Abstract
Aims
In heart failure (HF) with preserved ejection fraction (HFpEF), microvascular inflammation is proposed as an underlying mechanism. Myeloperoxidase (MPO) is associated with vascular dysfunction and prognosis in congestive HF.
Methods and results
MPO, MPO‐related biomarkers, and echocardiography were assessed in 86 patients, 4–8 weeks after presentation with acute HF (EF ≥ 45%), and in 46 healthy controls. Patients were followed up for median 579 days (Q1;Q3 276;1178) regarding the composite endpoint all‐cause mortality or HF hospitalization. Patients were 73 years old, 51% were female, EF was 64% (Q1;Q3 58;68), E/e′ was ratio 10.8 (8.3;14.0), and left atrial volume index (LAVI) was 43 mL/m2 (38;52). Controls were 60 (57;62) years old (vs. patients; P < 0.001), 24% were female (P = 0.005), and left ventricular EF was 63% (59;66; P = 0.790). MPO was increased in HFpEF compared with controls, 101 (81;132) vs. 86 (74;101 ng/mL, P = 0.015), as was uric acid 369 (314;439) vs. 289 (252;328 μmol/L, P < 0.001), calprotectin, asymmetric dimethyl arginine (ADMA), and symmetric dimethyl arginine (SDMA), while arginine was decreased. MPO correlated with uric acid (r = 0.26; P = 0.016). In patients with E/e′ > 14, uric acid and SDMA were elevated (421 vs. 344 μM, P = 0.012; 0.54 vs. 0.47 μM, P = 0.039, respectively), and MPO was 121 vs. 98 ng/mL (P = 0.090). The ratios of arginine/ADMA (112 vs. 162; P < 0.001) and ADMA/SDMA (1.36 vs. 1.17; P = 0.002) were decreased in HFpEF patients, suggesting reduced NO availability and increased enzymatic clearance of ADMA, respectively. Uric acid independently predicted the endpoint [hazard ratio (HR) 3.76 (95% CI 1.19–11.85; P = 0.024)] but not MPO [HR 1.48 (95% CI 0.70–3.14; P = 0.304)] or the other biomarkers.
Conclusions
In HFpEF, MPO‐dependent oxidative stress reflected by uric acid and calprotectin is increased, and SDMA is associated with diastolic dysfunction and uric acid with outcome. This suggests microvascular neutrophil involvement mirroring endothelial dysfunction, a central component of the HFpEF syndrome and a potential treatment target.
Keywords: Myeloperoxidase, Microvascular inflammation, Endothelial dysfunction, Heart failure with preserved ejection fraction, Prognosis
Introduction
The diagnosis of heart failure (HF) is associated with either reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF). The latter is a heterogeneous group of patients making up nearly 50% of the HF population 1 and with a 65% mortality rate 5 years after hospitalization, making the prognosis as ominous as that of HFrEF. 1 Despite improvements in understanding the underlying disease mechanisms in HFrEF, many of the mechanisms in HFpEF remain unknown, and the syndrome has been referred to as the greatest unmet need in cardiovascular medicine. 2
In HFpEF, non‐cardiac co‐morbidities such as diabetes, obesity, hypertension, and chronic kidney disease are common 3 and have the ability to induce systemic inflammation proposed to cause microvascular endothelial dysfunction, in the myocardium as well as in the periphery. 4 , 5
Myeloperoxidase (MPO) is a leukocyte‐derived heme protein associated with vascular dysfunction and a prognostic biomarker in cardiovascular disease, including congestive HF. MPO catalyses the formation of hypochlorous acid, among other reactive oxidants. In cardiovascular disease, this is suggested to occur in the subendothelial glycocalyx, where MPO is electrostatically trapped to proteoglycans causing vascular dysfunction mediated by direct and indirect oxidation of nitric oxide (NO). 6 This may result in hypoxia, ATP depletion, and increased purine catabolism and accumulation of uric acid. 7
MPO may also affect vascular function by lowering availability of the NO‐substrate arginine through symmetric dimethyl arginine (SDMA) and asymmetric dimethyl arginine (ADMA). 8 In cardiovascular disease and HF, MPO and calprotectin, also released in response to oxidative stress, are both involved in amplifying the inflammatory response. 9 , 10
Elevated levels of MPO have been reported in patients with chronic HFrEF compared with controls and correlate with disease severity as defined by New York Heart Association (NYHA) class, N‐terminal brain natriuretic peptide (NT‐proBNP), and echocardiographic measurements of systolic and diastolic function 11 , 12 , 13 and remodelling 14 after myocardial infarction. Further, increasing levels of MPO are associated with mortality in patients with HFrEF 13 and acute HF. 15
The aim of the present study was to investigate MPO‐related oxidative stress through biomarkers reflecting neutrophil involvement (calprotectin), tissue hypoxia (uric acid), and NO availability (arginine, ADMA, SDMA), by comparing concentrations with those of healthy controls, associations with markers of HF and mortality, and HF hospitalization in patients with HFpEF.
Methods
Patients and controls
The Karolinska Rennes (KaRen) was a prospective observational multicentre study characterizing patients with HFpEF. 16 The biochemistry programme recruited 86 patients presenting to hospital with acute signs and symptoms of HF according to the Framingham criteria, NT‐proBNP > 300 ng/L, and a left ventricular EF (LVEF) ≥ 45% between 21 May 2007 and 29 December 2011. Blood sampling and echocardiography were performed at stable follow‐up 4–8 weeks after enrolment. Patients were followed up until 30 September 2012 for the composite outcome time to death from any cause or first hospitalization due to HF.
At the follow‐up visit, blood samples were collected in EDTA tubes in a fasting condition in the morning and centrifuged, and plasma was stored in aliquots in −70°C until analysis.
Healthy controls were consecutively recruited by newspaper advertisement. Controls were free of cardiovascular symptoms, clinical signs of ongoing disease, ongoing medication, and structural heart disease on echocardiography, and all had normal resting electrocardiograms.
