Abstract
Breast cancer is the most common cancer among Latina women, and Latina women are at higher risk for breast cancer mortality than white women. Lifestyle factors, such as consuming a nutritious diet and engaging in regular physical activity, promote health and are protective against heart disease, type 2 diabetes, and breast cancer recurrence. Previous studies have developed and tested interventions for Latina breast cancer survivors to improve diet and increase physical activity, however, no studies to date have developed a smartphone delivered intervention. The purpose of the current study was to compare two Smartphone delivered interventions, My Health, which focused on diet and physical activity, and My Guide, which focused on psychosocial functioning, on dietary and physical activity outcomes, post-intervention, and at a two-week follow-up assessment. Overall, participants receiving the My Health intervention reported a greater reduction in daily fat sources than the My Guide group over time. However, daily sources of fat did not differ between conditions. Walking, measured by estimated weekly metabolic equivalents (METs), increased across time points in both groups. These preliminary findings suggest that eHealth interventions aimed at improving lifestyle factors may favorably impact nutritional intake and physical activity. Future research should utilize more comprehensive and objective measures of diet and physical activity, and incorporate more behavioral lifestyle components into the intervention in larger samples with a longer follow-up period.
Keywords: Breast cancer, lifestyle, diet, physical activity, eHealth, Latina
Introduction
Breast cancer is the most common cancer among Latina women and accounts for the most cancer related deaths within this population (American Cancer Society, 2018). Latinas are at increased risk for estrogen- and progesterone-negative tumors (Bauer et al., 2007; Lara-Medina et al., 2011), which are associated with higher rates of recurrence and death. Further, Latinas have lower 5-year survival rates than non-Latina whites (DeSantis et al., 2011). Given these breast cancer-related disparities, it is important to identify modifiable risk factors within this population to promote health and prevent the development of chronic diseases and breast cancer recurrence (Lafourcade et al., 2018).
Lifestyle factors, such as consuming a nutritious diet (e.g., diet high in fruits, vegetables, whole grains, and legumes and low in saturated fat; Chi et al., 2013; Chlebowski et al., 1991) and engaging in regular physical activity, may be important in preventing breast cancer recurrence (Ibrahim & Al-Homaidh, 2011; Lahart et al., 2015; Spei et al., 2019). In a sample of 1,490 women treated for breast cancer, women who consumed 5 or more servings of fruit and vegetables per day and engaged in 30 minutes of walking 6 days per week reduced their breast cancer mortality risk by 50% (Pierce, Natarajan et al., 2007). Indeed, the American Cancer Society and the American Society of Clinical Oncology recommend that breast cancer survivors (BCS): 1) consume a diet high in fruits, vegetables, whole grains, and legumes and low in saturated fats; and 2) engage in at least 150 minutes of moderate physical activity or 75 minutes of vigorous physical activity per week. Despite these recommendations, research has found that Latina BCS tend to consume diets low in whole grains and high in starchy vegetables, refined grains, red meat, and calories from solid fats and sugars and engage in low levels of physical activity (Chlebowski et al., 1991; Ortiz et al., 2018).
A few lifestyle interventions have been developed for Latina BCS and have been found to be efficacious (Greenlee et al., 2015). The iCocinar para su salud! intervention (Greenlee et al., 2015) was a dietary intervention for Latina BCS aimed at increasing fruit and vegetable consumption and reducing fat consumption. The intervention included 9 in-person sessions (24 hours across 12 weeks), and the curriculum focused on nutrition education, cooking classes, and shopping trips. At 6-month follow up, women in the intervention group significantly increased their fruit and vegetable consumption, but there were no statistically significant differences between groups on fat or weight outcomes. One limitation of the study was that only 38% of participants attended all 9 sessions, and one potential barrier noted was time constraints (Greenlee et al., 2015). Project Viva! (Mama et al., 2017) was a randomized physical activity intervention developed for Latina BCS. At baseline, participants reported spending more than 11 hours per day of sedentary time. At 16-week follow up, participants across the intervention and control group increased levels of physical activity and decreased levels of sedentary time. However, participants only attended 57.5% of the in-person exercise group sessions given barriers to childcare and other scheduling conflicts (Mama et al., 2017). Therefore, there is a need for targeted lifestyle interventions for Latina BCS that address barriers to participation.
