Abstract
Aims
Patients with non‐ischaemic dilated cardiomyopathy (DCM) are at increased risk of sudden cardiac death. Identification of patients that may benefit from implantable cardioverter‐defibrillator implantation remains challenging. In this study, we aimed to determine predictors of sustained ventricular arrhythmias in patients with DCM.
Methods and results
We searched MEDLINE/Embase for studies describing predictors of sustained ventricular arrhythmias in patients with DCM. Quality and bias were assessed using the Quality in Prognostic Studies tool, articles with high risk of bias in ≥2 areas were excluded. Unadjusted hazard ratios (HRs) of uniformly defined predictors were pooled, while all other predictors were evaluated in a systematic review. We included 55 studies (11 451 patients and 3.7 ± 2.3 years follow‐up). Crude annual event rate was 4.5%. Younger age [HR 0.82; 95% CI (0.74–1.00)], hypertension [HR 1.95; 95% CI (1.26–3.00)], prior sustained ventricular arrhythmia [HR 4.15; 95% CI (1.32–13.02)], left ventricular ejection fraction on ultrasound [HR 1.45; 95% CI (1.19–1.78)], left ventricular dilatation (HR 1.10), and presence of late gadolinium enhancement [HR 5.55; 95% CI (4.02–7.67)] were associated with arrhythmic outcome in pooled analyses. Prior non‐sustained ventricular arrhythmia and several genotypes [mutations in Phospholamban (PLN), Lamin A/C (LMNA), and Filamin‐C (FLNC)] were associated with arrhythmic outcome in non‐pooled analyses. Quality of evidence was moderate, and heterogeneity among studies was moderate to high.
Conclusions
In patients with DCM, the annual event rate of sustained ventricular arrhythmias is approximately 4.5%. This risk is considerably higher in younger patients with hypertension, prior (non‐)sustained ventricular arrhythmia, decreased left ventricular ejection fraction, left ventricular dilatation, late gadolinium enhancement, and genetic mutations (PLN, LMNA, and FLNC). These results may help determine appropriate candidates for implantable cardioverter‐defibrillator implantation.
Keywords: Dilated cardiomyopathy, Sudden cardiac death, Implantable cardiac‐defibrillator, Prognosis, Risk
Introduction
Non‐ischaemic dilated cardiomyopathy (DCM) is characterized by systolic dysfunction and dilatation of the left ventricle (LV) in the absence of coronary artery disease or abnormal loading conditions. 1 Patients with DCM are at increased risk of sudden cardiac death (SCD) and may benefit from an implantable cardioverter‐defibrillator (ICD). 2 , 3 Prior studies have shown that ICD implantation substantially reduces mortality in patients with heart failure, and consequently, left ventricular ejection fraction (LVEF) continues to be the main criterion to select patients for prophylactic ICD implantation. 1 , 2 , 4 However, these prior data were primarily obtained in patients with ischaemic heart disease as illustrated by the DANISH trial. 4 Even though an updated meta‐analysis on ICD trials still showed that ICD implantation is effective, it shows that these recommendations cannot be rightfully extrapolated to those with non‐ischaemic DCM. 4 , 5
Over the past years, many studies described risk factors for ventricular arrhythmias in non‐ischaemic DCM. These studies uniformly reported previous sustained ventricular arrhythmias and late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (cMRI) as important predictors of arrhythmic events. 6 Of note, the prognostic value of many other investigated clinical risk factors remains unclear. In addition, most results were obtained in observational cohorts with relatively small patient numbers and high variation in reported associations. Prior reviews summarizing the available evidence dealt with this issue by combining both arrhythmic and heart failure outcomes, which however limits their ability to draw definite conclusions about SCD prevention. 7 , 8
In light of these shortcomings, we performed a meta‐analysis and systematically reviewed the studies that assessed predictors of sustained ventricular arrhythmias in DCM. We evaluated quality of evidence and summarized the reported associations using pooled analysis where appropriate. The obtained results may be of value for making management recommendations for this growing group of at‐risk DCM subjects.
Methods
We performed a systematic search of MEDLINE and Embase in February 2018 for clinical studies on risk factors for sustained ventricular arrhythmias in patients with DCM that was updated on January 2020. In short, ischaemia detection was mandatory for diagnosis of DCM in adult patients, and because our outcome of interest is sustained ventricular arrhythmia, articles with only a composite outcome of heart failure without sub‐analysis of arrhythmic outcome were excluded (e.g. DANISH trial). A detailed description of our search strategy, inclusion and exclusion criteria, as well as data extraction table can be found in the Supporting Information, Data S1 . This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. 9
Study eligibility
Any original study involving an adult population with DCM as defined by the European Society of Cardiology 1 that investigated an association of more than one risk factor with a predefined arrhythmic outcome was considered eligible for inclusion. Bibliographies of relevant reviews were checked for additional references. Only studies that specifically reported outcome associations for ventricular arrhythmias were included; hence, those studies with composite endpoints that included non‐arrhythmic events were not considered eligible for inclusion.
Primary outcome
Our primary outcome of interest was sustained ventricular arrhythmias, which was defined as spontaneous sustained ventricular tachycardia (VT), ventricular fibrillation (VF), (resuscitated) SCD, or appropriate ICD intervention for a ventricular arrhythmia. Non‐sustained VT was excluded as an outcome. Because the majority of studies exclusively reported risk estimates for combined arrhythmic outcome, we were obliged to consider all arrhythmic outcomes as equal.
Quality assessment
Individual study quality and risk of bias were assessed using the Quality in Prognostic Studies tool. 10 Study quality was assessed independently by two investigators (A. S. and E. K.); in case of disagreement, a third investigator (F. S.) also assessed study quality to reach consensus.
Statistical analysis
Our analyses were divided into two components: (i) a systematic review and (ii) a meta‐analysis of studies that was amenable for pooled analyses. First, we extracted all study characteristics, risk ratios, odds ratios (ORs), hazard ratios (HRs), confidence intervals (CIs), and P‐values per risk factor. If HRs and CIs were not reported, authors were contacted to obtain these data. The obtained associations on risk ratios, ORs, and P‐values were systematically reported in a table format and summarized in the text. Second, we performed a meta‐analysis of all studies that reported HRs, provided that the risk factor in question had uniform definitions across studies. We excluded studies only reporting ORs from the meta‐analysis, as ORs can only be reliably pooled when follow‐up time is equal. Furthermore, because adjustment of HRs was performed differently in studies, only crude (i.e. unadjusted) HRs were included in the meta‐analysis.
