2) * | <0.001 |
| Heart failure aetiology n (%) | 0.001 | ||||
| Hypertensive | 65 (36.5) * | 29 (17.3) * | 50 (29.6) | 32 (19.5) * | |
| Ischaemic | 32 (18.0) | 38 (22.6) | 41 (24.3) | 37 (22.6) | |
| Chagasic | 28 (15.7) | 23 (13.7) | 28 (16.6) | 37 (22.6) * | |
| Idiopathic | 24 (13.5) | 39 (23.2) * | 19 (11.2) | 23 (14.0) | |
| Other | 29 (16.3) | 39 (23.2) | 31 (18.3) | 35 (21.3) | |
| Weight (kg) | 78.7 ± 17.7 | 77.9 ± 17.9 | 75.6 ± 21.9 | 70.9 ± 17.5 * | 0.001 |
| Body mass index (BMI) | 28.9 ± 5.6 | 28.8 ± 5.6 | 27.8 ± 6.8 | 26.0 ± 5.5 * | <0.001 |
| Respiratory rate (irm) | 17.3 ± 1.4 | 17.3 ± 1.8 | 17.0 ± 1.3 | 16.7 ± 1.5 * | <0.001 |
| Heart rate (bpm) | 68.9 ± 12.7 | 69.5 ± 12.8 | 71.5 ± 14.2 | 74.9 ± 16.5 * | <0.001 |
| Systolic BP (mmHg) | 128.9 ± 24.7 | 122.3 ± 22.3 * | 124.4 ± 23.6 | 118.1 ± 23.6 * | <0.001 |
| Diastolic BP (mmHg) | 78.1 ± 14.6 | 74.7 ± 13.7 | 76.3 ± 14.4 | 75.6 ± 15.6 | 0.172 |
| Dyspnoea n (%) | 153 (86.0) | 148 (88.1) | 143 (84.6) | 144 (87.8) | 0.761 |
| Orthopnoea n (%) | 63 (35.4) | 69 (41.1) | 55 (32.5) | 68 (41.5) | 0.249 |
| Paroxysmal nocturnal dyspnoea n (%) | 36 (20.2) | 24 (14.3) * | 37 (21.9) | 47 (28.7) * | 0.015 |
| Jugular venous distension n (%) | 43 (24.2) * | 51 (30.4) | 55 (32.5) | 96 (58.5) * | < 0.001 |
| Pulmonary rales n (%) | 10 (5.6) | 10 (6.0) | 11 (6.5) | 20 (12.2) | 0.074 |
| Peripheral oedema n (%) | 29 (16.3) | 23 (13.7) | 29 (17.2) | 44 (26.8) * | 0.012 |
| Third heart sound n (%) | 3 (1.7) * | 4 (2.4) * | 12 (7.1) | 30 (18.3) * | <0.001 |
| Hepatojugular reflux n (%) | 8 (4.5) * | 7 (4.2) * | 12 (7.1) | 44 (26.8) * | < 0.001 |
| Capillary filling time (3–5 s) n (%) | 3 (1.7) | 3 (1.8) | 1 (0.6) * | 12 (7.3) * | 0.001 |
| Ascites n (%) | 3 (1.7) | 1 (0.6) | 1 (0.6) | 13 (7.9) | < 0.001 |
| Hepatomegaly n (%) | 14 (7.9) | 7 (4.2) | 15 (8.9) | 43 (26.2) | <0.001 |
| Creatinine (mg/dL) | 1.16 ± 0.52 | 1.30 ± 0.85 | 1.30 ± 0.99 | 1.31 ± 0.66 | 0.180 |
| Blood urea nitrogen (mg/dL) | 43.5 ± 18.7 | 50.2 ± 22.5 * | 49.3 ± 24.9 | 54.6 ± 27.1 * | <0.001 |
| Glomerular filtration rate (mL/min) | 67.5 ± 24.2 | 68.2 ± 21.7 | 67.4 ± 22.2 | 69.6 ± 23.5 | 0.811 |
| Sodium (mg/dL) | 139.8 ± 2.7 | 140.2 ± 2.6 | 139.4 ± 2.5 | 140.0 ± 3.1 | 0.056 |
| Haemoglobin (mg/dL) | 14.0 ± 1.6 | 13.9 ± 1.9 | 13.9 ± 1.7 | 13.9 ± 1.7 | 0.917 |
| Blood glucose fasting (mg/dL) | 109.5 ± 35.8 | 116.6 ± 55.2 | 117.5 ± 55.6 | 110.9 ± 56.2 | 0.366 |
| Glycated haemoglobin (n = 269) | 6.2 ± 1.2 | 6.5 ± 1.7 | 6.7 ± 1.8 | 6.5 ± 1.6 | 0.111 |
| BNP (pg/mL) | 70.5 (28.8–179.5) | 107.5 (40.5–249.0) | 165.0 (67.0–359.5) | 350.5 (127.5–980.3) * | <0.001 |
BNP, B‐type natriuretic peptide; BP, blood pressure; HF, heart failure; LVDD, left ventricular diastolic diameter; NYHA, New York Heart Association; RV, right ventricle.