Technology delivered interventions may be beneficial to Latina women, who generally participate in cancer-related research at lower rates than non-Latina women (Murthy et al., 2004). Barriers to participating in research include mistrust of researchers, low socioeconomic status, time burden, and lack of resources in Spanish (Wendler et al., 2006). eHealth approaches may be particularly beneficial to Latina women who utilize technology to receive health information as much as non-Latina women (Lopez et al., 2013). Further, technology delivered interventions may be more scalable than traditional in-person interventions (Prochaska et al., 2017). However, no study to date has tested the efficacy of a smartphone delivered lifestyle intervention for Latina BCS.
We developed two smartphone delivered interventions: My Health, which aimed to increase health-promoting behaviors among Latina BCS, and My Guide, which aimed to increase psychosocial functioning and reduce symptom burden among Latina BCS. In the original trial, My Guide was the experimental condition and our primary outcomes were symptom burden and health-related quality of life, while My Health was the comparison condition (Yanez et al., 2019). Descriptions of content in both applications are presented in Tables 1 and 2, The purpose of the current study was to determine whether participants receiving My Health increased our comparison intervention targets, healthful eating and physical activity, compared to participants receiving My Guide. We hypothesized that participants receiving My Health would report fewer daily fat sources and more daily servings of fruits and vegetables than participants receiving My Guide at post-intervention (T2) and two weeks post-intervention (T3). We also hypothesized that participants receiving My Health would engage in more physical activity and less sitting than participants receiving My Guide at post-intervention (T2) and two weeks post-intervention (T3). Results from this study may inform the development of future technology-delivered interventions targeting lifestyle behaviors among Latina BCS.
Table 1.
My Health main components
| My Health Components | Description |
|---|---|
| Food and Nutrition | This section discusses the importance of good nutrition for breast cancer survivors, and provides guidance on which foods to eat and which to limit. |
| Eating Well | This section instructs participants on how to maintain a balanced diet, including techniques on portion control, tips for eating out, and healthy recipes. |
| Preventing Diabetes and Heart Disease | Participants, who are largely older and post-menopausal, are at greater risk for developing diabetes and heart disease. This section presents information on diabetes and heart disease prevention, as well as a quiz component to dispel myths about both. |
| Exercise | This section discusses the benefits of exercise and provides non-traditional, culturally appropriate approaches to increasing activity levels and raising heart rates. |
| Lifestyle | This section presents important information on healthy lifestyle habits, including sun safety and smoking cessation. |
| Doctor’s Recommendations | This section provides guidance on medication adherence and tips for interacting with a doctor. |
Note. The “components” listed above represent the different button options on the home screen. Efforts were made to ensure that the amount of material included in each application was similar. Coaching call protocols and content were also identical across groups.
Table 2.
My Guide main components
| My Guide Components | Description |
|---|---|
| Managing My Symptoms | This section highlights the physical and psychological symptoms commonly experienced after cancer treatment, and provides methods to manage them. |
| Managing My Health | This section provides general information on breast cancer, adjuvant treatments and related side effects, and strategies to manage side effects. |
| Friends and Family | This section discusses ways in which relationships with family, friends, and other acquaintances might change after a cancer diagnosis, and presents strategies to address these changes. |
| Managing My Emotions | This section discusses the emotions most commonly experienced after cancer treatment, and provides stress management and relaxation techniques. |
| Breast Cancer Medications | This section presents important information specifically for survivors on different types of hormone therapies and related side effects. |
| Community and Everyday Support | This section provides a comprehensive list of external resources and community organizations for breast cancer survivors, including health-related and financial support. |
| Videos, Audio Programs | Video and audio recordings are integrated throughout the sections to supplement information provided in the text. Videos provide expert explanations of side effects and breast health, while audio recordings walk participants through relaxation exercises. |
| Bookmarks | An added feature allows participants to mark their preferred sections and sub-sections for easy reference. |
Note. The “components” listed above represent the different button options on the home screen. Efforts were made to ensure that the amount of material included in each application was similar. Coaching call protocols and content were also identical across groups.