Quantification of biomarkers
Plasma MPO and calprotectin concentrations were determined by ELISA (BioLegend, San Diego, CA; and BMA Biomedicals, Augst, Switzerland, respectively). Uric acid, l‐arginine, ADMA, and SDMA were quantified by isotope dilution liquid chromatography coupled to tandem mass spectrometry. 17 , 18 NT‐proBNP was quantified by Elecsys electrochemiluminescence “sandwich” immunoassay, proBNPII (Roche Diagnostics, Bromma, Sweden) with a lower detection limit of 5 ng/L, and interassay coefficients of variation of ≤20%.
Estimated glomerular filtration rate (eGFR) was calculated according to the CKD‐EPI creatinine equation. 19
The echocardiographic assessment was performed on a ViVid 7 echo‐platform (GE VingMed, Horten, Norway) and analysed in core centre Hôpital Pontchaillou, Rennes, France. Examinations were interpreted once and measurements were performed three times and averaged by a sonographer (E. D.) blinded to the clinical history of the patient.
Structural heart disease was assessed as left atrial (LA) volume index (LAVI) calculated as LA volume in mL divided by body surface area in m2 and categorized as >34 mL/m2. Left ventricular hypertrophy (LVH) was assessed as left ventricular mass index and dichotomized as left ventricular mass divided by body surface area (>95 g/m2 in women and >116 g/m2 in men) according to the European Society of Cardiology (ESC) guidelines. 20 Diastolic dysfunction was assessed as E′ and E/e′ ratio and categorized as average E/e′ ratio > 14 according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines. 21
Statistics
Continuous variables are expressed as median and quartiles (Q1;Q3) and differences between groups determined by the Wilcoxon rank sum test. Categorical variables are expressed as numbers and percentages and analysed using Fisher's exact test. Correlations between plasma MPO and echocardiographic measurements of cardiac function were determined using Spearman's correlation coefficient. Correlations between MPO and clinical and biochemical characteristics included in Table 1 were performed, as they may influence MPO levels and/or outcome.
Table 1.
Baseline characteristics in the 86 patients in Karolinska Rennes
| Variable | HFpEF patients (n = 86) |
|---|---|
| Patient history | |
| Age (years) | 73 (66;79) |
| Gender (female) | 44 (51%) |
| Medical history | |
| Smoking | 45 (52%) |
| Hypertension | 68 (79%) |
| COPD | 14 (16%) |
| Diabetes mellitus type 2 | 27 (31%) |
| Coronary heart disease | 29 (34%) |
| Stroke | 9 (10%) |
| Atrial fibrillation | 49 (57%) |
| NYHA class I | 19 (22%) |
| NYHA class II | 47 (55%) |
| NYHA class III | 20 (23%) |
| Measurements | |
| Weight (kg) | 83.5 (72;98) |
| BMI (kg/m2) | 28.5 (25.0;32.9) |
| Systolic blood pressure (mmHg) | 140 (90;210) |
| Diastolic blood pressure (mmHg) | 80 (70;85) |
| Heart rate (b.p.m.) | 70 (60;80) |
| Treatment | |
| ARB | 28 (33%) |
| ACE‐inhibitor | 42 (49%) |
| Thiazide diuretics | 14 (16%) |
| Potassium‐sparing diuretics | 18 (21%) |
| Loop diuretics | 63 (73%) |
| Calcium channel blocker | 27 (31%) |
| Beta‐blocker | 69 (80%) |
| Anticoagulants | 47 (55%) |
| Antiplatelet | 29 (34%) |
| Statins | 38 (44%) |
| Nitroglycerine | 12 (14%) |
| Glucose‐lowering medication | 17 (20%) |
| Pacemaker | 20 (23%) |
| ECHO parameters | |
| LVEF (%) | 64 (58; 68) |
| LAVI (mL/m2) | 44 (38; 52) |
| LA volume (mL) | 86.5 (75; 104) |
| Left ventricular mass index (g/m2) | 114 (95;142) |
| Male | 125 (102;157) |
| Female | 109 (94;136) |
| LVEDd (mm) | 47 (43;53) |
| E/A ratio | 1.3 (0.9;2.1) |
| E/e′ ratio | 10.8 (8.3;14.0) |
| E′ | 8.0 (7.0;10.0) |
| IVRT (diastole) | 94 (77;113) |
| Mitral VTI | 23 (16;30) |
| E‐wave deceleration time (ms) | 203 (156;228) |
| Biochemistry | |
| NT‐proBNP (ng/L) | 1000 (469;2330) |
| Glucose fasting (mmol/L) | 5.6 (5.1;7.5) |
| Creatinine (μmol/L) | 84 (73;107) |
| eGFR (mL/min/1.73 m2) | 70 (53;85) |
| Haemoglobin (g/L) | 131 (122;142) |
| White blood cells count (x 109 cells/L) | 7.2 (5.6;8.5) |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IVRT, isovolumic relaxation time; LAVI, left atrial volume index; LVEDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal brain natriuretic peptide; NYHA class, New York Heart Association class; VTI, velocity‐time integral.
Continuous variables are presented as median and lower and upper quartiles (Q1;Q3) and categorical variables as numbers (n) and percentages (%).
Kaplan–Meier analyses and Cox proportional hazards models were used to analyse MPO and uric acid as a predictors of outcome and presented as hazard ratio (HR) and 95% confidence interval (CI). The same variables as in the correlation analyses were used as covariates in multiple Cox regression models. The final multiple model included NT‐proBNP, age, and gender.
Owing to non‐normal distribution, all biomarkers were log‐transformed prior to analysis. All P‐values were two‐sided, and statistical significance was set at 0.05. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Ethics
The KaRen study was conducted according to the International Conference on Harmonisation and Good Clinical Practice guidelines, and the investigation conforms to the principles outlined in the Declaration of Helsinki. The biochemistry substudy was approved by the ethical review board at Karolinska Institutet and the controls by the Local Ethics Committee in Gothenburg. Written and oral informed consent was obtained from all patients and controls prior to enrolment.
Results
The characteristics of all 86 patients with HFpEF are presented in Table 1 . The median age of patients was 73 years, and 51% were female. LVEF was 64% (Q1;Q3 58;68), E/e′ ratio 10.8 (8.3;14.0), and LAVI 43 mL/m2 (38;52). A proportion of 23% had E/e′ > 15, 67% had E′ < 9, 89% had LAVI > 34 mL/m2, and 58% had LVH. The 46 healthy controls were 24% women (vs. patients; P‐value = 0.005) and 60 (57;62) years of age, and the body mass index (BMI) was 24.4 (22.9;26.0) kg/m2 (both vs. patients; P‐value < 0.001). LVEF was 63% (59;66; P‐value = 0.790).