Using the meta package in R [version 3.5.1 R Core Team (2018)], random‐effects meta‐analysis for the HRs were conducted. 11 Statistical heterogeneity between studies was assessed using the χ 2 test for homogeneity, expressed by I 2 index. P‐values were interpreted in a descriptive manner using a significance value of <0.05.
Subgroup analyses were performed to assess the influence of ICD implantation. For sensitivity analyses, fixed‐effects meta‐analyses were performed, and the difference to the results of the random‐effects analysis was discussed.
Results
Search results
Figure 1 shows our search results and selection process. In short, our literature search yielded 1996 unique citations that were carefully screened based on title and abstract. Of these, 1793 citations were excluded as they did not report risk factors for arrhythmic outcomes in the appropriate population. The remaining 203 candidate publications received a thorough full‐text assessment, resulting in a total of 51 studies that met the inclusion criteria. After updating the search in 2020, this yielded an additional four papers totalling 55 included studies. Of the included studies, 29 reported HRs uniformly and were thus included in the meta‐analysis.
Figure 1.
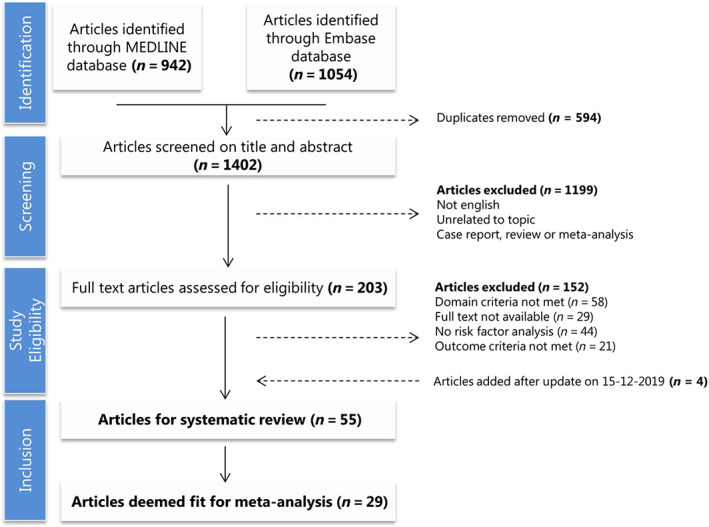
Flowchart of search results and selection process.
Study characteristics
Study characteristics are provided in Supporting Information, Table S1 . The 55 included studies were published between 1992 and 2019 and comprised a total number of 11 451 patients with DCM of whom 76% were male and had mean age of 54 ± 7.9 years. Mean follow‐up time was 3.7 ± 2.3 years with a crude annual event rate of 4.5% [95% CI (4.30–4.76)]. The 28 meta‐analysed studies included a total number of 6287 patients with DCM with 73% male and a mean age of 55.0 ± 4.3 years. Mean follow‐up time of the meta‐analysed studies was 3.9 ± 2.6 years with a crude annual event rate of 4.29% [95% CI (4.02–4.57)].
Quality assessment
Using the Quality in Prognostic Studies tool, the risk of bias was evaluated in six areas in observational prognostic research: (i) study participation, (ii) study attrition, (iii) prognostic facture measurement, (iv) outcome measurement, (v) study confounding, and (vi) statistical analysis and reporting. Results are shown in Figure 2 . The highest risk of bias was introduced by study attrition, limited adjustment for confounders, and limitations in statistical analysis. Details can be found in the Supporting Information, Data S1 .
Figure 2.
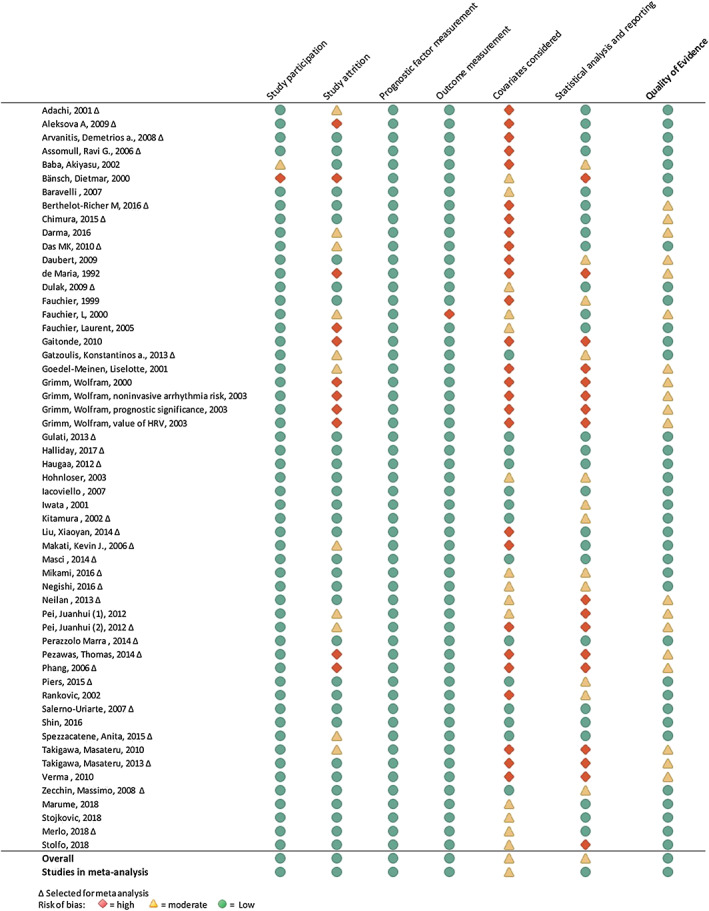
Quality assessment using Quality in Prognostic Studies tool of 51 articles included in the systematic review and meta‐analysis.
Risk factors for life‐threatening arrhythmias
The main risk factor associations are reported by category below. All extracted data are available in the Supporting Information, Data S1 . The pooled HRs from our meta‐analyses are summarized in Figure 3 ; the corresponding forest plots can be found in the Supporting Information, Data S1 .