ANOVA post‐hoc test Dunnet lowest quartile as reference.
Variables with P < 0.05.
Breath acetone and heart failure clinical characteristics
Concentration of EBA was analysed according to the number of symptoms/signs HF patients presented at their first evaluation. In this analysis, we included all typical symptoms and signs of HF syndrome: dyspnoea, orthopnoea, paroxysmal nocturnal dyspnoea, jugular venous distension, pulmonary rales, peripheral oedema, third heart sound, hepatojugular reflux, capillary filling time higher than 3 seconds, ascites, and hepatomegaly. Figure 1 , panel A, shows that acetone levels go up as the number of HF symptoms/signs increase. The relation between acetone and left or right HF symptoms/signs is depicted in Figure 1 , panels B and C, respectively. Acetone levels rise significantly when patients have three or more left or right HF symptoms/signs.
Figure 1.
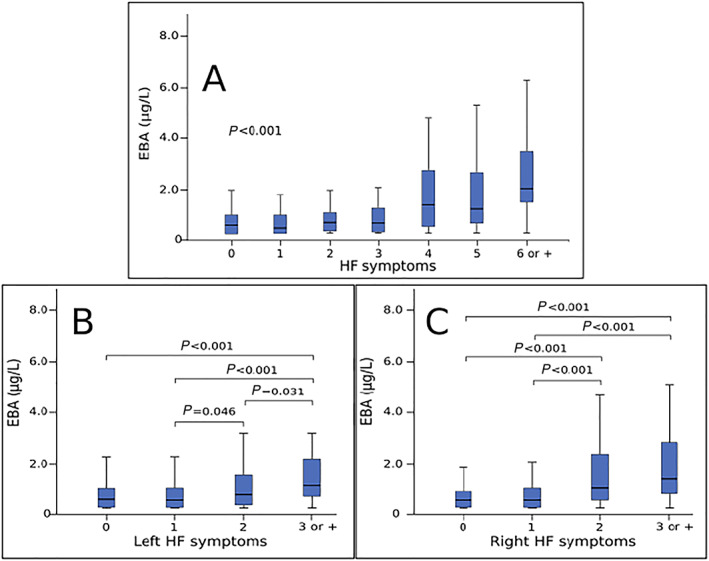
Exhaled breath acetone concentration according to number of heart failure symptoms. Panel A: Exhaled breath acetone (EBA) levels according to the number of heart failure symptoms. Panel B: EBA levels according to the number of left heart failure symptoms. Panel C: EBA levels according to the number of right heart failure symptoms.
By logistic regression, we analysed which clinical variables could predict EBA concentration, and we found that presence of a third heart sound [HR = 5.09(2.66–9.76), P < 0.001], hepatojugular reflux [HR = 3.38(1.78–6.39), P < 0.001], and jugular venous distension [HR = 1.99(1.23–2.95), P = 0.004] are independent predictors of EBA concentration. These findings are in Supporting Information Table S1 .
