Abstract
Aims
Diuretic resistance is common in acute decompensated heart failure (ADHF). When loop diuretic monotherapy is ineffective, thiazides are often recommended as adjunctive therapy, but these agents have many side effects and are associated with worsened survival. In contrast, sodium glucose cotransporter 2 inhibitors (SGLT‐2i's), initially developed as glucose‐lowering medications for type 2 diabetes, improve heart failure outcomes. A candidate contributory mechanism for this benefit is their diuretic effects. We sought to describe the safety and efficacy of SGLT‐2i's as loop diuretic adjuvants in ADHF.
Methods and results
We retrospectively analysed patients who received adjuvant SGLT‐2i therapy between August 2016 and June 2018 at Yale‐New Haven Hospital. Thirty‐one patients comprised the cohort, 58% of whom had type 2 diabetes. Compared with the 24 h prior to SGLT‐2i initiation, average weight loss improved (1.0 ± 2.2 kg, P = 0.03 at Day 1; 1.7 ± 4.9 kg, P = 0.08 at Day 2; and 2.1 ± 5.6 kg, P = 0.06 at Day 3), as did urine output (3.7 ± 2.0 L, P = 0.002 at Day 1; 3.4 ± 1.7 L, P = 0.02 at Day 2; and 3.1 ± 1.7 L, P = 0.02 at Day 3) while loop diuretic dosing remaining stable. Creatinine remained unchanged during the 3 days after initiation, as did blood pressure and the incidence of hypokalaemia (P = NS for all).
Conclusions
In this cohort of patients with ADHF, SGLT‐2i's improved weight loss, urine output, and diuretic efficiency without worsening of creatinine, potassium, or blood pressure. Further study of SGLT‐2i's as a loop diuretic adjuvant is warranted.
Keywords: Empagliflozin, Canagliflozin, Diuretic resistance
Background
Loop diuretics are the mainstay of therapy for patients with acute decompensated heart failure (ADHF) and volume overload. 1 However, many patients demonstrate a poor response, with up to 50% considered diuretic resistant. 2 In such cases, thiazides are often recommended as the first‐line diuretic adjuvant, but they have multiple potentially severe side effects that may explain the observed signal for worsened clinical outcomes with these agents. 3 , 4 Therefore, there is a substantial unmet need to safely improve effective diuresis in loop diuretic‐resistant patients.
Sodium glucose cotransporter 2 inhibitors (SGLT‐2i's) increase urinary excretion of both glucose and sodium and appear to produce a durable reduction in blood volume. 5 , 6 However, unlike traditional diuretics, they may do so with limited activation of the neurohormonal or electrolyte profile of the patient. 7 Although originally developed as glucose‐lowering medications for patients with type 2 diabetes, SGLT‐2i's have been shown to improve event‐free survival in patients with chronic heart failure, irrespective of the degree of hyperglycaemia or even diabetic status. 8 , 9 Furthermore, a recent pilot study examining SGLT‐2i's in ADHF suggested a potential benefit with respect to secondary 60 day clinical outcomes of worsening heart failure, death, or heart failure readmission. 10 We added this therapeutic class to our inpatient formulary as a diuretic adjuvant for loop diuretic resistance based on the known pharmacodynamic actions of the drug, along with early mechanistic studies suggesting that SGLT‐2i's were clinically meaningful natriuretic agents in combination with loop diuretics. 11
Aims
Herein, we report our experiences with the SGLT‐2i's in ADHF and describe the safety and efficacy of this approach in a real‐world cohort. Specifically, we describe the effects of SGLT‐2i's on diuretic efficiency, urine output, and weight loss, as well as renal function, blood pressure, and plasma glucose concentrations.
Methods
Patients included in this report received an SGLT‐2i between August 2016 and June 2018 during a hospitalization for ADHF at Yale‐New Haven Hospital, a 1541‐bed academic medical centre in New Haven, CT, USA. Canagliflozin (100 mg, 300 mg) and then subsequently empagliflozin (10 mg, 25 mg; after the EMPA‐REG OUTCOME trial was reported) were approved by our hospital's pharmacy and therapeutics committee for the treatment of diuretic resistance on the inpatient heart failure service. Patients were judged to have diuretic resistance by clinical assessment of the treating heart failure cardiologist. Charts were subsequently reviewed, and patients receiving SGLT‐2i strictly for diabetes management in the absence ADHF were excluded. To examine the potential efficacy, comparisons were pre‐SGLT‐2i to post‐SGLT‐2i administration, and therefore, patients receiving an SGLT‐2i on admission were excluded. For those who received an SGLT‐2i during multiple admissions, only the first encounter was analysed.