Figure 3.
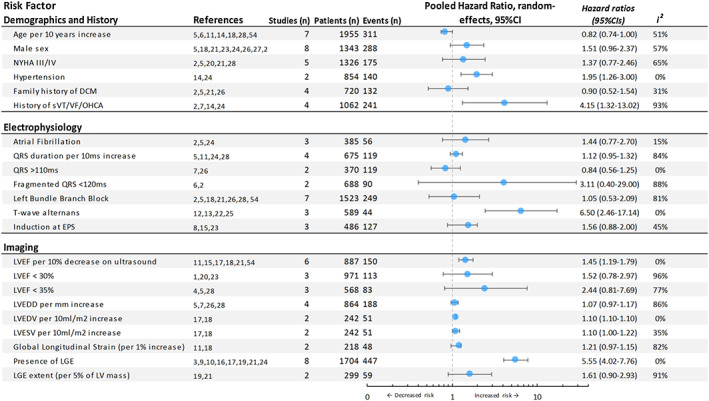
Summary of meta‐analysis. Pooled hazard ratios with 95% CIs are plotted. Results are grouped in ‘Demographics and History', ‘Electrophysiology' and ‘Imaging'. For references and individual study data, see Supporting Information, Data S1 . CI, confidence interval; EPS, electrophysiological study; LGE, late gadolinium enhancement; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association; OHCA, out‐of‐hospital cardiac arrest; sVT, sustained ventricular tachycardia; VT, ventricular tachycardia. For reference numbering see supplemental references
History and demographics
Age was investigated as a predictor in nine studies, of which seven were pooled in the meta‐analysis. This resulted in a small yet significant protective effect of age per 10 years increase [pooled HR 0.82; 95% CI (0.74–1.00)] with moderate heterogeneity (I 2 = 51%). The remaining three studies that were not meta‐analysed reported the same direction of effect, although this did not reach statistical significance.S31, S47, S40
Male sex was investigated as a predictor in 16 studies, of which eight were pooled in the meta‐analysis. The pooled results revealed a non‐significant higher risk of arrhythmias in men [pooled HR 1.51; 95% CI (0.96–2.37)] with moderate heterogeneity (I 2 = 57%). In two of the remaining eight studies that were not meta‐analysed, male sex was associated with an increased risk in arrhythmia.S43
NYHA class was investigated as a predictor in 10 studies. Meta‐analysis of five of these studies showed an increased arrhythmic risk for NYHA classes III/IV compared with classes I/II, but this did not reach statistical significance [pooled HR 1.37; 95% CI (0.77–2.46)]. The heterogeneity was significant (I 2 = 65%). Likewise, four additional studies that were not meta‐analysed did not show a significant association between NYHA class and arrhythmic risk in the long‐term.S41,S43,S42,S44,S55
Hypertension was investigated as a predictor in four studies, of which two were meta‐analysed.S13,S23 Both of these studies reported a significant association of hypertension with life‐threatening ventricular arrhythmias, leading to a pooled HR of 1.95 [95% CI (1.26–3.00)]. The two remaining studies that were not pooled did not show any significantly increased risk.S51,S7
Family history of DCM was investigated as a predictor in four studies, which were all pooled in the meta‐analysis. Pooled results did not direct towards an increased risk of arrhythmia [HR 0.90; 95% CI (0.52–1.54)] with moderate heterogeneity (I 2 = 31%).
History of sustained ventricular arrhythmia was investigated as a predictor in 10 studies, of which four were pooled in the meta‐analysis. All these studies revealed an association between history of sustained ventricular arrhythmia and recurrent future arrhythmias, resulting in a strong pooled HR of 4.15 [95% CI (1.32–13.02)]; however, significant heterogeneity was observed (I 2 = 93%). Of the six remaining studies, three showed a significantly higher arrhythmic risk (P ≤ 0.03), whereas the other studies did not reach statistical significance.S9,S22,S29,S37,S47,S49
Syncope was investigated as a predictor in two studies, which were not meta‐analysed due to missing HRs. None of these studies show any significant associations between syncope and arrhythmic outcome.S44,S14
Genetics
Mutations in genes coding for Lamin A/C (LMNA), Phospholamban (PLN), RNA binding motif protein 20 (RBM20), Myosin Binding Protein C (MYBPC3), Myosin Heavy Chain (MYH7), Cardiac Troponin T (TNNT2), and cardiac troponin I (TNNI3) were studied in a previously published meta‐analysis. 12 Mutations in LMNA and PLN significantly led to more ventricular arrhythmias (P < 0.05). Truncating mutations in Filamin C (FLNC) were investigated in three studies which reported frequent premature sudden death and ventricular arrhythmias (82%) in the study participants. 13 , 14
Additionally, Ser96Ala polymorphisms in Histidine‐Rich Calcium binding protein were investigated by one study and were strongly associated with life‐threatening ventricular arrhythmias [HR 9.62; 95% CI (2.18–42.39)].S2
Electrophysiology
Atrial fibrillation was investigated as a predictor in seven studies, of which three were pooled in the meta‐analysis. While all these studies reported an increased risk of ventricular arrhythmias in patients with DCM with atrial fibrillation, none of them reached statistical significance, resulting in a non‐significant pooled HR of 1.44 [95% CI (0.77–2.70)]. Of the four remaining studies that were not pooled, only one reported a significant association between atrial fibrillation and ventricular arrhythmias.S41,S47,S51,S34
QRS duration per 10 ms increase was investigated as a predictor in five studies of which four were meta‐analysed. Three studies directed towards an increased risk, but only two reached statistical significance leading to a non‐significant pooled HR of 1.12 [95% CI (0.95–1.32)] with significant heterogeneity (I 2 = 84%). One additional study showed no long‐term increased risk with an HR of 1.00 [95% CI (0.98–1.02)].S55
QRS duration > 110 ms was investigated as a predictor in two studies which were both meta‐analysed. The pooled HRs however did not reach statistical significance and direction of effect contrasted QRS duration per 10 ms increase [pooled HR 0.84; 95% CI (0.56–1.25)].S7,S25
Fragmented QRS (fQRS) was defined as any QRS morphology <120 ms with additional R waves or notching of the R or S waves in at least two contiguous leads. fQRS was investigated as a predictor in two studies, which were both meta‐analysed leading to a non‐significant association with arrhythmic events [pooled HR 4.11; 95% CI (0.40–42.41)].S19,S5
Left bundle branch block (LBBB) on 12‐lead ECG was investigated as a predictor in seven studies, which were all pooled in the meta‐analysis. This resulted in a non‐significant association between LBBB and ventricular arrhythmias [pooled HR 1.05; 95% CI (0.532–2.09)], although significant heterogeneity was observed (I 2 = 81%).