Biomarker concentration
Concentrations of biomarkers in HFpEF patients and controls are depicted in Figure 1 .
Figure 1.
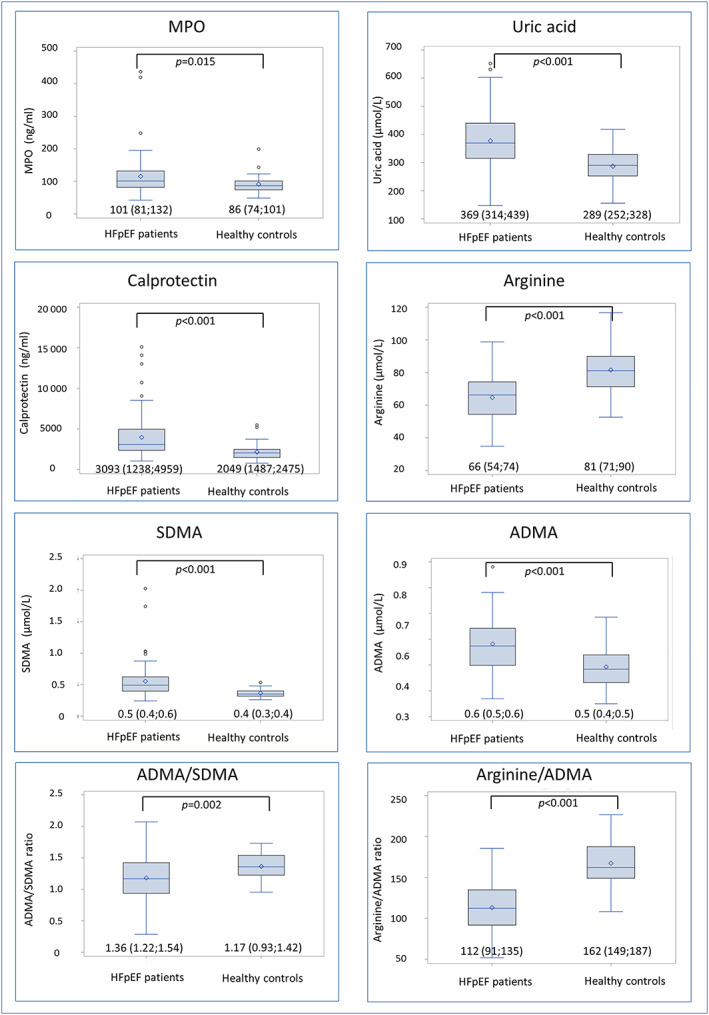
Concentrations of biomarkers in heart failure with preserved ejection fraction (HFpEF) patients and healthy controls presented as boxplots displaying interquartile range (IQR), median, mean (diamond), and outliers (circle). Whiskers represent maximum observation within 1.5 IQR above the 75th percentile.
Concentrations of all biomarkers differed in HFpEF patients compared with controls, showing higher plasma concentrations of MPO [101 (81;132) vs. 86 (74;101) ng/mL; P‐value = 0.015], uric acid, ADMA, SDMA, and calprotectin, while concentrations of arginine were lower. The ratios between arginine and ADMA and between ADMA and SDMA were decreased in HFpEF patients, suggesting reduced NO availability and increased enzymatic clearance of ADMA, respectively.
Correlations between biomarkers and clinical and echo characteristics
NYHA class correlated with uric acid (r = 0.22; P‐value = 0.045), SDMA (r = 0.33; P‐value = 0.002), calprotectin (r = 0.34; P‐value = 0.001) and negatively with ADMA/SDMA ratio (r = −0.30; P‐value = 0.005). Further, BMI correlated with MPO (r = 0.29; P‐value = 0.007), uric acid (r = 0.39; P‐value < 0.001), and calprotectin (r = 0.38; P‐value < 0.001). Hypertension correlated with MPO (r = 0.26; P‐value = 0.015), uric acid (r = 0.26; P‐value = 0.016), and calprotectin (r = 0.32; P‐value = 0.002).
Figure 2 depicts difference in biomarker concentration according to presence of diastolic dysfunction, reflected as E/e′ ratio > 14, and structural heart disease, assessed as LAVI > 34 mL/m2. Patients tend to have higher diastolic dysfunction MPO [121 (97;144) vs. 98 (77;112) ng/mL, P‐value = 0.090], and they had elevated concentrations of uric acid [421 (370;486) vs. 344 (284;420) μM, P‐value = 0.012] and SDMA [0.54 (0.44;0.70) vs. 0.47 (0.38;0.52) μM, P‐value = 0.039]. In patients with structural heart disease, arginine/ADMA ratio was lower [116 (103;133) vs. 155 (137;176), P‐value = 0.014], whereas the other biomarkers were not significantly different (Figure 2 ).
Figure 2.