Medical record data regarding diuretics administered, urine output, and laboratory values were extracted in 24 h intervals; administration of the first dose of an SGLT‐2i was used as the reference time point. Administered doses of loop diuretics were totalled and converted to intravenous (IV) furosemide equivalents with 40 mg IV furosemide equivalent to 80 mg oral (PO) furosemide, 1 mg IV or PO bumetanide, and 20 mg PO torsemide. 12 Similarly, doses of thiazide diuretic agents were extracted and converted to metolazone equivalents, where 10 mg of hydrochlorothiazide were equal to 1 mg of metolazone. 13 Daily weights were extracted from the chart. For days in which multiple weights were recorded, priority was given to standing weight over bed weight and then to the first measurement of the day.
The main outcomes for efficacy (weight, urine output, and diuretic efficiency) as well as safety (serum creatinine, potassium, glucose, and blood pressure) were collected at 24, 48, and 72 h after SGLT‐2i administration. The equation used to calculate diuretic efficiency, or the increase in urine output that would follow a doubling of diuretic dose, has been described previously. 2 Wilcoxon signed rank test or paired t‐test was used to compare baseline to post‐treatment values. Baseline characteristics are presented as median (interquartile range) or mean (±standard deviation). A two‐tailed P‐value less than 0.05 was considered significant. Analyses were performed using IBM SPSS Statistics (version 26, Armonk, New York).
Results
A total of 31 patients received an SGLT‐2i for diuretic resistance during a hospital admission for ADHF. More than half of the population had diabetes (18/31, 58%), and the average A1c for the cohort was 7.0 ± 1.5%. The average left ventricular ejection fraction was 40 ± 20%, and 58% (18/31) had a left ventricular ejection fraction <40%. There were several indicators of high illness severity, including median length of stay of 25 days (15–58 days), 29% (9/31) of patients having a ventricular assist device, and 32% (10/31) requiring inotropes and/or vasopressors at baseline. Central venous pressure was measured in seven patients (median = 15 mmHg, interquartile range 11.5 to 15.25 mmHg). On the day of SGLT‐2i therapy initiation, patients were receiving 160 mg (30–320 mg) of furosemide equivalents. Patients were hospitalized for a median of 6.4 days (interquartile range 3.5 to 15.1 days) prior to SGLT‐2i initiation. Full baseline characteristics can be found in Table 1 .
Table 1.
Characteristics of the patients at baseline
| Characteristic | Cohort N = 31 |
|---|---|
| Age (years) | 63 ± 13 |
| Female sex, n (%) | 6 (19) |
| Black race, n (%) a | 4 (13) |
| Weight (kg) | 90 ± 23 |
| Systolic blood pressure (mmHg) | 113 ± 21 |
| Diastolic blood pressure (mmHg) | 64 ± 10 |
| Mean arterial pressure (mmHg) | 83 ± 15 |
| Heart rate (beats/min) | 80 ± 16 |
| SpO2 (%) | 96 ± 2 |
| Serum creatinine (mg/dL) | 1.7 ± 1.0 |
| BUN (mg/dL) | 48 ± 28 |
| Blood glucose (mg/dL) | 151 ± 61 |
| Haemoglobin (mg/dL) | 10.5 ± 2.7 |
| Haematocrit (%) | 33 ± 7 |
| Left ventricular ejection fraction (%) | 40 ± 20 |
| HFrEF, n (%) | 17 (55) |
| Haemoglobin A1C ≥ 6.5 | 18 (58) |
| Haemoglobin A1C | 7.0 ± 1.5 |
| Estimated GFR by CKD‐EPI | |
| Mean (mL/min/1.73 m2) | 50.2 ± 24.0 |
| Rate of <60 mL/min/1.73 m2, n (%) | 19 (61) |
| In‐hospital therapy b | |
| LVAD, n (%) | 9 (29) |
| Vasopressor and/or inotrope, n (%) | 10 (32) |
| Loop diuretic dose (furosemide equivalents) | 160 (30–320) |
| Loop diuretic administered | |
| Furosemide | 16 (52) |
| Bumetanide | 15 (48) |
| Thiazides, n (%) | 8 (26) |
| Thiazide dose (mg of metolazone equivalents) | 13 |
BUN, blood urea nitrogen; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction; LVAD, left ventricular assist device; SpO2, peripheral capillary oxygen saturation.Body mass index was calculated as weight in kilograms divided by the square of the height in metres.