Non‐sustained VT (nsVT) was defined as ≥3 ventricular beats at ≥100 beats per minute either in patient's history or observed on 24 h Holter monitoring. nsVT was investigated as a risk factor in 14 studies, which were not pooled due to missing HRs. In the majority of these studies (n = 9), nsVT directed towards a significantly increased arrhythmic risk (P ≤ 0.05).S29,S36,S37,S38,S41,S42,S46,S12,S51
Heart rate variability standard deviations of all NN intervals (HRV SDNN) was defined as the standard deviation of intervals between normal sinus beats on Holter monitoring. While six studies investigated HRV as a predictor, none were pooled in the meta‐analysis due to the use of different cut‐off values and definitions. Three of six studies showed a significant association between HRV and arrhythmic risk, while the other three studies reported no significant association.S21,S36,S37,S42,S44,S46
T‐wave alternans (TWA) was defined as a change in T‐wave morphology that occurs in each alternant beat and measured during exercise test by spectral analysis. TWA was investigated as a predictor in six studies, of which three were pooled in the meta‐analysis. This resulted in a significant association with ventricular arrhythmias [pooled HR 6.5; 95% CI (2.46–17.14)]. The remaining three studies confirmed this association by reporting a significantly increased arrhythmic risk in the presence of TWA.S33,S42,S45
Signal‐averaged ECG was investigated as a predictor in three studies, which were not pooled given the inconsistent methods of measurement and variable definitions of late potentials. None of the studies showed significant association with arrhythmic events.S29,S41,S42
Imaging
Left ventricular ejection fraction
Left ventricular ejection fraction per 10% decrease was investigated as a predictor in 13 studies using both echocardiography and cMRI, of which eight were meta‐analysed. This showed a non‐significant association with ventricular arrhythmias [pooled HR 1.30; 95% CI (0.98–1.71)] with moderate heterogeneity (I 2 = 58%). When pooling LVEF that was measured solely on echocardiography, this led to a significant association with ventricular arrhythmias [pooled HR 1.45; 95% CI (1.19–1.78)] with little heterogeneity (I 2 = 0%). Additionally, seven other studies investigated LVEF per 5% or 10% decrease of which four reported a statistically significant effect directed towards increased arrhythmic risk.S43,S42,S46,S55
Left ventricular ejection fraction <30% was investigated as a predictor in six studies of which three were meta‐analysed. The heterogeneity was large (I 2 = 96%), leading to a non‐significant pooled HR of 1.52 [95% CI (0.78–2.97)]. In contrast, the three remaining studies all described a statistically significant increased arrhythmic risk.S41,S44,S47
Left ventricular ejection fraction <35% was investigated as a predictor in three studies, which were all meta‐analysed leading to a pooled HR of 2.4 [95% CI (0.81–7.69)].S4,S5,S28
Left ventricle volumes
Left ventricular end‐diastolic diameter per mm increase on echocardiography was investigated as a predictor in four studies which were all meta‐analysed. While all studies were directed towards an increased arrhythmic risk with increasing left ventricular end‐diastolic diameter, only one reached statistical significance resulting in a non‐significant pooled HR [HR 1.07; 95% CI (0.97–1.17)] with significant heterogeneity (I 2 = 86%).
Left ventricular end‐diastolic volume and left ventricular end‐systolic volume per 10 mL/m2 increase on cMRI were assessed as a predictor in two studies, which were pooled in the meta‐analysis. Both parameters led to a small but significantly increased arrhythmic risk [pooled HR 1.10; 95% CI (1.10–1.11) for left ventricular end‐diastolic volume per 10 mL/m2 and pooled HR 1.10; 95% CI (1.00–1.22) for left ventricular end‐systolic volume per 10 mL/m2].
Late gadolinium enhancement
The presence of LGE was investigated as a predictor in eight studies, which were all pooled in the meta‐analysis. This revealed a strong association between the presence of LGE and ventricular arrhythmias [pooled HR 5.55; 95% CI (4.02–7.67)]. Because definitions of localization patterns of LGE were not uniform, the association of its specific patterns with arrhythmic events could not be further evaluated.
Late gadolinium enhancement per 5% increase in absolute LV mass was investigated in two studies, which were both meta‐analysed. Pooled results showed a non‐significant increased risk for ventricular arrhythmias [HR 1.61; 95% CI (0.90–2.93)], however with a significant heterogeneity (I 2 = 91%). The combined presence of LGE and QRS duration > 120 ms was investigated in one study and was associated with an increased arrhythmic risk [HR 9.53; 95% CI (2.84–31.98)].S52
Other imaging parameters
Global longitudinal strain (GLS) was defined by the average maximum systolic shortening in a 16‐segment LV model. Global longitudinal strain per 1% increase was assessed as a predictor in two studies, which were both meta‐analysed leading to a non‐significant pooled HR of 1.21 [95% CI (0.97–1.51)] with high heterogeneity (I 2 = 82%).
Other imaging parameters on echocardiography and right heart catheterization were not associated with arrhythmic risk. One study investigated myocardial tissue damage in single photon emission computed tomography using a semi‐quantified myocardial severity index and reported a mild, yet significantly increased arrhythmic risk for higher severity indices [HR 1.01; 95% CI (1.00–1.02)].S26 Subjective description of segmental wall motion abnormalities on echocardiography was associated with an increased arrhythmic risk [HR 4.1; 95% CI (1.90–9.00)].S39
Miscellaneous
Several blood biomarkers such as atrial natriuretic peptide, B‐type natriuretic peptide, estimated glomerular filtration rate, norepinephrine, and potassium did not significantly affect arrhythmic risk.S47,S31,S13,S22,S4 One study reported that plasma creatinine per μmol/L increase was associated with increased arrhythmic risk [HR 1.01; 95% CI (1.00–1.02)].S13 Another study reported every one standard deviation increase of growth differentiation factor 15 in blood was associated with increased arrhythmic risk [HR 2.1; 95% CI (1.1–4.3)].S52
Sensitivity analyses
Because 18 out of 29 studies included ICD recipients, all analyses were repeated by excluding studies with ICD carriers. As shown in the Supporting Information, Data S1 , this revealed that pooled effects remained similar for all meta‐analysed risk factors. In addition, changing our meta‐analysis strategy from random‐effects model to fixed‐effects model did not reveal any considerable differences in pooled risk estimates.