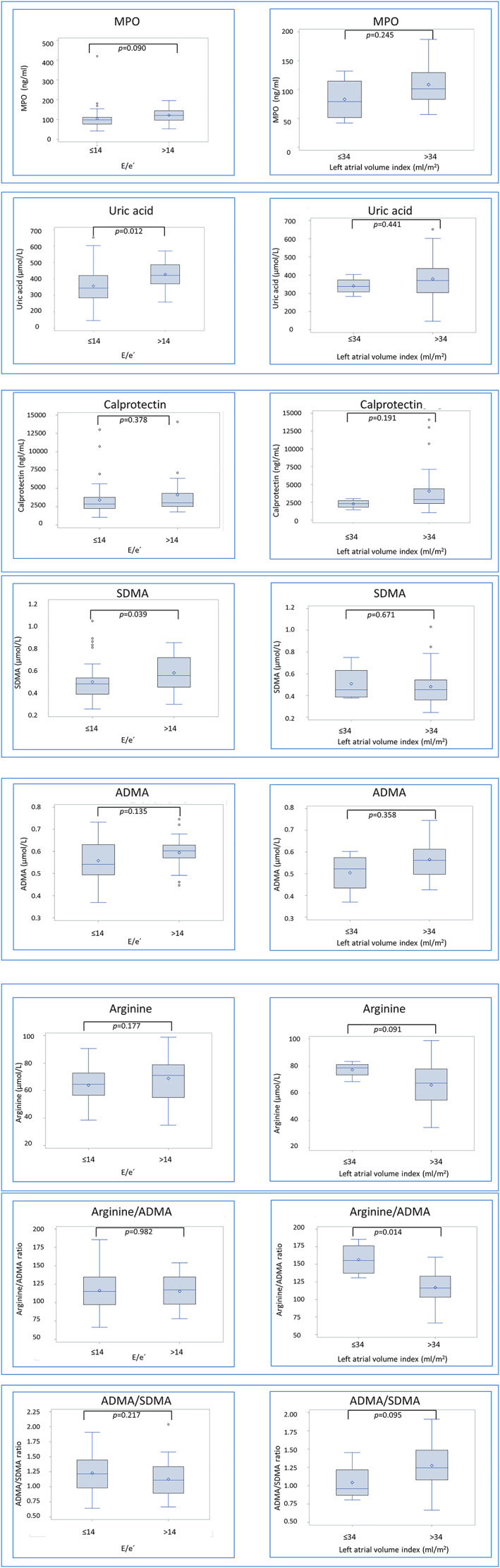
Biomarker concentrations according to presence of structural heart disease and diastolic dysfunction in heart failure with preserved ejection fraction (HFpEF) patients. Presented as boxplots displaying interquartile range (IQR), median, mean (diamond), and outliers (circle). Whiskers represent maximum observation within 1.5 IQR above the 75th percentile.
Correlation between biomarkers
In Figure 3 , bivariate correlations between MPO and biomarkers display correlations with uric acid (r = 0.22; P‐value = 0.038) and calprotectin (r = 0.34; P‐value = 0.001). There was an inverse correlation with arginine/ADMA, but it did not reach statistical significance (r = −0.18; P‐value = 0.09).
Figure 3.
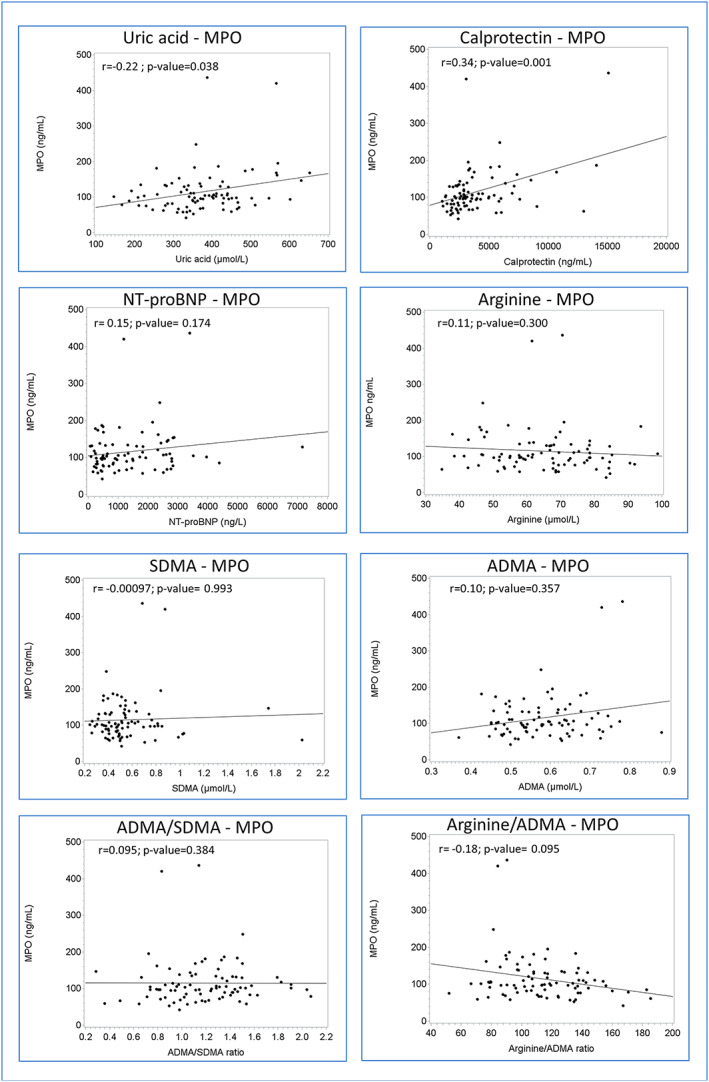
Correlations between myeloperoxidase and uric acid, N‐terminal brain natriuretic peptide (NT‐proBNP), calprotectin (neutrophil biomarker) arginine, symmetric dimethyl arginine (SDMA), and asymmetric dimethyl arginine (ADMA) (NO availability and endothelial function) in heart failure with preserved ejection fraction (HFpEF) patients.
Although not displayed in Figure 3 , uric acid was found to be correlated with calprotectin (r = 0.34; P‐value = 0.001) and negatively correlated with arginine (r = −0.27; P‐value = 0.012), the arginine/ADMA ratio (r = −0.37; P‐value < 0.001), and the ADMA/SDMA ratio (r = −0.34; P‐value = 0.001). NT‐proBNP correlated with uric acid (r = 0.24; P‐value = 0.025) and SDMA (r = 0.31; P‐value = 0.004) and negatively correlated with arginine (r = −0.22; P‐value = 0.041) and arginine/ADMA ratio (r = −0.37; P‐value < 0.001) and ADMA/SDMA ratio (r = −0.22; P‐value = 0.047). eGFR correlated with uric acid (r = −0.36; P‐value < 0.001), calprotectin (r = −0.43; P‐value < 0.001), SDMA (r = −0.76; P‐value < 0.001), arginine/ADMA ratio (r = −0.27; P‐value = 0.012), and ADMA/SDMA ratio (r = −0.69; P‐value < 0.001).
Prognosis
Median follow‐up time was 579 days (Q1;Q3 276;1178). No patient was lost to follow‐up. The composite endpoint of HF hospitalization or all‐cause death occurred in 36 patients, and 11 patients died during follow‐up.
