Abstract
Aims
Heart failure (HF) is a leading cause of hospitalization and is associated with high morbidity and mortality post‐diagnosis. Here, we examined the impact of recurrent HF hospitalization (HFH) on cardiovascular (CV) and all‐cause mortality among HF patients.
Methods and Results
Adult HF patients identified in the Clinical Practice Research Datalink with a first (index) hospitalization due to HF recorded in the Hospital Episode Statistics data set from January 2010 to December 2014 were included. Patients were followed up until death or end of study (December 2017). CV mortality as primary and as any reported cause and all‐cause mortality were evaluated. An extended Cox regression model was used for reporting adjusted relative CV mortality rates for time‐dependent recurrent HFHs. Overall, 8603 HF patients with an index hospitalization were included, providing 15 964 patient‐years of follow‐up. Patients were relatively old (median age: 80 years) and were mostly male (54.6%), with main co‐morbidities being hypertension and atrial fibrillation. Recurrent HFHs occurred one, two, three, and more than four times in 1561 (18.2%), 518 (6.02%), 206 (2.4%), and 153 (1.8%) patients, respectively. The median time to mortality was 215 (38–664) days for 50.8% of patients who died for any cause during the study period and 139 (27–531) days for 31.3% who died with CV reasons as primary cause. Compared with those of patients without recurrent HFHs, the adjusted hazard ratios (95% CI) for CV mortality as primary cause were 2.65 (2.35–2.99), 3.69 (3.06–4.43), 5.82 (4.48–7.58), and 5.95 (4.40–8.05) for those with one, two, three, and more than four recurrent HFHs.
Conclusions
There is a strong association between recurrent HFH and CV mortality, with the risk increasing progressively with each recurrent HFH.
Keywords: Heart failure, Mortality/survival
Introduction
Heart failure (HF) is a major public health issue that impacts 37.7 million people globally 1 and results in >1 million hospitalizations annually in both the USA and Europe. 2 The estimates for the prevalence of HF vary but are ~1–2% of the adult population in developed countries, rising to ≥10% among people >70 years of age. 3 With the increasing prevalence of chronic HF, there is a concomitant increase in the number of related hospitalizations; as chronic HF progresses, the risk of episodes of acute worsening increases. Furthermore, after discharge, patients with HF are at a high risk for re‐hospitalization. 2
In the UK alone, more than half a million people live with this chronic condition. 4 From the healthcare system perspective, the burden is high; ~1–2% of the National Health Service (NHS) budget is estimated to be spent on HF, of which 60–70% is driven by the costs of hospitalization. 5 This cost is estimated to increase, driven by an aging population.
Previous observational studies conducted in the USA and Canada have found that the number of recurrent hospitalizations is a strong predictor of mortality. 6 , 7 Similar findings were noted in a post hoc analysis of the CHARM trial, where rates of cardiovascular (CV) mortality or HF hospitalization (HFH) were the highest in patients who had been previously hospitalized for HF. 8 However, there are limited data to evaluate this in a contemporary, real‐world European setting.
The objective of this study was to describe recurrent hospitalizations in an HF population and to evaluate the association of recurrent HFHs with CV mortality and all‐cause mortality. In the database, CV cause of mortality could have been recorded as primary cause or as any cause; both were assessed in this study. This study is important to evaluate the association between recurrent hospitalization and mortality risk and thereby aid understanding of disease progression in HF, in a contemporary, real‐world UK data set.
Methods
This was a retrospective non‐interventional cohort study conducted using primary care data from the Clinical Practice Research Datalink (CPRD) database. CPRD is a longitudinal primary care electronic medical record database from the UK, which contains >10 million active patient records (and over 35 million overall) drawn from ~1100 primary care practices, with data extending back as far as 1987. It is the world's largest database of anonymized, longitudinal primary care medical records.
The CPRD database is linked to other electronic health records, including (i) Hospital Episode Statistics (HES; a data warehouse containing details of all admissions to NHS hospitals in England), (ii) practice level Index of Multiple Deprivation (IMD), and (iii) the mortality data obtained from the Office of National Statistics (ONS).
This study was approved by the Independent Scientific Advisory Committee (ISAC) at CPRD (ISAC protocol reference number: 18_252R).
Study design and population
Adult patients (aged ≥ 18 years) with a HF diagnosis in the primary care database from 1 January 2006 to 31 December 2017 and at least one HFH from 1 January 2010 to 31 December 2014 were identified. Patients were eligible for inclusion if their record was approved for linkage to the HES, IMD, and ONS death register databases and had continuous practice registration up‐to‐standard for at least a year before the index date and the follow‐up period (or until death) for each patient. Patients were followed up until the end of study (31 December 2017), death, practice last collection date or transfer‐out date (whichever event was the earliest). The International Classification of Diseases, 10th Revision (ICD‐10) codes (I11.0, I13.0, I13.2, I42.0, I42.1, I42.2, I42.9, or I50.X) were used to identify HFHs, and primary care (Read) coding schemes were used to identify HF diagnoses in the primary care database.
The date of admission for the first HFH in the identification period was defined as the index date (Figure 1 ). Patients without acceptable data (as per the quality standards defined in the CPRD database) were excluded. Patients were also excluded if they had experienced an HFH during the 4 year pre‐index period (1 January 2006 to 1 January 2010), described as the ‘clean period'.
Figure 1.