Patients in the upper EBA quartile (EBA > 1.2ug/L) tend to have higher levels of liver biochemical tests, which suggests that acetone levels increase in patients with liver congestion. It reinforces the idea that acetone reflects clinical characteristics of HF, especially right HF. Table 3 depicts liver biochemical tests according to EBA quartile.
Table 3.
Liver biochemical test according to exhaled breath acetone concentration
| Variable | Quartile 1 (n = 178) | Quartile 2 (n = 168) | Quartile 3 (n = 169) | Quartile 4 (164) | P |
|---|---|---|---|---|---|
| Serum albumin (g/dL) | 4.1 ± 0.4 | 4.1 ± 0.3 | 4.0 ± 0.4 | 4.0 ± 0.4* | 0.039 |
| Alanine aminotransferase (mg/dL) | 33.7 ± 19.0 | 29.2 ± 13.3* | 32.0 ± 16.7 | 34.4 ± 17.2 | 0.022 |
| Aspartate aminotransferase (mg/dL) | 27.5 ± 12.6 | 26.2 ± 12.3 | 27.9 ± 13.7 | 32.8 ± 18.3* | <0.001 |
| GGT (mg/dL) | 77.6 ± 86.6 | 76.4 ± 96.5 | 114.1 ± 278.4 | 176.3 ± 203.3* | <0.001 |
| Alkaline phosphatase (mg/dL) | 87.4 ± 59.7 | 89.6 ± 40.3 | 95.8 ± 65.2 | 109.6 ± 59.2* | 0.002 |
| Total bilirubin (mg/dL) | 0.55 ± 0.41 | 0.57 ± 0.37 | 0.62 ± 0.34 | 0.85 ± 0.57* | <0.001 |
GGT, gamma glutamyl transpeptidase
Breath acetone and outcomes
The median follow‐up time was 12.4 months, and no patient was lost to follow‐up. During this period, 47 (6.7%) patients died. Thirty‐nine (5.6%) died from cardiac cause, being 61% from HF and eight patients from non‐cardiovascular cause (five with infection and three unknown cause). Survival curves for acetone stratified according to EBA quartile against cardiac and all‐cause mortality are shown in Figure 2 . Concentrations of EBA above 1.2 ug/dL (upper quartile) in patients with chronic HF were associated with a progressively worse prognosis in 18‐month follow‐up (log rank test, P < 0.001 for both outcomes). There was no interaction between acetone and the presence of diabetes (P = 0.90 for interaction) or renal failure (P = 0.99 for interaction) to predict mortality. The comparison between baseline characteristics and medication in use of patients in EBA upper quartile (quartile 4) and inferior quartiles (quartiles 1–3) is depicted in Supporting Information Tables S2 and S3 , respectively.
Figure 2.
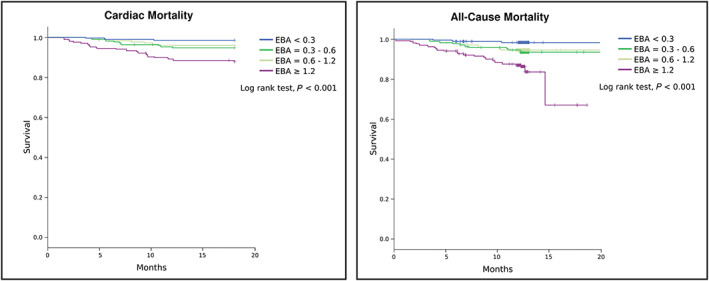
Kaplan–Meier curves for cardiac and all‐cause death according to exhaled breath acetone quartile. EBA, exhaled breath acetone.
Variables considered potential predictors of mortality in HF patients (age, gender, left ventricular ejection fraction, NYHA class, body mass index, presence of diabetes, serum creatinine, sodium, haemoglobin, BNP, high‐risk aetiology, chagasic and ischaemic disease, and EBA) were included in univariable Cox regression model.