As shown in Figure 4A and Table 2 , increasing concentrations of MPO did not predict the composite endpoint in unadjusted analysis [HR 1.48 (95% CI 0.70–3.14; P‐value = 0.304)].
Figure 4.
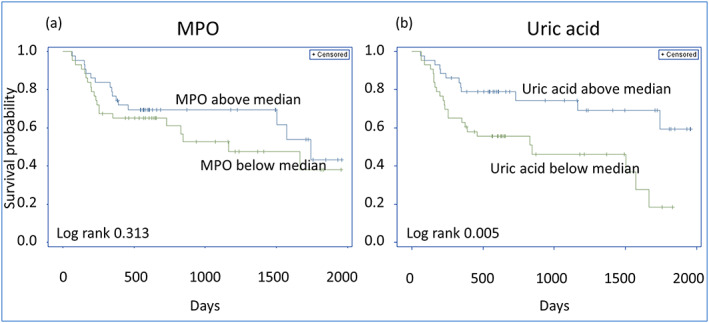
Kaplan–Meier curves displaying cumulative survival free from the composite endpoint in heart failure with preserved ejection fraction (HFpEF) patients below vs. above median concentration of (A) myeloperoxidase (MPO) and (B) uric acid.
Table 2.
Associations between biomarkers and the composite outcome in the 86 heart failure with preserved ejection fraction patients
| Parameter | All‐cause mortality or HF hospitalization (n = 36) unadjusted |
All‐cause mortality or HF hospitalization (n = 36) (adjusted age, gender, and NT‐proBNP) |
|||||
|---|---|---|---|---|---|---|---|
| n/events | Hazard ratio | 95% CI | P‐value | Hazard ratio | 95% CI | P‐value | |
| MPO | 86/36 | 1.48 | 0.70–3.14 | 0.304 | 1.23 | 0.56–2.74 | 0.606 |
| MPO (adjusted age) | 86/36 | 1.51 | 0.71–3.23 | 0.286 | |||
| MPO (adjusted gender) | 86/36 | 1.40 | 0.65–3.01 | 0.386 | |||
| MPO (adjusted eGFR) | 84/36 | 1.36 | 0.66–2.82 | 0.403 | |||
| MPO (adjusted NT‐proBNP) | 85/36 | 1.21 | 0.56–2.62 | 0.634 | |||
| Uric acid | 86/36 | 4.79 | 1.55–14.83 | 0.007 | 3.74 | 1.19–11.75 | 0.024 |
| NT‐proBNP | 85/36 | 1.49 | 1.05–2.12 | 0.027 | 1.49 a | 1.02‐2.17 | 0.038 |
| Arginine | 86/36 | 0.70 | 0.17–2.60 | 0.562 | 1.12 | 0.28–4.44 | 0.874 |
| SDMA | 86/36 | 1.39 | 0.65–2.95 | 0.399 | 1.08 | 0.48–2.42 | 0.859 |
| Calprotectin | 86/36 | 1.38 | 0.74–2.24 | 0.374 | 1.09 | 0.61–1.94 | 0.767 |
| ADMA | 86/36 | 1.34 | 0.16–11.08 | 0.789 | 0.91 | 0.11–7.86 | 0.929 |
| ADMA/SDMA ratio | 86/36 | 0.73 | 0.33–1.61 | 0.436 | 0.92 | 0.41–2.05 | 0.833 |
| Arginine/ADMA ratio | 86/36 | 0.63 | 0.18–2.26 | 0.477 | 1.18 | 0.28–5.06 | 0.821 |
Adjusted age and gender only.
Figure 4B displays that uric acid above median was associated with decreased survival and predicted the composite endpoint in univariable analysis [HR 4.79 (95% CI 1.55–14.82); P‐value = 0.007].
Uric acid remained as a significant predictor when adjusting for NT‐proBNP, age, and gender [HR 3.76 (95% CI 1.19–11.85); P‐value = 0.024]. Calprotectin, ADMA, SDMA, or arginine did not predict the composite endpoint (Table 2 ).
Discussion
In HFpEF, we found higher plasma concentrations of MPO and biomarkers, reflecting inflammation and neutrophil involvement (Figure 5 ). MPO‐dependent oxidative stress may be reflected by uric acid and calprotectin. NO availability and endothelial dysfunction reflected by SDMA were associated with diastolic dysfunction and arginine/ADMA ratio with structural remodelling. Uric acid was a significant prognostic predictor, which may mirror MPO activity contributing to endothelial microvascular dysfunction. This implies MPO‐dependent oxidative stress as a component of the HFpEF syndrome.
Figure 5.
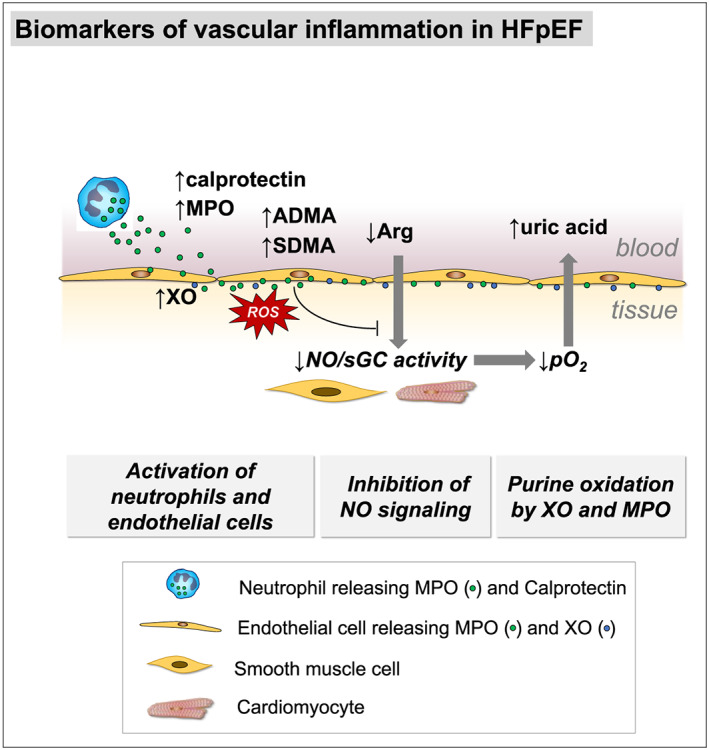
Kaplan–Meier curves displaying cumulative survival free from the composite endpoint in heart failure with preserved ejection fraction (HFpEF) patients below vs. above median concentration of (A) myeloperoxidase (MPO) and (B) uric acid.