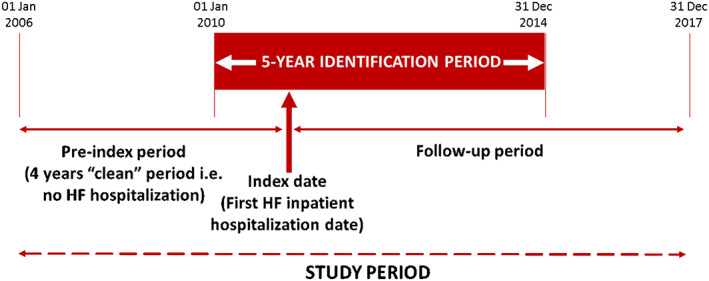
Overview of study design.
Note: Identification period: patients with at least one heart failure hospitalization (HFH) were identified during this time. Index date: date of first HFH during the identification period. Pre‐index period: 4 years prior to index date. During this time, patients had no HFH (‘clean' period). Follow‐up period: until study end (31 Dec 2017), death, and transfer‐out date (whichever event was earliest).
Study variables
Patients were grouped according to the number of recurrent HFHs (one, two, three, and more than four) after the index HFH. A patient who died after the index HFH date or who survived until end of follow‐up with no subsequent recurrent HFHs was classified as zero recurrent HFH. A patient who died after the first HF re‐admission but before the second HF re‐admission or who survived until the end of follow‐up with no further recurrent HFHs was classified as one recurrent HFH, and so on. Baseline characteristics assessed at the index date included age, gender, body mass index (BMI), smoking status, socio‐economic status, co‐morbidities, estimated glomerular filtration rate (eGFR), cardiac resynchronization therapy (CRT) device use, and concomitant medications. Co‐morbidities and CRT device use were assessed during the 1 year pre‐index period (including the index date), whereas concomitant medications were assessed during the 3 month pre‐index period. Concomitant medications of interest were angiotensin‐converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB), angiotensin receptor neprilysin inhibitor (ARNI), beta‐blocker (BB), and mineralocorticoid receptor antagonist (MRA). ACEi and ARB were grouped together, as they have similar effects and were never taken together. ARNI was not present in the baseline tables, as it was not approved until after November 2015; however, patients who were followed up beyond this date may have received prescriptions for ARNI, and therefore, ARNI could be present as a time‐dependent variable during the follow‐up analysis.
During the study follow‐up, all‐cause mortality and CV mortality (both where CV disease was listed as primary cause of death and where CV disease was listed as any cause of death) were recorded for overall HF population. Among patients with zero recurrent HFH, mortality outcomes were evaluated from the date of admission. All‐cause mortality was assessed by linking the patients to the ONS database and establishing a date of death in the ONS database. CV mortality as primary cause was assessed using ICD‐10 codes (i.e. Chapter I00‐99) in the primary cause of death field. CV mortality as any cause was assessed using ICD‐10 codes (i.e. Chapter I00‐99) in the primary cause of death field and in any of the 15 causes of death fields in the patient's ONS file. Date of death was also extracted from the ONS database.
Data analysis
Baseline demographics and clinical characteristics were summarized using descriptive analyses. Imputation was not performed for the missing data. Continuous variables were summarized as either mean ± standard deviation (SD) or median [inter‐quartile range (IQR)], while all categorical variables were summarized as frequency and percentage. Categorical variables were compared using the χ 2 test. The Kolmogorov–Smirnov test was used to check the skewness of data. Continuous variables were compared using unequal variance two‐sample t‐test. For continuous variables with skewed data, the Mann–Whitney U test was used.
An extended Cox regression model was used for reporting adjusted relative CV mortality rates for time‐dependent recurrent HFH. Adjusted variables were included based on clinical importance or significance at baseline and if the missing values were below 30%. Estimates were adjusted for the following covariates (Supporting Information Supplement 1a and 1b): gender, age, BMI, IMD, smoking status, co‐morbidities, pacemaker/defibrillator devices, laboratory/test values, and concomitant medications. Gender, smoking status, BMI, index year, IMD, and laboratory/test values were taken at baseline. Given that age, co‐morbidities, concomitant medications, and pacemaker/defibrillator devices are time‐dependent covariates, they were assessed at baseline and at follow‐up. Competing risks were addressed using the Fine–Gray sub‐distribution hazard function. 9 The Fine–Gray method is implemented in the Survival package in R and is part of the PHREG procedure in SAS.
Results
Baseline characteristics
A total of 116 262 patient records between 1 January 2006 and 31 December 2017 were considered for this study. After the selection criteria were applied (Figure 2 ), 8603 HF patients with an index HFH were included in the analysis, resulting in a total of 15 964 patient‐years of follow‐up (mean ± SD: 677.8 ± 653.2 days).
Figure 2.
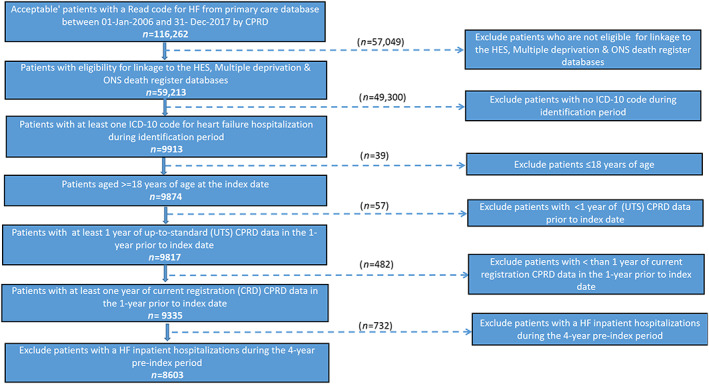
Patient selection process.
Note: CPRD, Clinical Practice Research Datalink; HES, Hospital Episodes Statistics; HF, heart failure; ICD‐10: International Classification of Diseases, 10th Edition; ONS, Office of National Statistics.