EBA levels higher than 1.2 ug/L increased the risk of cardiac death in 3.74 times [HR = 3.74 (1.95–7.20), P < 0.001]. After multivariable Cox regression analysis, the independent predictors of cardiac mortality were EBA upper quartile [HR = 2.52 (1.24–5.13), P < 0.001)], BNP [HR = 1.005 (1.002–1.009), P = 0.002)] and high‐risk HF aetiology [HR = 3.07 (1.52–6.19), P = 0.002)]. Table 4 depicts the univariable and multivariable Cox proportional regression analysis for cardiac mortality. EBA upper quartile was also an independent predictor of all‐cause mortality as well as BNP and high‐risk aetiology: EBA upper quartile [HR = 2.27 (1.22–4.20), P = 0.010)]; BNP [HR = 1.003 (1.001–1.006), P = 0.016)]; high‐risk HF aetiology [HR = 2.31 (1.24–4.31), P = 0.009)] (Supporting information, Table S4 ).
Table 4.
Univariable and Cox proportional multivariable regression for 18‐month cardiac mortality
| Univariable regression model for 18‐month cardiac mortality | |||
|---|---|---|---|
| Variable | HR | 95% CI | P |
| EBA upper quartile | 3.74 | 1.95–7.20 | <0.001 |
| High‐risk HF aetiology a | 3.38 | 1.70–6.69 | <0.001 |
| BNP (for each 10 units increase) | 1.007 | 1.004–1.010 | <0.001 |
| Serum creatinine | 1.32 | 1.02–1.70 | 0.033 |
| Left ventricular ejection fraction | 0.95 | 0.91–0.99 | 0.011 |
| Age | 1.024 | 0.996–1.052 | 0.092 |
| Gender | 1.64 | 0.76–3.52 | 0.206 |
| Haemoglobin | 0.85 | 0.71–1.02 | 0.087 |
| Sodium | 0.96 | 0.85–1.08 | 0.505 |
| Body mass index | 0.95 | 0.89–1.01 | 0.114 |
| Diabetes | 0.70 | 0.33–1.51 | 0.368 |
| NYHA 3 | 1.83 | 0.70–4.81 | 0.219 |
| NYHA 4 | 11.71 | 1.68–81.88 | 0.013 |
| Cox proportional multivariable regression model for 18‐month cardiac mortality | |||
| Variable | HR | 95% CI | P |
| EBA upper quartile | 2.52 | 1.24 – 5.13 | 0.011 |
| High‐risk HF aetiology a | 3.07 | 1.52–6.19 | 0.002 |
| BNP (for each 10 units increase) | 1.005 | 1.002–1.009 | 0.002 |
| Serum creatinine | 1.32 | 0.97–1.81 | 0.082 |
| Left ventricular ejection fraction | 0.97 | 0.92–1.01 | 0.115 |
| NYHA 4 | 2.59 | 0.28–23.95 | 0.401 |
BNP, B‐type natriuretic peptide; EBA, exhaled breath acetone; HF, heart failure; NYHA, New York Heart Association.
Chagasic and ischaemic aetiology.
During the period of study, 123 hospitalizations for HF were registered. EBA levels higher than 1.2 ug/L were associated with hospitalizations for HF [HR = 1.9 (1.3–2.7), P = 0.001], but after adjustment for other potential predictors, EBA was not an independent predictor of hospitalization. In multivariate Cox regression model, age [HR = 1.03 (1.02–1.05), P < 0.001], BNP [HR = 1.005 (1.002–1.008), P = 0.001], and NYHA class III [HR = 2.70 (1.35–5.39), P = 0.005] and NYHA IV [HR = 2.06 (2.01–2.12), P = 0.011] were the independent predictors of hospitalization for HF.