In our cohort of well‐characterized HFpEF patients, we found higher levels of MPO and related biomarkers compared with those of healthy controls. Increased concentrations of MPO compared with those of controls have previously been described in HFrEF 11 and in HF populations including HFpEF patients, but the latter have been in minority and not separately studied. 12 Recently, it was also reported that S100A8 (one of the components of calprotectin) is elevated in plasma of HFpEF patients independent of symptomatic severity. 22 Interestingly, the authors could demonstrate adverse effects on calcium handling, as recovery of the spontaneous Ca2+ transient following each depolarizing pulse was considerably slower in the presence of rS100A8. This suggests a causative potential of calprotectin in HFpEF pathophysiology.
Correlations with measurements of diastolic function and structural heart disease
In HFrEF and acute decompensated HF (ADHF), respectively, MPO concentration is not correlated 23 and weakly correlated with NYHA class. 24 Also, in the present study, NYHA class did not correlate with MPO concentration. However, a correlation was found between NYHA class and uric acid, demonstrating that this latter marker as a reflector of functional status in HFpEF. Further, we found that uric acid was associated with diastolic dysfunction, while MPO trended to be elevated in patients with E/e′ > 14 but did not reach significant difference. This is in line with findings in ADHF where no association between deteriorating diastolic stage and MPO was found 24 but is in contrast to HFrEF where MPO has been associated with deteriorating diastolic stage. 13 There are data suggesting MPO as an important part of the structural remodelling of the myocardium. For example, it may be involved in the pathophysiology of atrial fibrillation through atrial accumulation of MPO accompanied by augmented fibrosis as demonstrated in mice and humans with atrial fibrillation. 25 This suggests that MPO potentially may be of importance also for the development of HFpEF where atrial fibrillation and fibrosis are major components.
Importance of coronary microvascular dysfunction in heart failure with preserved ejection fraction
Both MPO and uric acid correlated with the inflammatory protein calprotectin, which, similar to MPO, is released from neutrophils. This association, and also with arginine and arginine/ADMA, suggests a correlation between neutrophil involvement and reduced NO production. Also ADMA has been suggested to regulate MPO release from neutrophils, and MPO oxidative activity has been shown to inhibit dimethylarginine dimethylaminohydrolase activity, the enzyme metabolizing ADMA. 8 In the current study, we did not find correlations supporting these mechanisms, but there was a weak correlation between MPO and ADMA, potentially confounded by the impact of eGFR on ADMA.
The elevated calprotectin concentrations in our HFpEF patients correlated with NYHA class and hypertension. Calprotectin binds to the RAGE receptor (receptor for the advanced glycation end‐products) and toll‐like receptor 4 (TLR4) shown be important for endotoxin‐induced dysfunction of the cardiomyocytes. 26 Further, there was an association between MPO, uric acid, and calprotectin and BMI linked to underlying co‐morbidities such as hypertension and diabetes. These conditions have been proposed as drivers of the HFpEF disease initiating endothelial dysfunction and microvascular inflammation. 4 , 27 In support of this hypothesis, we have recently revealed that 75% of patients with HFpEF do have coronary microvascular dysfunction assessed as depressed coronary flow reserve, which also was associated with endothelial dysfunction. 28
Uric acid as a reflector of myeloperoxidase
In endothelial dysfunction, there is a depletion of NO as a consequence of oxidative stress. It is not clear if oxidative stress or MPO elevation initiates the process, but MPO may be an important contributor. When exposed to oxidative stress, human endocardial endothelial cells express MPO. 29 As activated MPO consumes NO, this causes protein chlorination or nitration, eventually leading to tissue damage and subsequent remodulation. This has been demonstrated in chronic HF (mean EF 27%) where MPO was associated with the cytoplasmic protein heart‐type fatty acid‐binding protein reflecting myocardial damage. 30
It is important to stress that the current study investigated the correlation between systemic plasma levels of MPO and other biomarkers and features of HFpEF, and it is not known how well the plasma concentration of MPO reflects the enzymatic activity in the tissue, such as in the vascular wall. Possibly, only a portion of the circulating MPO is a result of activation and degranulation of neutrophils because MPO is also constitutively secreted by neutrophils. 31 A more plausible role of the plasma MPO pool is that it feeds the vasculature with MPO that is trapped, endocytosed, and bound to proteoglycans in the subendothelial compartment, where it may be enzymatically active. 32 Accordingly, biomarkers representing the tissue activity of MPO would possibly better reflect the contribution of MPO to HFpEF pathophysiology. Although the link between MPO, vascular dysfunction, and uric acid is yet to be proven, uric acid may represent such a biomarker. Uric acid is accumulated in hypoxic situations, because of a net increase in ATP consumption and increased purine catabolism by local (endothelial) xanthine oxidase (XO) that generates xanthine and uric acid, along with the reactive oxygen species superoxide anion and hydrogen peroxide. 33 Notably, MPO is also enzymatically involved in this process, by oxidizing xanthine into uric acid, 34 as well as uric acid into radicals that form adducts on proteins 35 inhibition of MPO.