The included patients were relatively old with a median (IQR) age of 80 (71–86) years at index and were more likely to be male (54.6%). The median BMI was 28, with 38.7% of patients being overweight or obese. The most common co‐morbidities observed at baseline were hypertension (65.0%) and atrial fibrillation (53.3%). Other frequent co‐morbidities included diabetes (28.4%), renal disease (27.8%), and chronic pulmonary disorder (27.3%) (Table 1 ). More than half of the patients (62.5%) were taking an ACEi or ARB at index.
Table 1.
Demographic and clinical characteristics of patients with heart failure hospitalization
| Characteristics | 0 rHFH | 1 rHFH | 2 rHFH | 3 rHFH | 4 rHFH | ≥4 rHFH | All patients |
|---|---|---|---|---|---|---|---|
| n (%) | 6165 (71.7%) | 1561 (18.1%) | 518 (6.0%) | 206 (2.4%) | 72 (0.8%) | 153 (1.8%) | 8603 (100.0%) |
| Male, n (%) | 3318 (53.8%) | 879 (56.3%) | 293 (56.6%) | 118 (57.3%) | 39 (54.2%) | 89 (58.2%) | 4697 (54.6%) |
| Age (years) at index, median (IQR) | 80 (71–87) | 80 (71–86) | 79 (71–85) | 78 (68–82) | 78 (69–83) | 75 (66–83) | 80 (71–86) |
| Follow‐up (days) | |||||||
| Patient‐years | 10 471.1 | 3270.2 | 1191.1 | 571.9 | 195.7 | 460.1 | 15 964.4 |
| Mean ± SD | 620.4 ± 644.8 | 765.2 ± 650.3 | 839.9 ± 634.2 | 1014.0 ± 612.1 | 992.8 ± 646.7 | 1098.3 ± 661.9 | 677.8 ± 653.2 |
| Smoking status | |||||||
| Current smoker | 731 (11.9%) | 172 (11.0%) | 58 (11.2%) | 23 (11.2%) | 7 (9.7%) | 25 (16.3%) | 1009 (11.7%) |
| Never smoker | 2510 (40.7%) | 644 (41.3%) | 208 (40.2%) | 88 (42.7%) | 25 (34.7%) | 53 (34.6%) | 3503 (40.7%) |
| Former smoker | 2456 (39.8%) | 636 (40.7%) | 224 (43.2%) | 86 (41.8%) | 34 (47.2%) | 68 (44.4%) | 3470 (40.3%) |
| Missing/unknown | 468 (7.6%) | 109 (7.0%) | 28 (5.4%) | 9 (4.4%) | 6 (8.3%) | 7 (4.6%) | 621 (7.2%) |
| BMI a , median (IQR) | 27.7 (23.8–32.4) | 27.8 (24.2–32.7) | 28.7 (24.5–32.6) | 28.9 (24.2–34.1) | 29.2 (23.0–34.2) | 29.3 (24.5–34.2) | 27.9 (24.0–32.5) |
| BMI category | |||||||
| Underweight: <18.5 kg/m2 | 101 (1.6%) | 18 (1.2%) | 7 (1.4%) | ND | ND | ND | 128 (1.5%) |
| Normal weight: ≥18.5 and <25 kg/m2 | 974 (15.8%) | 256 (16.4%) | 82 (15.8%) | 41 (19.9%) | 14 (19.4%) | 24 (15.7%) | 1377 (16.0%) |
| Overweight: ≥25 and <30 kg/m2 | 1041 (16.9%) | 286 (18.3%) | 98 (18.9%) | 38 (18.5%) | 10 (13.9%) | 32 (20.9%) | 1495 (17.4%) |
| Obese: ≥30 kg/m2 | 1254 (20.3%) | 350 (22.4%) | 129 (24.9%) | 60 (29.1%) | 20 (27.8%) | 44 (28.8%) | 1837 (21.4%) |
| Missing/unknown | 2795 (45.3%) | 651 (41.7%) | 202 (39.0%) | 67 (32.5%) | 27 (37.5%) | 51 (33.3%) | 3766 (43.8%) |
| Multiple deprivation index | |||||||
| Quintile 1 (least deprived) | 1213 (19.7%) | 307 (19.7%) | 88 (17.0%) | 28 (13.6%) | 15 (20.8%) | 29 (19.0%) | 1665 (19.4%) |
| Quintile 2 | 1352 (21.9%) | 346 (22.2%) | 113 (21.8%) | 41 (19.9%) | 15 (20.8%) | 27 (17.7%) | 1879 (21.8%) |
| Quintile 3 | 1397 (22.7%) | 321 (20.6%) | 114 (22.0%) | 44 (21.4%) | 10 (13.9%) | 23 (15.0%) | 1899 (22.1%) |
| Quintile 4 | 1159 (18.8%) | 323 (20.7%) | 100 (19.3%) | 44 (21.4%) | 20 (27.8%) | 40 (26.1%) | 1666 (19.4%) |
| Quintile 5 (most deprived) | 1036 (16.8%) | 263 (16.9%) | 103 (19.9%) | 49 (23.8%) | 12 (16.7%) | 34 (22.2%) | 1485 (17.3%) |
| Missing/unknown | 8 (0.1%) | 1 (0.1%) | 0 | 0 | 0 | 0 | 9 (0.1%) |
| CRT‐P | 825 (13.4%) | 238 (15.3%) | 95 (18.3%) | 34 (16.5%) | 13 (18.1%) | 36 (23.5%) | 1228 (14.3%) |
| CRT‐D | 232 (3.8%) | 83 (5.3%) | 21 (4.0%) | 14 (6.8%) | 4 (5.6%) | 10 (6.5%) | 360 (4.2%) |