Discussion
This study aimed to systematically review predictors of sustained ventricular arrhythmias in non‐ischaemic DCM, examine the quality of evidence, and establish potential risk factors of adverse clinical outcome. We found an annual risk of 4.5% of sustained ventricular arrhythmias, which underlines the importance of ICD implantation in this cohort. Of note, arrhythmic risk is considerably higher in younger patients with hypertension, prior (non‐)sustained ventricular arrhythmia, decreased LVEF in ultrasound, LV dilatation, the presence of LGE, and genetic mutations (PLN, LMNA, and FLNC). While these findings may help select appropriate candidates for ICD implantation, they must be interpreted in light of (i) the quality of evidence, (ii) clinical utility of promising risk factors, and (iii) future directives.
Quality of evidence
An important limitation of prior studies is the lack of statistical power and limited adjustment for confounders. Because the number of events per variable in our included studies was often <10 (a number generally recommended in regression analysis), statistical models had limited power for adequate confounder adjustment. 10 , 15 This resulted in variable risk of bias in overall studies that may in part explain the inconsistency of reported results. Furthermore, some included reports are up to 20 years old. During this period, medical treatment for heart failure has changed substantially, and event rates have changed too. In addition, diagnostic methods have evolved over the years, which may have led to more frequent diagnosis of DCM.
To compensate for power limitations, we attempted to pool results from studies that report HRs into a quantitative meta‐analysis. Our decision to only meta‐analyse studies that report HRs resulted in a lower risk of bias given their use of recommended statistical methods. However, because pooling is only appropriate in the setting of uniform definitions, the number of studies included in our meta‐analysis was unfortunately limited. Given these constraints in individual study quality, historical changes in heart failure workup, and our inability to pool all available results, we deem overall quality of evidence to be moderate, which should be taken into consideration when assessing the results of our study.
Promising risk factors
For a prognostic model to be useful in daily clinical practice, its predictors must be reproducible and easy to obtain. In this light, the utility of parameters limited in standardization such as TWA and HRV remains limited, whereas conventional measurements have consistently performed better in recently published risk prediction models for hypertrophic (HCM) and arrhythmogenic cardiomyopathy (ACM). 16 , 17 Based on our findings, promising risk factors include younger age, hypertension, prior (non‐)sustained ventricular arrhythmia, decreased LVEF, LV dilatation, LGE, and presence of genetic mutations in PLN, LMNA, and FLNC (Figure 4 ).
Figure 4.
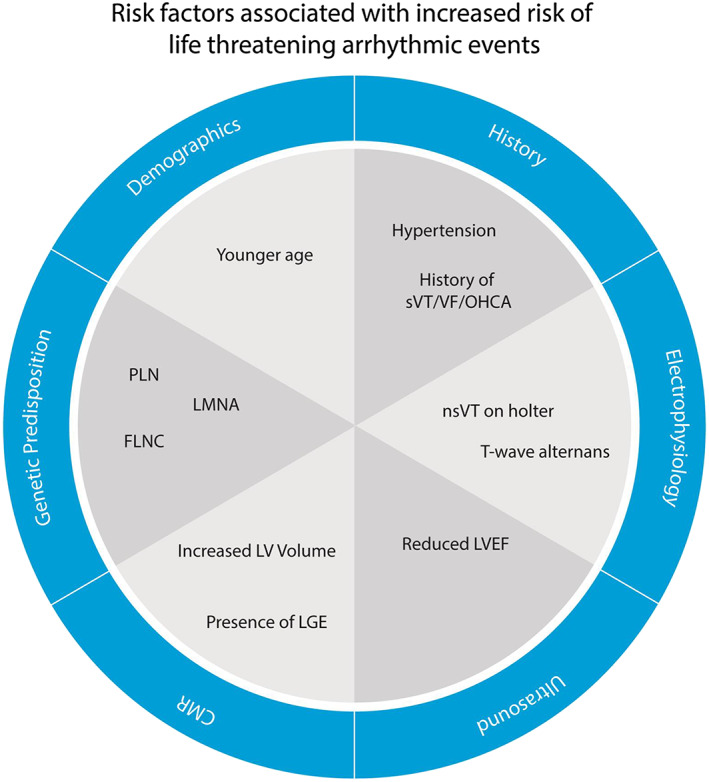
Title: Predictors of sustained ventricular arrhythmias in non‐ischaemic DCM. Caption: summarized findings of the systematic review and meta‐analysis. Abbreviations: see text. (Figure 4 is central illustration).
History and demographics
Younger age was associated with ventricular arrhythmias, which is in line with literature on other cardiomyopathies and primary arrhythmia syndromes. This finding may reflect faster conduction in younger heart and lower thresholds for arrhythmia due to changes in Ca2+ handling. 18 Given the competing risks of heart failure and arrhythmic outcome, the exact influence and mechanism of young age remain up to investigation.
Male sex was not significantly predictive of arrhythmias although the results do indicate an association. This is similar to HCM but distinctly different from ACM, in which male sex was a strong predictor of arrhythmias. 16 , 17 There is a growing body of literature suggesting sex differences in cardiovascular diseases and a lower incidence of SCD in women. Suggested mechanisms may be related to hormonal effects on Ca2+ handling, shorter QT interval in adult male patients, and differences in underlying pathology such as coronary artery disease.18 However, studies on direct effects of sex differences have not been conclusive, and its exact involvement in ventricular arrhythmia remains unclear. 19 , 20
In our pooled data, hypertension was significantly associated with outcome. This effect may be caused by cardiac remodelling with persistent systemic hypertension as experimental clinical studies have provided evidence for myocardial fibrosis and changes in LV function. 21 Whether this constitutes an opportunity for arrhythmia prevention by antihypertensive medication (i.e. risk factor modulation) remains up for investigation.