Discussion
There are two relevant findings in this study. First of all, it shows that EBA levels reflect clinical changes of HF in patients with chronic stable disease. In particular, we were able to disclose a significant correlation between breath acetone and right HF symptoms. Second, it shows EBA as an independent predictor of cardiac and overall mortality in chronic stable HF patients. Interestingly, the capacity of breath acetone to predict outcomes in patients with HF was not influenced by the presence of diabetes.
Breath acetone has already been described as a biomarker of HF. However, previous studies involved patients with ADHF. 4 , 11 , 12 Also, most of the studies excluded patients with diabetes and/or renal failure. 4 , 11 Kupari et al. showed that ADHF patients (n = 31) presented significantly higher levels of breath acetone than patients with heart disease with no HF and healthy individuals. 11 Our group, in a previous study, showed that not only HF patients (n = 89) have higher breath acetone concentration when compared with healthy people but also that acetone levels increase according to NYHA class, which suggests an association between acetone levels and the severity of the disease. 4 A group of researchers from the Cleveland Clinic also observed higher levels of breath acetone in 35 patients with ADHF when compared with healthy ones. 12 More recently, Yokokawa et al. showed a positive correlation between breath acetone and pulmonary capillary wedge pressure in patients with non‐ischaemic HF, suggesting breath acetone could be a marker of haemodynamic severity. They included patients with stable symptoms (NYHA II/III) but excluded patients with diabetes or severe renal dysfunction. 13 Considering all these findings, it is possible to say that the role of acetone as a biomarker of ADHF is well established. However, this is the first time EBA is unveiled as a biomarker of chronic HF, even considering patients with different comorbidities.
It is also important to highlight the association between breath acetone and clinical/laboratorial signs of right ventricular dysfunction such as hepatojugular reflux, jugular venous distension, and liver biochemical tests. This relation has been previously suggested, 4 but in the current study, this association has been confirmed because these signs are predictors of high levels of breath acetone. We hypothesize that hepatic congestion is involved in the mechanism of production of acetone.
Regarding the role of acetone as a biomarker of prognosis of HF, our group has previously suggested the ability of acetone to predict outcomes. In a cohort of 89 HF with reduced ejection fraction patients, we showed that EBA was associated with a worse prognosis. Levels of acetone higher than 3.7 ug/L increased the risk of death or heart transplantation about three times within 12 months. However, the study included mainly patients with ADHF, the number of patients with chronic HF disease (n = 30) was small, and all patients with diabetes were excluded. 6 The current study confirmed that EBA, collected at enrolment, is a predictor of outcomes in a broader population because it included greater number of patients who had had stable symptoms (81% NYHA I/II) during the previous 3 months and no hospitalization during this period. Also, diabetic patients were not excluded.
Heart metabolism in HF has been the focus of several studies in the last decades. Indeed, the human healthy heart requires enormous amounts of energy to sustain contractile function. It is known that ATP production in myocardium is based on mitochondrial oxidative phosphorylation, which means that energy source comes mainly from fatty acids (about 95%), while glucose is responsible for the other 5%. 14 , 15 However, the myocardial fuel utilization changes in the failing heart. There is a shift from free fatty acids (FFA) to glucose as a source of energy in advanced HF, increasing the reliance on glycolysis. 16 , 17
Two recent articles 18 , 19 analysed different metabolites, proteins, and gene transcripts in the failing heart muscle and concluded that enzymes regulating glucose and fatty acid metabolism are down‐regulated while those regulating ketone body metabolism are up‐regulated. One of these studies showed that mouse models of heart failing use ketone bodies as a fuel instead of fatty acids. They proposed that this metabolic shift is triggered by reduced capacity for fatty acids oxidation. 18 The second study analysed non‐diabetic patients with advanced dilated non‐ischaemic cardiomyopathy and showed an increase in FFA serum concentration and a significant increase in the serum concentration of β‐hydroxybutyrate (βOHB) at the time of heart transplantation. The high circulating levels of FFA and βOHB drive hepatic ketogenesis to synthesize ketones. Despite this increase in serum βOHB, they observed a decrease in myocardial βOHB, which suggests augmented utilization of ketone body in the myocardium of patients with end‐stage HF disease. 19
These studies 18 , 19 reinforce the hypothesis that adrenergic stimulation induces lipolysis, increases FFA concentration and ketone bodies production in advanced HF patients, 20 , 21 which can be measured in the blood 22 or in the breath. 4 , 11 But also add the concept that ketone bodies could be used as a source of energy for the myocardium in end‐stage HF. In fact, changes in ketone body metabolism are clearly present in failing heart but if it is the cause or consequence of HF is still to be determined.