Race was identified through review of the electronic health record.
Therapy received during the day of SGLT‐2i initiation.
Compared with weight 24 h prior to treatment, average weight decreased by 1.0 ± 2.2 kg at Day 1 (P = 0.03), 1.7 ± 4.9 kg at Day 2 (P = 0.08), and 2.1 ± 5.6 kg at Day 3 (P = 0.06) after SGLT‐2i administration. Daily urine output improved during Day 1 (P = 0.002), Day 2 (P = 0.02), and Day 3 (P = 0.02) compared with the 24 h prior to treatment (Figure 1 A ). While the diuretic dose remained constant at Day 1, there was a tendency towards reduction during Day 2 and Day 3, but this did not reach statistical significance (Figure 1 B ). There was no change in thiazide use before and after therapy (seven patients prior, eight patients post, P = 0.40). Diuretic efficiency was stable prior to SGLT‐2i administration but increased on Day 1 (P = 0.02), Day 2 (P = 0.03), and Day 3 (P = 0.12) (Figure 1 C ). Haematocrit improved overall with 19/26 patients (73%) achieving haemoconcentration after SGLT‐2 administration.
Figure 1.
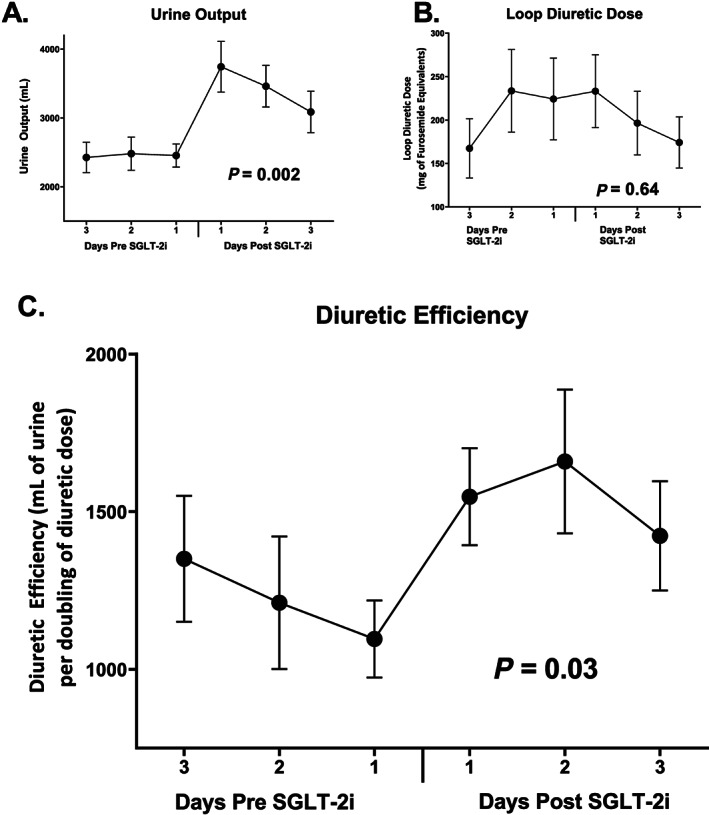
Trends in diuretic efficiency before and after SGLT‐2i therapy (A) as well as urine output (B) and loop diuretic dose (C), which are the primary determinants of diuretic efficiency. Diuretic efficiency is defined as the amount of urine output that would result from a doubling of the diuretic dose. SGLT‐2i, sodium glucose cotransporter 2 inhibitor.