Method
Participants
Participants were 80 Latina women who completed treatment for breast cancer. However, two participants were withdrawn due to technical issues and not included in the study analyses (one participant from each condition) and thus 78 participants were analyzed. Inclusion criteria included: 1) stage 0-III breast cancer diagnosis; 2) at least 21 years old; and 3) within 2–24 months of completing primary breast cancer treatment with or without adjuvant endocrine therapy. Women were excluded if they had a prior cancer diagnosis or treatment for serious mental illness, suicidal ideation, and could not speak or read in English or Spanish. Having a smartphone was not included in the eligibility criteria, and we provided a study-appointed smartphone to participants as needed. The vast majority of participants (n=73, 91%) used their own smartphone for the duration of the study.
Procedures
All study procedures were approved by the Institutional Review Board (ClinicalTrial.gov ID NCT03645005). A full description of the study procedures and telecoaching protocol has been published elsewhere (Yanez et al., 2018). Participants were recruited through advertisements and referrals from physicians at two medical centers and at a community-based organization for Latina women with breast cancer, and disease and medical information were confirmed through medical chart review. Eligible women provided informed consent and completed baseline measures assessing sociodemographic information as well as psychosocial measures and health behaviors (T1; baseline). These measures were repeated post-intervention (T2; approximately 6 weeks), and two weeks post-intervention (T3; approximately 8 weeks).
Participants were randomized 1:1 to receive one of two smartphone applications: My Health or My Guide. Participants met with research staff to receive training on how to use the application, and were encouraged to use the application for 2 hours each week for 6 weeks. Each participant was assigned a telecoach to check in with them by phone. Telecoaches were bilingual and trained by a licensed clinical psychologist in motivational interviewing, problem solving, and goal setting. All participants received telecoching calls prior to weeks 1, 2, and 6. During weeks 3–5, calls were only completed if participants used the application for less than 90 minutes that week. Participants who used the application for 90 minutes or more during weeks 3–5 received an encouraging text message instead of a call. All telecoaching calls were recorded and reviewed by a licensed clinical psychologist, and telecoaches received weekly supervision to monitor protocol fidelity.
Study Applications
My Health and My Guide are HIPAA compliant web applications that were developed by the Center for Behavioral Intervention Technologies (CBITs) at the Northwestern University Feinberg School of Medicine. The content was developed through an iterative process of receiving feedback from our community partner, Latina BCS, and physicians. Both applications are available in English and Spanish, and all written content can be played as an audio file to address potential limitations in literacy. My Health was developed to improve health-promoting behaviors and reduce health risk behaviors among Latina BCS. Content focuses on healthy eating, culturally appropriate healthy recipes, the importance of engaging in physical activity, ways to prevent comorbid conditions like diabetes, and general health management (e.g., following doctor recommendations, healthy lifestyle behaviors). The My Guide content focuses on reduction of symptom burden and improving health-related quality of life. Specifically, My Guide includes information on coping with cancer late effects, communication with health care providers and loved ones, utilizing social support, and increasing breast cancer knowledge.
Measures
Sociodemographic and cancer-specific characteristics.
Participants self-reported their age, language preference, country of origin, Latina ancestry, highest level education completed, employment status, annual household income, and marital status. They also self-reported cancer-specific characteristics including their stage of disease and treatment(s) received (e.g., radiation therapy, chemotherapy). Cancer-specific characteristics were verified by medical chart review.
Nutrition.
The 23-item Brief Dietary Assessment Tool for Hispanics was developed specifically for Hispanic/Latinx populations and is comprised of two subscales that yield estimates of dietary fat sources and fruit/vegetable servings consumed per day (Wakimoto et al., 2006). For dietary fat sources, respondents indicate how often they consumed 16 sources of fats over the past month (e.g., “French fries or fried potatoes’) on a Likert scale from once per month or less to five or more times per week. Scores were calculated to reflect average number of daily fat sources. For fruits and vegetables, respondents indicate how often they consumed seven sources of fruits and vegetables over the past month (e.g., “green salad like lettuce or spinach salad”) on a Likert scale from less than once per week to two or more times per day. Scores were calculated to reflect average number of daily servings of fruits and vegetables. Cronbach’s alphas for the daily fat sources subscale were acceptable at each time point (range 0.72–0.75). Cronbach’s alpha for the daily fruits and vegetables servings subscale was acceptable at T1 (0.72), but was poor at both T2 and T3 (0.48).
Physical activity.