| Any CRT device | 910 (14.8%) | 261 (16.7%) | 101 (19.5%) | 40 (19.4%) | 13 (18.1%) | 38 (24.8%) | 1350 (15.7%) |
| Any co‐morbidity a | 5947 (96.5%) | 1517 (97.2%) | 499 (96.3%) | 200 (97.1%) | 72 (100.0%) | 152 (99.4%) | 8315 (96.7%) |
| Atrial fibrillation | 3294 (53.4%) | 817 (52.3%) | 282 (54.4%) | 116 (56.3%) | 37 (51.4%) | 76 (49.7%) | 4585 (53.3%) |
| Anaemia | 1166 (18.9%) | 319 (20.4%) | 107 (20.7%) | 48 (23.3%) | 10 (13.9%) | 21 (13.7%) | 1661 (19.3%) |
| Angina pectoris | 1439 (23.3%) | 394 (25.2%) | 132 (25.5%) | 72 (35.0%) | 23 (31.9%) | 47 (30.7%) | 2084 (24.2%) |
| Dyslipidaemia | 1198 (19.4%) | 345 (22.1%) | 111 (21.4%) | 51 (24.8%) | 19 (26.4%) | 40 (26.1%) | 1745 (20.3%) |
| Hypertension | 3964 (64.3%) | 1034 (66.2%) | 359 (69.3%) | 136 (66.0%) | 50 (69.4%) | 99 (64.71%) | 5592 (65.0%) |
| Peripheral artery disease | 304 (4.9%) | 92 (5.9%) | 36 (7.0%) | 18 (8.7%) | 6 (8.3%) | 8 (5.2%) | 458 (5.3%) |
| Oedema | 1389 (22.5%) | 337 (21.6%) | 98 (18.9%) | 39 (18.9%) | 16 (22.2%) | 26 (17.0%) | 1889 (22.0%) |
| Cerebrovascular disease | 561 (9.1%) | 141 (9.0%) | 33 (6.4%) | 17 (8.3%) | 7 (9.8%) | 10 (6.5%) | 762 (8.9%) |
| Chronic pulmonary disorder | 1683 (27.3%) | 424 (27.2%) | 147 (28.4%) | 57 (27.7%) | 18 (25.0%) | 39 (25.5%) | 2350 (27.3%) |
| Diabetes | 1639 (26.6%) | 472 (30.2%) | 179 (34.6%) | 90 (43.7%) | 26 (36.1%) | 60 (39.2%) | 2440 (28.4%) |
| Myocardial infarction | 1028 (16.7%) | 278 (17.8%) | 96 (18.5%) | 42 (20.4%) | 15 (20.8%) | 27 (17.7%) | 1471 (17.1%) |
| Peripheral vascular disease | 603 (9.8%) | 166 (10.6%) | 76 (14.7%) | 26 (12.6%) | 9 (12.5%) | 16 (10.5%) | 887 (10.3%) |
| Renal disease | 1682 (27.3%) | 429 (27.5%) | 166 (32.1%) | 66 (32.0%) | 16 (26.4%) | 46 (30.1%) | 2389 (27.8%) |
| Pneumonia | 980 (15.9%) | 218 (14.0%) | 67 (12.9%) | 37 (18.0%) | 14 (19.4%) | 29 (19.0%) | 1331 (15.5%) |
| Ischaemic heart disease | 2874 (46.6%) | 768 (49.2%) | 280 (54.1%) | 120 (58.3%) | 43 (59.7%) | 86 (56.2%) | 4128 (48.0%) |
| Any concomitant medication | 4697 (76.2%) | 1237 (79.2%) | 415 (80.1%) | 176 (85.4%) | 61 (84.7%) | 131 (85.6%) | 6656 (77.4%) |
| ACEis | 2781 (45.1%) | 734 (47.0%) | 260 (50.2%) | 99 (48.1%) | 34 (47.2%) | 80 (52.3%) | 3954 (46.0%) |
| ARB | 1064 (17.3%) | 305 (19.5%) | 106 (20.5%) | 48 (23.3%) | 16 (22.2%) | 36 (23.5%) | 1559 (18.1%) |
| BB | 3003 (48.7%) | 780 (50.0%) | 286 (55.2%) | 115 (55.8%) | 40 (55.6%) | 92 (60.1%) | 4276 (49.7%) |
| MRA | 957 (15.5%) | 293 (18.8%) | 102 (19.7%) | 55 (26.7%) | 17 (23.6%) | 44 (28.8%) | 1451 (16.9%) |
| ACEis or ARB | 3756 (60.9%) | 1011 (64.8%) | 354 (68.3%) | 144 (69.9%) | 48 (66.7%) | 111 (72.6%) | 5376 (62.5%) |
| eGFR (mL/min/1.73 m2), mean ± SD | 66.3 ± 25.2 | 64.3 ± 25.0 | 62.9 ± 25.6 | 65.9 ± 26.8 | 66.9 ± 25.7 | 66.1 ± 25.1 | 65.7 ± 25.2 |
| eGFR category | |||||||
| eGFR ≥ 60 mL/min/1.73 m2 | 3167 (51.4%) | 765 (49.0%) | 242 (46.7%) | 98 (47.6%) | 38 (52.8%) | 76 (49.7%) | 4348 (50.5%) |
| eGFR < 60 mL/min/1.73 m2 | 2232 (36.2%) | 625 (40.0%) | 223 (43.1%) | 89 (43.2%) | 30 (41.7%) | 66 (43.1%) | 3235 (37.6%) |
: in 1 year prior to index date.
ACEis, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blocker; BB, beta‐blocker; BMI, body mass index; CRT‐D, cardiac resynchronization defibrillator; CRT‐P, cardiac resynchronization pacemaker; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; rHFH, recurrent heat failure hospitalization.