Creatinine remained unchanged from baseline during the 3 day follow‐up period (0.0 ± 0.5 mg/mL change, P > 0.99 on Day 1), with similar trends seen at Day 2 and Day 3 (P > 0.95 for both, Figure 2 A ). Blood urea nitrogen was similarly stable (Figure 2 B ). There was no significant difference in blood glucose from baseline to Day 1 (P = 0.91), Day 2 (P = 0.40), nor Day 3 (P = 0.84, Figure 2 C ). The change in blood pressure from baseline to Day 1 was small and not statically significant (−3.0 ± 11.4 mmHg, P = 0.17, Figure 2 D ), with this trend persisting throughout the last 2 days analysed (P = NS for both). Three patients developed hypokalaemia (potassium ≤3 mg/dL) in the 3 days prior to initiation, whereas only one patient experienced hypokalaemia in the 3 days following. No patients were diagnosed with a urinary tract infection or genital mycotic infection during the observation period.
Figure 2.
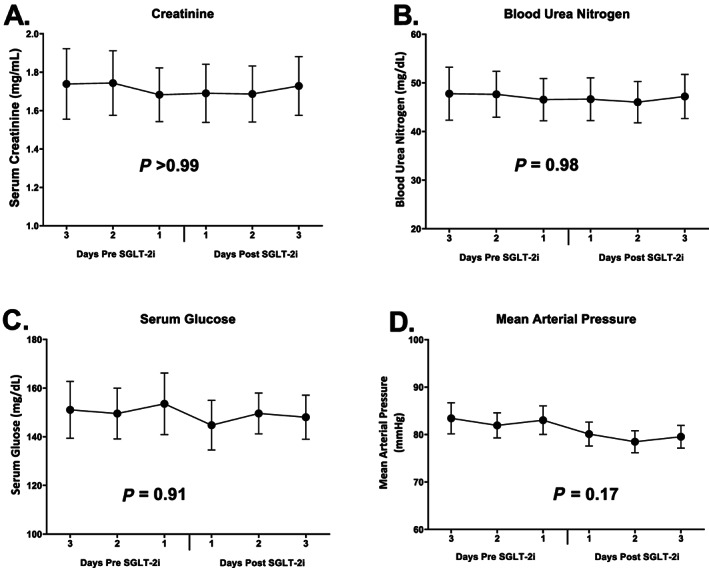
Trends in serum creatinine (A), blood urea nitrogen (B), serum glucose (C), and mean arterial pressure (D) before and after SGLT‐2i therapy. SGLT‐2i, sodium glucose cotransporter 2 inhibitor.
Conclusions
In this case series of patients with ADHF and generally high disease severity, therapy with an SGLT‐2i was associated with improved urine output and weight loss after therapy. These effects were observed without escalation of loop diuretic or thiazide therapy, and the resultant diuretic efficiency was markedly improved. Lastly, we detected no signals for harm, including deterioration of renal function, adverse change in blood pressure or electrolytes, or genitourinary infections while on therapy.
This study adds incrementally to the recent prospective study of SGLT‐2i's in ADHF by Damman et al. as these investigators did not find a significant change in weight or diuretic efficiency, despite significantly improved urine output. 10 This incongruency with our findings may be explained in part by higher disease severity in our population. Nearly one in three required mechanical circulatory support and a similar proportion were receiving inotropes and/or vasopressors, meaning they would have been excluded from the Damman et al. study. Renal dysfunction was also highly prevalent, with nearly two in three having an estimated glomerular filtration rate <60 mL/min/1.73 m2 at baseline. Lastly, loop diuretic dosing was substantially higher, as patients received an average of 230 mg of furosemide equivalents on just the first day of SGLT‐2i therapy. This is a critical point as mechanistic studies have suggested that the synergism observed between loop diuretics and SGLT‐2i's is only present in patients previously receiving loop diuretics. 6 , 11
This report is limited by the fact that it was a case series lacking randomization, blinding, or a control group. Because physicians were starting SGLT‐2i's largely due to lack of response of the patient to loop diuretics, it is possible that other parallel interventions were occurring (beyond thiazide and loop diuretic dosing, which we were able to track). As such, we cannot conclude that any of the observed differences were causally induced by the SGLT‐2i's. Given these limitations, the results should be considered as hypothesis‐generating only.