Some limitations should be acknowledged. First of all, we evaluated patients from a specialized cardiology centre of a tertiary university hospital. Second, exhaled acetone measures were taken once, at inclusion in the study, and changes in acetone levels after enrolment were not analysed. Finally, it is a prospective observational single‐centre study. Therefore, our results might not be generalized to other HF populations.
Conclusions
The present study shows that breath acetone is a non‐invasive HF biomarker, whose levels reflect clinical features of chronic HF, especially right HF symptoms. Moreover, breath acetone proved to be an independent predictor of cardiac and overall mortality in chronic HF patients. In this way, studies designed to test the use of this biomarker as a guide to HF treatment should be of great interest.
Clinical implications
The findings of this study reveal a biomarker of HF, which reflects metabolic changes of this syndrome and is related to worse prognosis. Our data suggest that the increase in breath acetone in HF patients indicates a need for improvement in the treatment of such patients.
Conflict of interest
There is no conflict of interest to declare.
Funding
The study was supported by Proadi SUS‐SIPAR (grant 25000.180672/2011–8) and Sao Paulo Research Foundation‐FAPESP for ACP (grant 2013/17368–0) and by an unrestricted grant from Novartis (grant 3001765759) to pay the costs related to laboratorial tests.
Supporting information
Table S1. Logistic Regression of clinical variables to predict the exhaled breath acetone upper quartile
Table S2. Baseline characteristics of study population according to inferior and superior quartiles
Table S3. Medication in use according to exhaled breath acetone quartile
Table S4. Univariable and COX proportional multivariable regression for 18‐month all‐cause mortality
Acknowledgement
We are grateful to the Central Analytical Facility of the Chemistry Institute for the gas chromatography mass spectrometry analysis.
Marcondes‐Braga, F. G. , Gioli‐Pereira, L. , Bernardez‐Pereira, S. , Batista, G. L. , Mangini, S. , Issa, V. S. , Fernandes, F. , Bocchi, E. A. , Ayub‐Ferreira, S. M. , Mansur, A. J. , Gutz, I. G. R. , Krieger, J. E. , Pereira, A. C. , and Bacal, F. (2020) Exhaled breath acetone for predicting cardiac and overall mortality in chronic heart failure patients. ESC Heart Failure, 7: 1744–1752. 10.1002/ehf2.12736.
Trial registration: Current Controlled Trials NTC02043431.