Overall, 28% (n = 2438) of the patients were hospitalized for HF more than once. Among these 2438 patients, 1561 (18.14%) had one, 518 (6.02%) had two, 206 (2.39%) had three, and 153 (1.78%) had more than four recurrent HFHs. At baseline, compared with patients with zero or one recurrent HFH, patients with two, three, or more than four recurrent HFHs had a higher percentage of co‐morbidities, such as ischaemic heart disease, diabetes, renal disease, and angina pectoris. Overall, the baseline condition of patients with recurrent HFHs appeared worse in several aspects than the patients with zero recurrent HFH, for example, a higher percentage of overweight/obese patients, higher co‐morbidities, lower eGFR levels, and increased use of concomitant medications.
Hospitalization and mortality rates
Among patients with more than one recurrent HFH, the number of all‐cause hospitalizations [median (IQR): 5 (3–8)] was much higher than that of patients without recurrent HFH [median (IQR): 2 (1–4); P < 0.0001]. In the total study population, 50.8% of patients died from any cause (Table 2 ). Of these total deaths, 88.6% were attributable to CV reasons as any cause, and 61.7% were listed as having CV reason as the primary cause. The median time to mortality was 215 (38–664) days for 50.8% of patients who died for any cause during the study period and 139 (27–531) days for 31.3% who died with CV reasons as primary cause. Across the groups of patients with recurrent HFHs, 88–95% of the total deaths had CV disease listed as any cause. Among the patients with more than four recurrent HFHs (n = 153), 60% (n = 91) of patients died by the end of the study, of which CV mortality (primary cause) contributed to 74% (n = 67) of the total deaths. Of the patients who were alive at the end of the study (n = 4231), 74% did not experience recurrent HFHs.
Table 2.
Proportion of deaths across study groups
| Death events | 0 rHFH | 1 rHFH | 2 rHFH | 3 rHFH | ≥4 rHFH | All patients |
|---|---|---|---|---|---|---|
| n = 6165 | n = 1561 | n = 518 | n = 206 | n = 153 | n = 8603 | |
| All‐cause, n (%) | 3026 (41.0) | 839 (53.8) | 290 (56.0) | 126 (61.2) | 91 (59.5) | 4372 (50.8) |
| CV (as any cause), n (%) | 2621 (86.6) | 770 (91.8) | 275 (94.8) | 120 (95.2) | 86 (94.5) | 3872 (88.6) |
| CV (as primary cause), n (%) | 1788 (59.1) | 552 (65.8) | 199 (68.6) | 91 (72.2) | 67 (73.6) | 2697 (61.7) |
CV, cardiovascular; rHFH, recurrent heart failure hospitalization.
Some hospitalizations were associated with inpatient mortality. The duration of the final hospitalization associated with inpatient mortality was similar among all categories of recurrent HFH. The median length of hospitalization leading to all‐cause mortality ranged between 11 and 12 days across the groups, with a median value of 11 (5–22) days for the overall population. A similar range was noted for median length of hospitalization leading to CV mortality (as any cause or as primary cause). The median length of hospitalization leading to CV mortality as primary cause ranged between 10.5 and 14.0 days, with a median value of 11 (5–22) days for the total study population.
Time to immediate subsequent recurrent HFH decreased with each re‐hospitalization, with a median of 143 (35–464) days from index to first recurrent HFH, 84 (21–272) days from first recurrent HFH to second recurrent HFH, 77 (26–237) days from second recurrent HFH to third recurrent HFH, and 46 (11–127) days from third recurrent HFH to fourth recurrent HFH.
Correlation between recurrent heart failure hospitalization and all‐cause mortality / cardiovascular mortality (as primary or any cause of mortality)
Recurrent HFHs were associated with a statistically significant increase in all‐cause mortality and CV mortality (both as primary and as any cause of death). There was a significant increase in annualized mortality rates with each additional recurrent HFH. Among patients with one recurrent HFH, the annualized rate for CV mortality as primary cause was 32 deaths/100 person‐years at risk, which increased significantly to 63 deaths/100 person‐years at risk for patients with four recurrent HFHs. Similarly, the annualized rate for all‐cause death increased significantly from 49 to 89 deaths/100 person‐years at risk (Figure 3). Compared with that in the four recurrent HFH group, a decline was observed in the more than four recurrent HFH group for both all‐cause and CV mortality.
Figure 3.
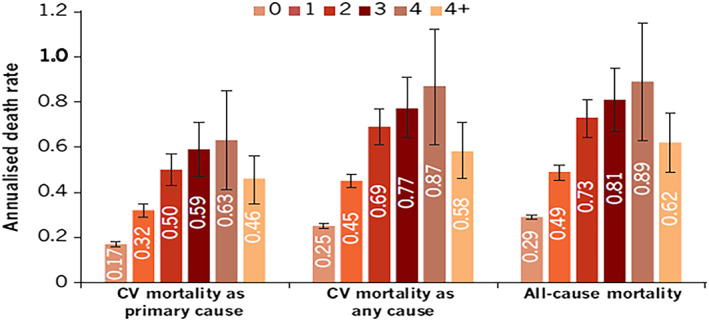
Annualized mortality rates according to number of recurrent HF hospitalizations.
Note: Error bars represent 95% CI range. CI, confidence interval; CV, cardiovascular; HF, heart failure.