Genetics
The evidence for an association between genotype and outcomes has been recently reported in a meta‐analysis.13 Ventricular arrhythmias in PLN, LMNA, and FLNC in patients with DCM are markedly higher. 12 , 13 Phospholamban is known for an overlap syndrome between DCM and ACM. 22 It regulates the sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) pump and interplays with Na+/Ca2+ exchanger (SLC8A1 or NCX1), which is important for maintaining calcium homeostasis in cardiomyocytes. 23 Calcium dysregulation can elicit early/delayed after depolarizations thereby increasing arrhythmogenicity. 24 Lamin A/C is a nuclear envelope protein and has been association with arrhythmias in many studies. 12 Mutations in Lamins A and C may lead to nuclear abnormalities. Because these proteins also interact with the cytoskeleton and extracellular matrix, they can affect the structural myocardial stability, which explains the detrimental effect in the heart. 25 Filamin C is essential for cardiomyocyte structuring, anchoring membrane proteins to the cytoskeleton, and binding several proteins in the Z‐disc of the sarcomere. Mutations in Filamin C may lead to an overlapping DCM and ACM phenotype with LV dysfunction and frequent ventricular arrhythmias. 13 These association of genotype with outcome suggest a potential for individualized treatment strategies.
Electrophysiology
In our meta‐analysed data, prior (non‐)sustained ventricular arrhythmias were strongly associated with future arrhythmias. It seems obvious that those with prior sustained ventricular arrhythmias (i.e. the secondary prevention population) should receive an ICD, which is incorporated as a recommendation in many guidelines. 3 However, recommendations for the primary prevention population are less straightforward. Our results suggest that also prior non‐sustained VT is associated with subsequent sustained ventricular arrhythmias, which is in line with the AMIOVIRT and DEFINITE trials that solely included patients with prior non‐sustained VTs or frequent extrasystoles therefore increasing the arrhythmic burden. 26 , 27 In addition, it is important to keep in mind that VTs and VF may reflect a different underlying substrate. Recently, a large study in ACM showed that prior VT did not predict subsequent VF events, which appeared to be more stochastic. 28
Although currently implantation of an ICD is recommended for primary prevention in patients with DCM and LV ejection fraction <35% and NYHA classes II‐III who have expected survival of at least 1 year, catheter ablation of VT might be a potential therapeutic approach in the future as stand‐alone therapy or as first step before implanting ICD. 29 Tung et al. showed that catheter ablation of monomorphic VTs in patients with structural heart disease (ischaemic or non‐ischaemic cardiomyopathy) resulted in 70% freedom from VT recurrence and that freedom from VT recurrence was associated with improved transplant‐free survival, independent of heart failure severity. 30 In a more recent study, Santoro et al. showed that whereas VT recurrence without clustering had no prognostic implication in patients with non‐ischaemic DCM, incidence of VT clustering (VTc) was an independent predictor of mortality. This group might be the better candidate for ICD implantation. 31 TWA was strongly associated with outcome, which may reflect autonomic dysfunction. Even though it holds potential in identifying high risk patients, its clinical role has not been fully defined therefore limiting its utility in daily clinical practice. 32
Imaging
Non‐ischaemic DCM is defined diagnostically as either increased LV dilatation or decreased LV function (LVEF <45%), and our pooled results showed that both LV dilatation and decreased LV function confer prognostic information. 33 Even though this was expected, the effect size was relatively small. To date, six trials have investigated the survival benefit of ICD therapy for primary prevention that included patients with DCM. All had an LVEF ≤35%, with an average of 24% in a recently updated meta‐analysis therefore limiting its value in patients with an LVEF higher than 35%. 5 , 33 Identification of patients with high arrhythmic risk with preserved or slightly reduced LVEF therefore remains uncertain. Reduced LVEF is related to extent of fibrosis, a substrate for zig‐zag pathways and re‐entrant arrhythmias, and its relation to arrhythmia seems logical. 34 Cardiac magnetic resonance has the ability to perform tissue characterization by LGE reflecting presence of localized (segmental) fibrosis. 6 Logically, the presence of LGE is strongly associated with arrhythmia throughout different studies. 8 , 35 The role of newer parameters that quantify diffuse fibrosis (e.g. T1 mapping) remains to be investigated.
Prognostic model for implantable cardioverter‐defibrillator implantation in dilated cardiomyopathy
The results obtained from this meta‐analysis may help for further model building to improve risk assessment in DCM. Because patients with DCM can experience both heart failure‐related outcomes (heart transplantation, assist device, and heart failure‐related death) as well as arrhythmic endpoints (VT, VF, and SCD), they are considered to be competing: one event hinders the occurrence of another event of interest. 25 Even though for prognostic research applying the subdistribution proportional hazards model is recommended, all articles solely reported HRs that can overestimate or underestimate risks. To adequately capture real clinical risks, future modelling should focus more on competing risks using methods such as the cumulative incidence competing risk or subdistribution proportional hazards. 32 Therefore, coming clinical decision support tools for ICD implantation in non‐ischaemic DCM should incorporate a multitude of relevant variables to better reflect real clinical practice as well as perform competing event analyses.
Conclusions
The annual risk of life‐threatening ventricular arrhythmia in DCM is approximately 4.5% and is considerably higher in patients at younger age, patients with hypertension, prior (non‐)sustained VT, decreased LVEF, LV dilatation, presence of LGE, and pro‐arrhythmic genetic mutations (PLN, LMNA, and FLNC). These results may help for further prognostic model building to improve personalized risk assessment in non‐ischaemic DCM.
Perspectives
Clinical competency in heart failure patient care and preventive medicine: SCD risk assessment in non‐ischaemic DCM can be improved using multiple tests rather than solely relying on LVEF, which is in line with the neutral results from the DANISH trial. 4
Translational outlook
The examples set by the HCM/ACM risk calculators should be followed to enable personalized risk assessment. 16 , 17 Such a model should be built by including the promising risk factors of our study but also several other suggested risk factors including sex.