In HF, inhibition of oxidative stress related to purine oxidation appears interesting, but XO inhibition investigated in clinical trials in HFrEF improvement of clinical outcomes and prognosis has so far been unsuccessful. 36 , 37 However, it may be more relevant to try this concept in HFpEF where oxidative stress and microvascular endothelial dysfunction are suggested as fundamental parts of the pathophysiology and development of the disease. 4 , 27
Prognosis
MPO has been demonstrated as a prognostic predictor in HFrEF and ADHF. 13 , 15 In HFpEF, prognostic implications of MPO have not previously been studied; however, in an HF cohort including 79 (28%) patients with HFpEF, diastolic vs. systolic HF did not influence the prognostic value of MPO. 12 We could not confirm MPO as a prognostic predictor in HFpEF; however, uric acid possibly reflecting MPO activity was associated with outcome. Elevation of uric acid is a well‐known predictor of mortality in HFrEF 38 also demonstrated in HFpEF. 39 , 40
Limitations
This is a relatively small single cohort study, and there is therefore a potential lack of power. The potential roles of MPO and uric acid as prognostic markers should be investigated in larger HFpEF studies. There was a difference in age and gender between the patients and the healthy controls. Further, the protocol did not require structural heart disease or diastolic dysfunction as it was designed prior to the 2012 and 2016 ESC guidelines; however, 94% of the study population did comply with the present criteria of HFpEF as recommended by ESC 2016 guidelines. 41 We analysed several biomarkers as reflectors of inflammation, endothelial function, and oxidative stress. We do, however, acknowledge that additional biomarkers such as 4‐hydroxy‐2‐nonenal or 3‐nitrotyrosine would provide even more evidence of oxidative and nitrosative stress. Drugs lowering uric acid levels such as allopurinol may have influenced the results but were not registered.
Conclusions
In HFpEF, we found higher concentrations of MPO and biomarkers reflecting inflammation and neutrophil involvement. MPO‐dependent oxidative stress may be reflected by uric acid and calprotectin. NO availability and endothelial dysfunction reflected by SDMA were associated with diastolic dysfunction and arginine/ADMA ratio with structural remodelling. Uric acid was a significant prognostic predictor, which may mirror MPO activity contributing to endothelial microvascular dysfunction. This may suggest MPO‐dependent oxidative stress and xanthine oxidase as components in the development of the HFpEF syndrome introducing potential future treatment targets.
Conflict of Interest
There are no specific conflicts of interest related to this study, but as the findings may be associated with HF drugs or devices and in ongoing or future trials, we disclose the following: C. H.: consulting fees from Novartis and speaker and honoraria from MSD and Roche; L. H. L.: research grants and speaker and honoraria from AstraZeneca, consulting honoraria from Novartis and St Jude Medical, and research grants from Boston Scientific; C. L.: principal investigator of REVERSE, a CRT study sponsored by Medtronic research grants, speaker honoraria, and consulting fees from Medtronic and speaker honoraria and consulting fees from St. Jude Medical; E. D.: speaker honoraria and consulting fees from Novartis and AstraZeneca; J. C. D.: research grants, speaker honoraria, and consulting fees from Medtronic and St Jude Medical. E. M., B. K., T. M., and L. M. G. are employees of AstraZeneca, which has an MPO inhibitor in development. All authors had full access to all the data and have participated in the work and agreed with the content of the article. The authors take full responsibility for its integrity and the data analysis.
Funding
The work was supported by grants from Fédération Française de Cardiologie/Société Française de Cardiologie, France; Medtronic Bakken Research Center, Maastricht, The Netherlands; Center for Gender Medicine, Karolinska Institutet, Stockholm, Sweden (to C. H.); the Swedish Research Council (grant 2013‐23897‐104604‐23); Swedish Heart Lung Foundation (grants 20080409 and 20100419); and the Stockholm County Council (grants 00556‐2009 and 20110120) (L. H. L.).
Acknowledgements
The authors wish to thank Kambiz Shahgaldi and Maria Westerlind for echocardiogram assessments and Gunilla Förstedt at Karolinska University hospital for blood sampling, laboratory analysis, and patient care.
Hage, C. , Michaëlsson, E. , Kull, B. , Miliotis, T. , Svedlund, S. , Linde, C. , Donal, E. , Daubert, J.‐C. , Gan, L.‐M. , and Lund, L. H. (2020) Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Failure, 7: 1534–1546. 10.1002/ehf2.12700.
References
- 1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 2. Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JG, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray J, Misselwitz F, Nodari S, O'Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV, Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail 2014; 2: 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012; 59: 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 5. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite‐Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC: Heart Failure 2016; 4: 312–324. [DOI] [PubMed] [Google Scholar]
- 6. Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte‐derived vascular no oxidase. Science 2002; 296: 2391–2394. [DOI] [PubMed] [Google Scholar]
- 7. Leyva F, Chua TP, Anker SD, Coats AJ. Uric acid in chronic heart failure: a measure of the anaerobic threshold. Metabolism 1998; 47: 1156–1159. [DOI] [PubMed] [Google Scholar]
- 8. von Leitner EC, Klinke A, Atzler D, Slocum JL, Lund N, Kielstein JT, Maas R, Schmidt‐Haupt R, Pekarova M, Hellwinkel O, Tsikas D. Pathogenic cycle between the endogenous nitric oxide synthase inhibitor asymmetrical dimethylarginine and the leukocyte‐derived hemoprotein myeloperoxidase. Circulation 2011; 124: 2735–2745. [DOI] [PubMed] [Google Scholar]
- 9. Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brummer J, Rudolph V, Munzel T, Heitzer T, Meinertz T, Baldus S. Myeloperoxidase mediates neutrophil activation by association with cd11b/cd18 integrins. Proc Natl Acad Sci U S A 2005; 102: 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100a8/a9 in inflammation. Front Immunol 2018; 9: 1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang WH, Brennan ML, Philip K, Tong W, Mann S, Van Lente F, Hazen SL. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol 2006; 98: 796–799. [DOI] [PubMed] [Google Scholar]
- 12. Michowitz Y, Kisil S, Guzner‐Gur H, Rubinstein A, Wexler D, Sheps D, Keren G, George J. Usefulness of serum myeloperoxidase in prediction of mortality in patients with severe heart failure. Isr Med Assoc J 2008; 10: 884–888. [PubMed] [Google Scholar]