Compared with that in patients with no recurrent HFHs, the risk of mortality due to CV reason as the primary cause increased by approximately six times in patients with three and more than four recurrent HFHs {hazard ratio (HR) [95% confidence interval (CI)]: 5.82 (4.48–7.58) and 5.95 (4.40–8.05), respectively}. The risk of mortality when CV reasons were listed as any cause of death [more than four recurrent HFHs; HR: 3.93 (3.00–5.15)] and the risk of all‐cause mortality [more than four recurrent HFHs; HR: 3.47 (2.75–4.38)] increased by approximately four times for patients with recurrent HFHs compared with those without recurrent HFHs.
As expected, co‐morbidities were associated with increased risk of both CV and all‐cause mortality (Figures 4 , 5 , and 6 ). Pneumonia emerged as the biggest risk factor for CV mortality, both as primary (HR: 1.61; P < 0.0001) and any cause of death (HR: 2.18; P < 0.0001). Other co‐morbidities such as metastatic tumour and chronic obstructive pulmonary disease did not appear to increase the risk of CV mortality, while they were risk factors for all‐cause mortality, with metastatic tumour associated with the highest risk (HR: 2.79; P < 0.0001).
Figure 4.
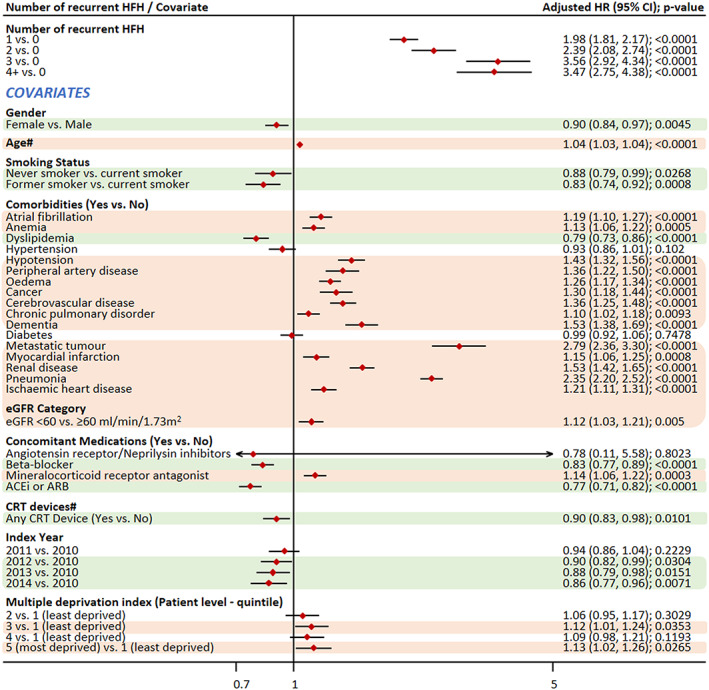
Impact of covariates on the association of recurrent HFH and all‐cause mortality.
Note: #Time‐dependent covariate. Covariates highlighted in green were associated with a significantly reduced risk of mortality, while those highlighted in light red colour were associated with a significantly increased risk of mortality. ACEis, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CI, confidence interval; CRT, cardiac resynchronization therapy; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Figure 5.
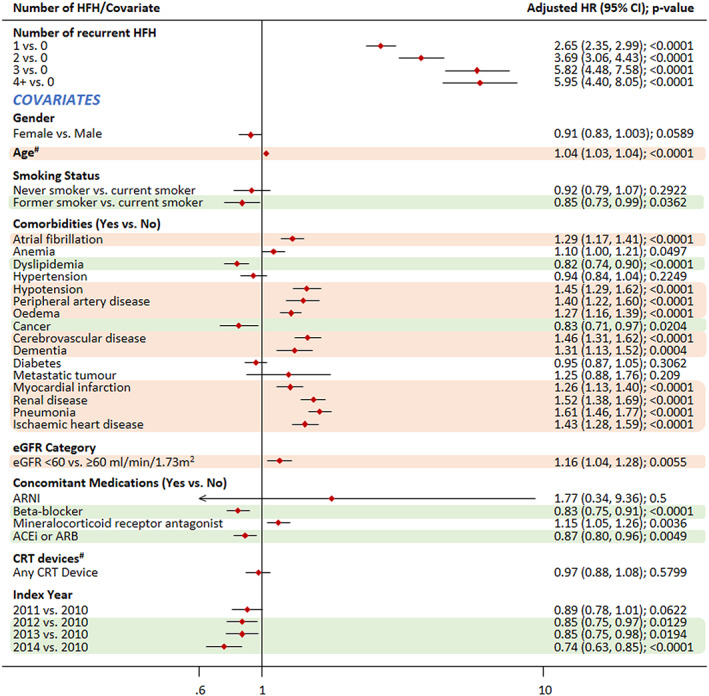
Impact of covariates on the association of recurrent HFH and CV mortality as primary cause.
Note: #Time‐dependent covariate. Covariates highlighted in green were associated with a significantly reduced risk of mortality, while those highlighted in light red colour were associated with a significantly increased risk of mortality; ACEis, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CI, confidence interval; CRT, cardiac resynchronization therapy; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Figure 6.