Conflict of interest
Prof. Dr Asselbergs reports grants from ERA‐CVD EU, during the conduct of the study; Prof. Dr Katus reports personal fees from Novo Nordisk, personal fees from Bayer Vital, personal fees from Astra Zeneca, personal fees from Daiichi Sankyo, outside the submitted work; Dr Kayvanpour, Dr Sedaghat‐Hamedani, Dr Jensen, Dr te Riele, Dr Bosman, Prof. Meder, Mrs Proctor, Ms Broezel, Dr Gi, and Dr Sammani have nothing to disclose.
Funding
This project received funding from the European Union's Horizon 2020 research and innovation programme under the ERA‐NET Co‐fund action no. 680969 (ERA‐CVD DETECTIN‐HF), jointly funded by the Dutch Heart Foundation (2016T096), and Netherlands Organization for Health Research and Development (ZonMw). A.S. is funded by the Alexandre Suerman Stipendium. A.T.R. is funded by the Dutch Heart Foundation (2015T058), the UMC Utrecht Fellowship Clinical Research Talent and CVON 2015‐12 eDETECT. E.K. is partially funded by the German Centre for Cardiovascular Research, DZHK (81X3500117). F.A. is supported by UCL Hospitals NIHR Biomedical Research and CVON 2015‐12 eDETECT.
Supporting information
Data S1. Supplementary tables and file.
Acknowledgements
We would like to thank all collaborators in the ERA‐CVD DETECTIN‐HF programme.
Sammani, A. , Kayvanpour, E. , Bosman, L. P. , Sedaghat‐Hamedani, F. , Proctor, T. , Gi, W.‐T. , Broezel, A. , Jensen, K. , Katus, H. A. , te Riele, A. S. J. M. , Meder, B. , and Asselbergs, F. W. (2020) Predicting sustained ventricular arrhythmias in dilated cardiomyopathy: a meta‐analysis and systematic review. ESC Heart Failure, 7: 1430–1441. 10.1002/ehf2.12689.
Subject: Heart Failure and Cardiac Disease, Cardiomyopathies
Arjan Sammani and Elham Kayvanpour contributed equally as first authors.
Benjamin Meder and Folkert W. Asselbergs contributed equally as senior authors.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200m.27206819 [Google Scholar]
- 2. Shun‐Shin MJ, Zheng SL, Cole GD, Howard JP, Whinnett ZI, Francis DP. Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta‐analysis of 8567 patients in the 11 trials. Eur Heart J 2017; 38: 1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekvål TM, Spaulding C, van Veldhuisen D, ESC Scientific Document Group . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015; 36: 2793–2867.26320108 [Google Scholar]
- 4. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp‐Pedersen C, Pehrson S, DANISH Investigators . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 5. Wolff G, Lin Y, Karathanos A, Brockmeyer M, Wolters S, Nowak B, Fürnkranz A, Makimoto H, Kelm M, Schulze V. Implantable cardioverter/defibrillators for primary prevention in dilated cardiomyopathy post‐DANISH: an updated meta‐analysis and systematic review of randomized controlled trials. Clin Res Cardiol 2017; 106: 501–513. [DOI] [PubMed] [Google Scholar]
- 6. Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA, Sramko M, Masci PG, Barison A, Mckenna P, Mordi I. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta‐analysis. JACC Heart Fail 2017; 5: 28–38. [DOI] [PubMed] [Google Scholar]
- 7. Goldberger JJ, Subačius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 2014; 63: 1879–1889. [DOI] [PubMed] [Google Scholar]
- 8. Halliday BP, Cleland JGF, Goldberger JJ, Prasad SK. Personalizing risk stratification for sudden death in dilated cardiomyopathy. Circulation 2017; 136: 215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Open Med 2009; 3: e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 10. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280. [DOI] [PubMed] [Google Scholar]
- 11. Schwarzer G. An R package for meta‐analysis. R news 2007; 7: 40–45. [Google Scholar]
- 12. Kayvanpour E, Sedaghat‐Hamedani F, Amr A, Lai A, Haas J, Holzer DB, Frese KS, Keller A, Jensen K, Katus HA, Meder B. Genotype‐phenotype associations in dilated cardiomyopathy: meta‐analysis on more than 8000 individuals. Clin Res Cardiol 2017; 106: 127–139. [DOI] [PubMed] [Google Scholar]
- 13. Ortiz‐Genga MF, Cuenca S, Dal Ferro M, Zorio E, Salgado‐Aranda R, Climent V, Padrón‐Barthe L, Duro‐Aguado I, Jiménez‐Jáimez J, Hidalgo‐Olivares VM, García‐Campo E, Lanzillo C, Suárez‐Mier MP, Yonath H, Marcos‐Alonso S, Ochoa JP, Santomé JL, García‐Giustiniani D, Rodríguez‐Garrido JL, Domínguez F, Merlo M, Palomino J, Peña ML, Trujillo JP, Martín‐Vila A, Stolfo D, Molina P, Lara‐Pezzi E, Calvo‐Iglesias FE, Nof E, Calò L, Barriales‐Villa R, Gimeno‐Blanes JR, Arad M, García‐Pavía P, Monserrat L. Truncating FLNC mutations are associated with high‐risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol 2016; 68: 2440–2451. [DOI] [PubMed] [Google Scholar]
- 14. Nozari A, Aghaei‐Moghadam E, Zeinaloo A, Mollazadeh R, Majnoon MT, Alavi A, Ghasemi Firouzabadi S, Mohammadzadeh A, Banihashemi S, Nikzaban M, Najmabadi H, Behjati F. A novel splicing variant in FLNC gene responsible for a highly penetrant familial dilated cardiomyopathy in an extended Iranian family. Gene 2018; 659: 160–167. [DOI] [PubMed] [Google Scholar]
- 15. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 16. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, Omar RZ, Elliott PM, for the Hypertrophic Cardiomyopathy Outcomes Investigators . A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk‐SCD). Eur Heart J 2014; 35: 2010–2020. [DOI] [PubMed] [Google Scholar]