In a retrospective analysis, this population of largely diuretic‐resistant ADHF patients receiving loop diuretics and an SGLT‐2i demonstrated a more robust diuresis without signals for worsening renal function or hypotension. This suggests a potential role for SGLT‐2i as a diuretic adjuvant in patients with ADHF unresponsive to loop diuretics, and further large‐scale prospective study is warranted.
Conflict of interest
Dr Testani reports grants and personal fees from Sequana Medical, grants and personal fees from BMS, personal fees from AstraZeneca, personal fees from Novartis, grants and personal fees from 3ive labs, personal fees from Cardionomic, personal fees from Bayer, personal fees from Boehringer‐Ingelheim, personal fees from MagentaMed, grants from Otsuka, personal fees from Renalguard, grants and personal fees from Sanofi/Lexicon, grants and personal fees from FIRE1, grants from Abbott, and personal fees from W.L. Gore, outside the submitted work. Dr Collins reports grants from NIH, PCORI, AHRQ, and AstraZeneca and personal fees from Ortho Clinical, Boehringer‐Ingelheim, Roche, and Relypsa Medical. Dr Riello reports consulting fees from Janssen, Johnson & Johnson, Pfizer, and Portola and serves on advisory boards at AstraZeneca, Janssen, Johnson & Johnson, Medicure, and Portola. Dr Inzucchi serves on committees at Boehringer‐Ingelheim and Lexicon/Sanofi, serves as a consultant for Novo Nordisk, AstraZeneca, VTV Therapeutics, Merck, Abott/Alere, and Zafgen, and has provided lectures for Merck and Boehringer‐Ingelheim. The other authors have no disclosures relevant to the content of this paper.
Acknowledgements
This work was supported by NIH Grants R01HL139629, R21HL143092, R01HL128973 (J.M.T.), and 5T32HL007950 (M.G.).
Griffin, M. , Riello, R. , Rao, V. S. , Ivey-Miranda, J. , Fleming, J. , Maulion, C. , McCallum, W. , Sarnak, M. , Collins, S. , Inzucchi, S. E. , and Testani, J. M. (2020) Sodium glucose cotransporter 2 inhibitors as diuretic adjuvants in acute decompensated heart failure: a case series. ESC Heart Failure, 7: 1966–1971. 10.1002/ehf2.12759.
References
- 1. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006; 119: S3–S10. [DOI] [PubMed] [Google Scholar]
- 2. Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J 2014; 35: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Commi W, Force AAT. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: E147–E239. [DOI] [PubMed] [Google Scholar]
- 4. Brisco‐Bacik MA, ter Maaten JM, Houser SR, Vedage NA, Rao V, Ahmad T, Wilson FP, Testani JM. Outcomes associated with a strategy of adjuvant metolazone or high‐dose loop diuretics in acute decompensated heart failure: a propensity analysis. J Am Heart Assoc 2018; 7: e009149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013; 15: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffin MR, Ivey-Miranda V, Fleming J, Maulion J, Moskow C, Mahoney J, Jeon D, Inzucchi S, Testani SE, JM . Late‐breaking science abstracts and featured science abstracts from the American Heart Association's Scientific Sessions 2019: empagliflozin in heart failure: diuretic and cardio‐renal effects. Circulation 2019; 140: 20180. [Google Scholar]
- 7. Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D‐HT and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 9. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E‐RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 10. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, van Eck JWM, Heerspink HJL, Voors AA. Randomized, double‐blind, placebo‐controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA‐RESPONSE‐AHF). Eur J Heart Fail 2020; 22: 713–722. [DOI] [PubMed] [Google Scholar]
- 11. Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction between the sodium‐glucose‐linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc 2018; 7: e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheen AJ, Vancrombreucq JC, Delarge J, Luyckx AS. Diuretic activity of torasemide and furosemide in chronic heart failure: a comparative double blind cross‐over study. Eur J Clin Pharmacol 1986; 31: 35–42. [DOI] [PubMed] [Google Scholar]
- 13. Sica DA, Carter B, Cushman W, Hamm L. Thiazide and loop diuretics. J Clin Hypertens (Greenwich) 2011; 13: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]