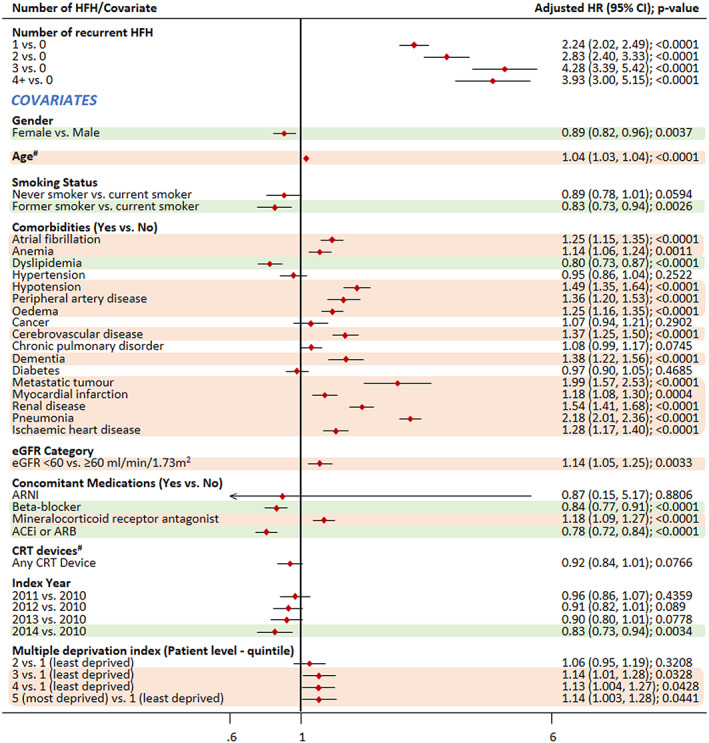
Impact of covariates on the association of recurrent HFH and CV mortality as any cause.
Note: #Time‐dependent covariate. Covariates highlighted in green were associated with a significantly reduced risk of mortality, while those highlighted in light red colour were associated with a significantly increased risk of mortality. ACEis, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CI, confidence interval; CRT, cardiac resynchronization therapy; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Treatment with BBs was associated with a decreased risk of CV mortality as primary cause of death (HR: 0.83), CV mortality as any cause of death (0.84), and all‐cause mortality (0.83). The same pattern was observed for ACEi/ARB, which was also associated with a decreased risk of mortality (HR: 0.87, 0.78, and 0.77, respectively). In case of patients on MRA, there was an increased risk of mortality. Positively, later index year was associated with decreased mortality. Gender was associated with CV mortality as any cause and all‐cause mortality; female gender was associated with a decreased risk of mortality. Former smokers were shown to be at a significantly lower risk than current smokers for CV mortality (as primary cause or any cause) and all‐cause mortality.
Discussion
This retrospective analysis of 8603 patients with incident HFH highlights that recurrent HFHs are associated with an increased risk of CV mortality (as primary cause or any cause), and this is also seen with all‐cause mortality. Risk of mortality increases significantly with increasing number of recurrent HFHs. Compared with that in patients with no recurrent HFH, the adjusted risk of CV mortality (as primary cause) was three‐fold higher in patients with one recurrent HFH and six‐fold higher in patients with more than four recurrent HFHs. Similarly, the adjusted risk of all‐cause mortality increased more than two‐fold in patients with one recurrent HFH and was four‐fold higher in patients with more than four recurrent HFHs. Moreover, the time to each subsequent re‐hospitalization became shorter, highlighting the progressive nature of the disease. The findings of our study further strengthen the existing literature evaluating the relationship between mortality and recurrent HFH by providing contemporary data from a large, real‐world database. This study also brings new information about the burden of HFH for the community, including the length of stay at hospital (median > 10 days). The study data point to the importance of HF management; treatment options that can improve prognosis and reduce re‐hospitalizations will also have a positive impact on healthcare systems.
Overall, the included patients were elderly with a median age of 80 years and mostly male. Other studies evaluating the relationship of recurrent HFH on mortality or re‐admissions following incident HFH also reported similar baseline characteristics. 6 , 10 In comparison with patients without recurrent HFH, patients with recurrent HFHs had a higher percentage of co‐morbidities, and a higher proportion were overweight/obese, along with a higher usage of baseline medications, indicating that the baseline condition of patients with a higher number of recurrent HFHs was likely to be worse than the patients with zero recurrent HFH.
Patients with more than one recurrent HFH were hospitalized for any reason more often than patients without recurrent HFH (median number of hospitalizations: 5 and 2, respectively). This finding implies that patients frequently hospitalized for HF are more prone to experience a worsening of their general condition. Furthermore, compared with patients without recurrent HFH, patients with more recurrent HFHs were slightly younger and had a longer follow‐up time, which could have resulted in more hospitalizations due to any cause. Besides the mortality risk associated with increasing numbers of hospitalizations, frequent hospital admissions and reduced time between hospitalizations (median of 143 days from index HFH to 46 days from third recurrent HFH to fourth recurrent HFH) increase the burden for the patient and their family. Recent publications have suggested that the concept of reduced ‘home‐time'—defined as the time a patient spends alive and out of a healthcare institution 11 —is associated with decreased time between hospitalizations and is of critical importance to the overall burden on patients and their families.
In the total population, 51% of patients died from any cause, and among these, 88.6% of patients died from CV reasons as any cause and 61.7% of patients with CV reasons as the primary cause of death. Patients with CV mortality as primary cause had the lowest median time to mortality. In comparison with patients who were alive at the end of follow‐up, patients with CV mortality were slightly older, diagnosed earlier in the study (based on index year), more likely to have more than one recurrent HFH, and with a lower BMI and poor socio‐economic status. A significant increase was noted in the annualized mortality rates after each recurrent HFH. However, a lower mortality rate was observed in the more than four recurrent HFH group, which included patients who had four, five, and six (up to 12) recurrent HFHs. To ensure an adequate sample size, patients with more than four HFHs were grouped together; the lower annualized mortality rates in this group may be due to inclusion of patients who survived for a relatively long time while experiencing multiple hospitalizations. Additionally, although patients without recurrent HFH had a better survival, as the all‐cause annual mortality rate was 29 deaths/100 person‐years at risk, mortality remains high even in this lower risk group of HF patients.