- 13. Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, Jasper S, Hazen SL, Klein AL. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol 2007; 49: 2364–2370. [DOI] [PubMed] [Google Scholar]
- 14. Askari AT, Brennan M‐L, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med 2003; 197: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, Meissner J, Noveanu M, Reiter M, Twerenbold R, Schaub N, Buser A, Mueller C. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem 2010; 56: 944–951. [DOI] [PubMed] [Google Scholar]
- 16. Donal E, Lund LH, Linde C, Edner M, Lafitte S, Persson H, Bauer F, Ohrvik J, Ennezat PV, Hage C, Löfman I, Juilliere Y, Logeart D, Derumeaux G, Gueret P, Daubert JC. Rationale and design of the Karolinska‐Rennes (KaRen) prospective study of dyssynchrony in heart failure with preserved ejection fraction. Eur J Heart Fail 2009; 11: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwedhelm E, Tan‐Andresen J, Maas R, Riederer U, Schulze F, Boger RH. Liquid chromatography–tandem mass spectrometry method for the analysis of asymmetric dimethylarginine in human plasma. Clin Chem 2005; 51: 1268–1271. [DOI] [PubMed] [Google Scholar]
- 18. Luo X, Cai N, Cheng Z. Determination of uric acid in plasma by LC‐MS/MS and its application to an efficacy evaluation of recombinant urate oxidase. Anal Sci 2013; 29: 709–713. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 21. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 22. Raphael R, Purushotham D, Gastonguay C, Chesnik MA, Kwok WM, Wu HE, Shah SJ, Mirza SP, Strande JL. Combining patient proteomics and in vitro cardiomyocyte phenotype testing to identify potential mediators of heart failure with preserved ejection fraction. J Transl Med 2016; 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reina‐Couto M, Carvalho J, Valente MJ, Vale L, Afonso J, Carvalho F, Bettencourt P, Sousa T, Albino‐Teixeira A. Impaired resolution of inflammation in human chronic heart failure. Eur J Clin Invest 2014; 44: 527–538. [DOI] [PubMed] [Google Scholar]
- 24. Shah KB, Kop WJ, Christenson RH, Diercks DB, Kuo D, Henderson S, Hanson K, Mehra MR, deFilippi C. Lack of diagnostic and prognostic utility of circulating plasma myeloperoxidase concentrations in patients presenting with dyspnea. Clin Chem 2009; 55: 59–67. [DOI] [PubMed] [Google Scholar]
- 25. Rudolph V, Andrie RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch‐Hoffmann B, Schwoerer AP, Lau D, Fu X, Klingel K, Sydow K. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med 2010; 16: 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kruzliak P, Novak J, Novak M, Fodor GJ. Role of calprotectin in cardiometabolic diseases. Cytokine Growth Factor Rev 2014; 25: 67–75. [DOI] [PubMed] [Google Scholar]
- 27. Lam CS, Lund LH. Microvascular endothelial dysfunction in heart failure with preserved ejection fraction. Heart 2016; 102: 257–259. [DOI] [PubMed] [Google Scholar]
- 28. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink‐Nelson L, Ljung Faxén U, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS‐HFpEF. Eur Heart J 2018; 39: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. La Rocca G, Di Stefano A, Eleuteri E, Anzalone R, Magno F, Corrao S, Loria T, Martorana A, Di Gangi C, Colombo M, Sansone F. Oxidative stress induces myeloperoxidase expression in endocardial endothelial cells from patients with chronic heart failure. Basic Res Cardiol 2009; 104: 307–320. [DOI] [PubMed] [Google Scholar]
- 30. Gedikli O, Kiris A, Hosoglu Y, Karahan C, Kaplan S. Serum myeloperoxidase level is associated with heart‐type fatty acid‐binding protein but not troponin T in patients with chronic heart failure. Med Princ Pract 2015; 24: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansson M, Olsson I, Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch Biochem Biophys 2006; 445: 214–224. [DOI] [PubMed] [Google Scholar]
- 32. Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Investig 2001; 108: 1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cantu‐Medellin N, Kelley EE. Xanthine oxidoreductase‐catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biol 2013; 1: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stamp LK, Turner R, Khalilova IS, Zhang M, Drake J, Forbes LV, Kettle AJ. Myeloperoxidase and oxidation of uric acid in gout: implications for the clinical consequences of hyperuricaemia. Rheumatology (Oxford) 2014; 53: 1958–1965. [DOI] [PubMed] [Google Scholar]
- 35. Turner R, Brennan SO, Ashby LV, Dickerhof N, Hamzah MR, Pearson JF, Stamp LK, Kettle AJ. Conjugation of urate‐derived electrophiles to proteins during normal metabolism and inflammation. J Biol Chem 2018; 293: 19886–19898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hare JM, Mangal B, Brown J, Fisher C Jr, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP, OPT‐CHF Investigators . Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT‐CHF study. J Am Coll Cardiol 2008; 51: 2301–2309. [DOI] [PubMed] [Google Scholar]
- 37. Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tang WH, Dunlap ME, LeWinter M, Mann DL, Felker GM, O'Connor CM, Goldsmith SR, Ofili EO, Saltzberg MT, Margulies KB, Cappola TP, Konstam MA, Semigran MJ, McNulty S, Lee KL, Shah MR, Hernandez AF, NHLBI Heart Failure Clinical Research Network . Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT‐HF) study. Circulation 2015; 131: 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tamariz L, Harzand A, Palacio A, Verma S, Jones J, Hare J. Uric acid as a predictor of all‐cause mortality in heart failure: a meta‐analysis. Congest Heart Fail 2011; 17: 25–30. [DOI] [PubMed] [Google Scholar]
- 39. Shimizu T, Yoshihisa A, Kanno Y, Takiguchi M, Sato A, Miura S, Nakamura Y, Yamauchi H, Owada T, Abe S, Sato T, Suzuki S, Oikawa M, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Takeishi Y. Relationship of hyperuricemia with mortality in heart failure patients with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2015; 309: H1123–H1129 [DOI] [PubMed] [Google Scholar]
- 40. Manzano L, Babalis D, Roughton M, Shibata M, Anker SD, Ghio S, van Veldhuisen DJ, Cohen‐Solal A, Coats AJ, Poole‐Wilson PPA, Flather MD. Predictors of clinical outcomes in elderly patients with heart failure. Eur J Heart Fail 2011; 13: 528–536 [DOI] [PubMed] [Google Scholar]
- 41. Persson H, Donal E, Lund LH, Matan D, Oger E, Hage C, Daubert JC, Linde C, KaRen Investigators . Importance of structural heart disease and diastolic dysfunction in heart failure with preserved ejection fraction assessed according to the ESC guidelines—a substudy in the Ka (Karolinska) Ren (Rennes) study. Int J Cardiol 2018; 274: 202–207. [DOI] [PubMed] [Google Scholar]