- 17. Cadrin‐Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie ØH, Saguner AM, Svensson A, Andorin A, Tichnell C, Murray B, Zeppenfeld K, van den Berg MP, Asselbergs FW, Wilde AAM, Krahn AD, Talajic M, Rivard L, Chelko S, Zimmerman SL, Kamel IR, Crosson JE, Judge DP, Yap SC, van der Heijden JF, Tandri H, Jongbloed JDH, Guertin MC, van Tintelen JP, Platonov PG, Duru F, Haugaa KH, Khairy P, Hauer RNW, Calkins H, te Riele ASJM, James CA. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019; 40: 1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strait JB, Lakatta EG. Aging‐associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 2012; 8: 143–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolbrette D, Naccarelli G, Curtis A, Lehmann M, Kadish A. Gender differences in arrhythmias. Clin Cardiol 2002; 25: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linde C, Bongiorni MG, Birgersdotter‐Green U, Curtis AB, Deisenhofer I, Furokawa T, Gillis AM, Haugaa KH, Lip GYH, van Gelder I, Malik M, Poole J, Potpara T, Savelieva I, Sarkozy A, ESC Scientific Document Group , Fauchier L, Kutyifa V, Ernst S, Gandjbakhch E, Marijon E, Casadei B, Chen YJ, Swampillai J, Hurwitz J, Varma N. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018; 20: 1565–1565ao. [DOI] [PubMed] [Google Scholar]
- 21. Kenchaiah S, Pfeffer MA. Cardiac remodeling in systemic hypertension. Med Clin North Am 2004; 88: 115–130. [DOI] [PubMed] [Google Scholar]
- 22. van Rijsingen IAW, van der Zwaag PA, Groeneweg JA, Nannenberg EA, Jongbloed JD, Zwinderman AH, Pinto YM, Dit Deprez RH, Post JG, Tan HL, de Boer RA, Hauer RN, Christiaans I, van den Berg M, van Tintelen J, Wilde AA. Outcome in Phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet 2014; 7: 455–465. [DOI] [PubMed] [Google Scholar]
- 23. Langenbacher AD, Dong Y, Shu X, Choi J, Nicoll DA, Goldhaber JI, Philipson KD, Chen JN. Mutation in sodium‐calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc Natl Acad Sci U S A 2005; 102: 17699–17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu GS, Morales A, Vafiadaki E, Lam CK, Cai WF, Haghighi K, Adly G, Hershberger RE, Kranias EG. A novel human R25C‐phospholamban mutation is associated with super‐inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res 2015; 107: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoorntje ET, Bollen IA, Barge‐Schaapveld DQ, van Tienen FH, te Meerman GJ, Jansweijer JA, van Essen AJ, Volders PG, Constantinescu AA, van den Akker PC, van Spaendonck‐Zwarts KY, Oldenburg RA, Marcelis CL, van der Smagt JJ, Hennekam EA, Vink A, Bootsma M, Aten E, Wilde AA, van den Wijngaard A, Broers JL, Jongbloed JD, van der Velden J, van den Berg MP, van Tintelen JP. Lamin A/C‐related cardiac disease: late onset with a variable and mild phenotype in a large cohort of patients with the Lamin A/C p.(Arg331Gln) founder mutation. Circ Cardiovasc Genet 2017; 10: e001631. [DOI] [PubMed] [Google Scholar]
- 26. Sharma A, Al‐Khatib SM, Ezekowitz JA, Cooper LB, Fordyce CB, Michael Felker G, Bardy GH, Poole JE, Thomas Bigger J, Buxton AE, Moss AJ. Implantable cardioverter‐defibrillators in heart failure patients with reduced ejection fraction and diabetes. Eur J Heart Fail 2018; 20: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 27. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, de Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries D, Feldman AM, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140–2150. [DOI] [PubMed] [Google Scholar]
- 28. Cadrin‐Tourigny J, Bosman L, Bourfiss M, Wang W, Tadros R, Bhonsale A, Nozza A. Individualized arrhythmic risk prediction in a primary prevention arrhythmogenic right ventricular cardiomyopathy (ARVC) population: a transatlantic multinational collaboration. Heart Rhythm 2018; 15: S1–S107. [Google Scholar]
- 29. Muser D, Santangeli P, Castro SA, Pathak RK, Liang JJ, Hayashi T, Magnani S, Garcia FC, Hutchinson MD, Supple GG, Frankel DS. Long‐term outcome after catheter ablation of ventricular tachycardia in patients with nonischemic dilated cardiomyopathy. Circ Arrhythm Electrophysiol 2016; 9: e004328. [DOI] [PubMed] [Google Scholar]
- 30. Tung R, Vaseghi M, Frankel DS, Vergara P, di Biase L, Nagashima K, Yu R, Vangala S, Tseng CH, Choi EK, Khurshid S, Patel M, Mathuria N, Nakahara S, Tzou WS, Sauer WH, Vakil K, Tedrow U, Burkhardt JD, Tholakanahalli VN, Saliaris A, Dickfeld T, Weiss JP, Bunch TJ, Reddy M, Kanmanthareddy A, Callans DJ, Lakkireddy D, Natale A, Marchlinski F, Stevenson WG, Della Bella P, Shivkumar K. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group study. Heart Rhythm 2015; 12: 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santoro F, Metzner A, Scholz L, Brunetti ND, Heeger CH, Rillig A, Reissmann B, Lemeš C, Maurer T, Fink T, Inaba O, Hashiguchi N, Kuck KH, Ouyang F, Mathew S. Prognostic significance of ventricular tachycardia clustering after catheter ablation in non‐ischemic dilated cardiomyopathy. Clin Res Cardiol 2019; 108: 539–548. [DOI] [PubMed] [Google Scholar]
- 32. Mason JW, Hancock EW, Gettes LS. Recommendations for the standardization and interpretation of the electrocardiogram. Circulation 2007; 115: 1325–1332. [DOI] [PubMed] [Google Scholar]
- 33. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio AL, Charron P. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non‐dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016; 37: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen M‐N, Kiriazis H, Gao X‐M, Du X‐J. Cardiac fibrosis and arrhythmogenesis In Comprehensive Physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2017. p 1009–1049. [DOI] [PubMed] [Google Scholar]
- 35. Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi C, Arzanauskaite M, Lota A, Tayal U, Vassiliou VS, Gregson J. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging 2018; 12: 1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary tables and file.