As observed in other studies, this study also showed that co‐morbidities were associated with increased risk of both CV and all‐cause mortality, for example, hypotension, cerebrovascular disease, renal disease, metastatic tumour, and pneumonia. For CV mortality, pneumonia as a co‐morbidity had the highest HR estimate [2.18 (2.01–2.36); P < 0.0001], which is in line with other studies that reported pneumonia as a risk factor for increased risk of CV complications, 12 , 13 whereas metastatic tumour had the strongest positive correlation with all‐cause mortality [HR: 2.79 (2.36–3.30); P < 0.0001]. Additionally, for CV mortality as primary cause, cancer as a co‐morbidity was associated with a lower mortality [HR: 0.83 (0.71–0.97); P < 0.0204], as the patients who would die because of cancer will be accounted in the all‐cause mortality group.
Interestingly, later index year also had a positive effect on mortality outcome, which could be attributed to either an improvement in HF prognosis or a shorter follow‐up period in the study. The analysis also showed that use of MRA was associated with an increased risk of mortality (whereas BB and ACEi/ARB use was associated with decreased risk of mortality). This is most likely due to the reason that MRA therapy is typically prescribed in high‐risk patients with poor prognosis. Female gender was associated with a small decreased risk in mortality—one potential reason for this could be lower rates of ischaemic disease in females versus males. 14
To the best of our knowledge, this is the first study to quantify the magnitude of risk between recurrent HFH and mortality in a real‐world setting in Europe. The study population was taken from CPRD, which has over 10 million active patient records drawn from ~1100 primary care practices across the UK and, thus, was representative of the UK population.
Although CPRD is representative of the UK population and offers high‐quality data, there are some limitations. Firstly, our analysis included only patients with a 4‐year clean period, which is reasonable to assume as the study identified the actual first HFH, and HFHs prior to this clean period were not considered. Second, because the patient identifiers with HF codes from the CPRD primary care database were used to find linked patients in the HES database, there is a risk that the study might have missed some patients who were diagnosed with more severe HF in the inpatient setting and where the diagnosis was not recorded by a general practitioner (in CPRD). Third, the present study included patients with at least one HFH, and therefore, patients who would have died before the first HFH were not captured in the current study. Therefore, both very severe cases and mild cases of HF that do not require hospitalizations were not included in the study. Fourth, information on HF severity (New York Heart Association classification) was not available, and therefore, the relationship of recurrent HFH with mortality after adjustment for functional status could not be evaluated. Fifth, left ventricular ejection fraction (LVEF) data were infrequently recorded in the database, and therefore, the impact of recurrent HFH on outcomes could not be studied as per the LVEF classification. Sixth, medication use might have been overestimated as the prescription of a drug was used as a proxy for use. Lastly, there are inherent limitations associated with secondary use of data, including missing data, selection bias, and others.
Conclusions
Recurrent HFHs are strongly associated with CV mortality and all‐cause mortality, as the risk of CV mortality and all‐cause mortality increases progressively with each recurrent HFH. Furthermore, mortality was even high among patients with no recurrent HFHs. Thus, the number of hospitalizations is a hallmark of the patient's prognosis and highlights the need for treatments that improve the course of the disease and help patients stay out of hospital.
Conflict of interest
Raquel Lahoz, Clare Proudfoot, Stefano Corda, and Rachel Studer are employees of Novartis Pharma AG, Basel. Ailís Fagan is an employee of Novartis Ireland Limited, Dublin. Martin McSharry was an employee of Novartis Ireland Limited, Dublin, at the time of conduct of this study.
Funding
This study was funded by Novartis Pharma AG, Basel, Switzerland.
Supporting information
Data S1. Supporting Information
Acknowledgements
The authors thank Ana Filipa Fonscea, Rumjhum Agrawal, and Zineb Zerrad‐Igrouche for providing medical writing assistance with this manuscript.
Lahoz, R. , Fagan, A. , McSharry, M. , Proudfoot, C. , Corda, S. , and Studer, R. (2020) Recurrent heart failure hospitalizations are associated with increased cardiovascular mortality in patients with heart failure in Clinical Practice Research Datalink . ESC Heart Failure, 7: 1688–1699. 10.1002/ehf2.12727.
References
- 1. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker‐Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan‐Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere‐Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz‐Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill NN, McGrath JJ, Medina‐Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi‐Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez‐Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez‐Riera L, Sanman E, Schwebel DC, Scott JG, Segui‐Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4. Cowie MR. The heart failure epidemic: a UK perspective. Echo Res Pract 2017; 4: R15–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace 2011; 13: ii13–ii17. [DOI] [PubMed] [Google Scholar]
- 6. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007; 154: 260–266. [DOI] [PubMed] [Google Scholar]
- 7. Lee DS, Austin PC, Stukel TA, Alter DA, Chong A, Parker JD, Tu JV. “Dose‐dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med 2009; 122: 162–169 e161. [DOI] [PubMed] [Google Scholar]
- 8. Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7: 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 10. Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009; 54: 1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greene SJ, O'Brien EC, Mentz RJ, Luo N, Hardy NC, Laskey WK, Heidenreich PA, Chang CL, Turner SJ, Yancy CW, Hernandez AF, Curtis LH, Peterson PN, Fonarow GC, Hammill BG. home‐time after discharge among patients hospitalized with heart failure. J Am Coll Cardiol 2018; 71: 2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Violi F, Cangemi R, Falcone M, Taliani G, Pieralli F, Vannucchi V, Nozzoli C, Venditti M, Chirinos JA, Corrales‐Medina VF, Group SS . Cardiovascular complications and short‐term mortality risk in community‐acquired pneumonia. Clin Infect Dis 2017; 64: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 13. Corrales‐Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, Newman A, Loehr L, Folsom AR, Elkind MS, Lyles MF, Kronmal RA, Yende S. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet 2018; 391: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