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire D, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics‐2014 update: a report from the American Heart Association. Circulation 2014; 129: 399–410. [DOI] [PubMed] [Google Scholar]
- 2. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE‐HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF Registry. J Am Coll Cardiol 2007; 50: 768–777. [DOI] [PubMed] [Google Scholar]
- 3. Albuquerque DC, Neto JD, Bacal F, Rohde LE, Bernardez‐Pereira S, Berwanger O, Almeida DR, Investigadores Estudo BREATHE . I Brazilian Registry of Heart Failure‐clinical aspects, care quality and hospitalization outcomes. Arq Bras Cardiol 2015; 104: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcondes‐Braga FG, Gutz IGR, Batista GL, Saldiva PHN, Ayub‐Ferreira SM, Issa VS, Mangini S, Bocchi EA, Bacal F. Exhaled acetone as a new biomaker of heart failure severity. Chest 2012; 142: 457–466. [DOI] [PubMed] [Google Scholar]
- 5. Bocchi EA, Braga FGM, Ferreira SMA, Rohde LEP, de Oliveira WA, de Almeida DR, Moreira MC, Bestetti RB, Bordignon S, Azevedo C, Tinoco EM. III Brazilian Guidelines for Chronic Cardiac Insufficiency‐coordinator of regulations and guidelines of the SBC. Arq Bras Cardiol 2009; 93: 1–71.19838462 [Google Scholar]
- 6. Marcondes‐Braga FG, Batista GL, Gutz IG, Saldiva PH, Mangini S, Issa VS, Ayub‐Ferreira SM, Bocchi EA, Pereira AC, Bacal F. Impact of exhaled breath acetone in the prognosis of patients with heart failure with reduced ejection fraction (HFrEF). One year of clinical follow‐up. PLoS ONE 2016; 11: e0168790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gioli‐Pereira L, Bernardez‐Pereira S, Marcondes‐Braga FG, Spina JM, da Silva RM, Ferreira NE, Bacal F, Fernandes F, Mansur AJ, Krieger JE, Pereira AC. Genetic and ElectroNic medIcal records to predict oUtcomeS in Heart Failure patients (GENIUS‐HF)‐design and rationale. BMC Cardiovasc Disord 2014; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Batista GL. Breath acetone analysis: methodology for studying hospitalized patients[master's dissertation]. São Paulo, Brazil: Chemistry Institute, University of Sao Paulo; 2010. Available at teses.usp.br/teses/disponiveis/46/46133/tde13122010wh102634/publico/DissertGuilhermeLBatista. 2010.
- 9. Teshima N, Li J, Toda K, Dasgupta PK. Determination of acetone in breath. Anal Chim Acta 2005; 535: 189–199. [Google Scholar]
- 10. Berntsson S. Spectrophotometric determination of acetone by salicylaldehyde method. Anal Chem 1956; 28: 1337. [Google Scholar]
- 11. Kupari M, Lommi J, Ventilä M, Karjalainen U. Breath acetone in congestive heart failure. Am J Cardiol 1995; 76: 1076–1078. [DOI] [PubMed] [Google Scholar]
- 12. Samara MA, Tang WH, Cikach F, Gul Z, Tranchito L, Paschke KM, Viterna J, Wu Y, Laskowski D, Dweik RA. Single exhaled breath metabolomic analysis identifies unique breathprint in patients with acute decompensated heart failure. J Am Coll Cardiol 2013; 61: 1463–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yokokawa T, Sugano Y, Shimouchi A, Shibata A, Jinno N, Nagai T, Kanzaki H, Aiba T, Kusano K, Shirai M, Takeishi Y, Yasuda S, Ogawa H, Anzai T. Exhaled acetone concentration is related to hemodynamic severity in patients with non‐ischemic chronic heart failure. Circ J 2016; 80: 1178–1186. [DOI] [PubMed] [Google Scholar]
- 14. Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 1954; 16: 504–515. [DOI] [PubMed] [Google Scholar]
- 15. Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C‐labeled substrates in humans. J Clin Invest 1987; 79: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007; 356: 1140–1151. [DOI] [PubMed] [Google Scholar]
- 17. Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knöll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 2012; 125: 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 2016; 133: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016; 133: 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinberg D, Nestel PJ, Buskirk ER, Thompson RH. Calorigenic effect of norepinephrine correlated with plasma free fatty acid turnover and oxidation. J Clin Invest 1964; 43: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lommi J, Kupari M, Yki‐Järvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol 1998; 81: 45–50. [DOI] [PubMed] [Google Scholar]
- 22. Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, Härkönen M. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol 1996; 28: 665–672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Logistic Regression of clinical variables to predict the exhaled breath acetone upper quartile
Table S2. Baseline characteristics of study population according to inferior and superior quartiles
Table S3. Medication in use according to exhaled breath acetone quartile
Table S4. Univariable and COX proportional multivariable regression for 18‐month all‐cause mortality