The 7-item International Physical Activity Questionnaire Short Form (IPAQ-SF) assesses self-reported physical activity at three levels of intensity (i.e., walking, moderate physical activity, and vigorous physical activity) as well time spent sitting as an indicator of daily sedentary time (Craig et al., 2003; Lee et al., 2011). Respondents indicate the number of days and number of minutes each day they engaged in each type of activity over the past 7 days (e.g., “during the last 7 days, on how many days did you walk for at least 10 minutes at a time?” and “how much time did you usually spend walking on one of those days?”). Using the published IPAQ-SF scoring guidelines (Forde), physical activity values at each intensity level were truncated at a duration of 180 minutes, and scores are calculated to reflect a participant’s estimated weekly metabolic equivalent (MET) energy expenditure for each intensity level as follows: walking=3.3 METs per minute, moderate=4.0 METs per minute, and vigorous=8,0 METs per minute (Forde). Standardized Cronbach’s alpha for the vigorous and moderate physical activity subscales were acceptable across time (range 0.86–0.93 and 0.88–0.90, respectively). Standardized Cronbach’s alpha for the walking subscale was acceptable at T1 (0.89) but poor at both T2 and T3 (0.54 and 0.56, respectively).
Analyses were calculated using METs per minute. However, for ease of interpretation, scores were also presented as minutes per week (i.e., without MET energy expenditure) which was calculated by multiplying the number of times per week by the number of minutes per session. Time spent sitting was calculated to reflect a participant’s estimated daily time sitting. In accordance with the IPAQ-SF scoring guidelines, women were categorized as being highly, moderately, or minimally physically active at each time point as follows: highly physically active=vigorous activity on at least 3 days and achieving a minimum of 1500 total MET minutes per week, or any combination of walking, moderate, and vigorous activity each day and achieving a minimum of 3000 MET minutes per week; moderately physically active=at least 30 minutes of vigorous and/or moderate activity on at least 3 days, or at least 30 minutes of moderate activity and/or walking on at least 5 days, or any combination of walking, moderate, and vigorous activity on at least 5 days and achieving a minimum of 600 MET minutes per week; minimally physically active=does not meet criteria for categorization as highly or moderately physically active.
Statistical Analyses
We Winsorized outlier values >3 standard deviations from the mean and screened the outcome measures for normality (Wilcox, 1993). All of the physical activity variables, with the exception of time spent sitting, were positively skewed and thus logarithmically transformed using log(x+1) to maintain positive values. Transformed values were used in all subsequent analyses, and descriptive values were back-transformed to report the geometric means in MET minutes and minutes per week. To characterize the sample, we calculated means, standard deviations, and frequencies and conducted t-tests and chi-square analyses. Descriptive statistics of logarithmically transformed values are presented as medians rather than means to approximate more accurate measures of central tendency.
We conducted linear mixed effects modeling to assess for differences between the study conditions in changes overtime in nutrition and physical activity. This statistical approach accounts for an individual’s trajectory of scores and controls for correlations between repeated assessments. It enables the use of all available data, as opposed to listwise deletion, so participants were included at each time point for which they provided data. All models controlled for language preference. Six models were assessed with nutrition outcomes (i.e., daily fat sources and daily servings of fruits/vegetables) and physical activity outcomes (i.e., weekly MET minutes for walking, moderate, vigorous physical activity, and daily sitting minutes) as the dependent variables, respectively. In each model, we assessed the effects of time (T1, T2, T3), condition (My Health, My Guide), and the interaction of time and condition on the dependent variable. Models for which there were no significant interaction of time and condition were re-specified without the interaction term in order to evaluate the main effects of time and condition. Cohen’s d effect sizes were calculated for significant effects and interpreted as follows: 0.2=small, 0.5=medium, 0.8=large (Cohen, 1988).
Results
Sample Characteristics
See Figure 1 for study flow and retention and Table 3 for descriptive characteristics of the sample. On average, women were 52.54 years old (SD=11.36). Most women preferred to communicate in Spanish (64%) and were born outside the US (71%) with Mexican ancestry (64%). The majority of participants had a high school education or less (54%), were not employed (56%), had an annual household income of less than $25,000 (53%), and were married or partnered (64%). Most participants had stage I (36%) or stage II disease (41%) and had received radiation therapy (71 %) and/or chemotherapy (58%). Study groups were not different with respect to the participants’ sociodemographic and clinical characteristics, nutrition, and physical activity at baseline (all ps>0.05).
Figure 1.
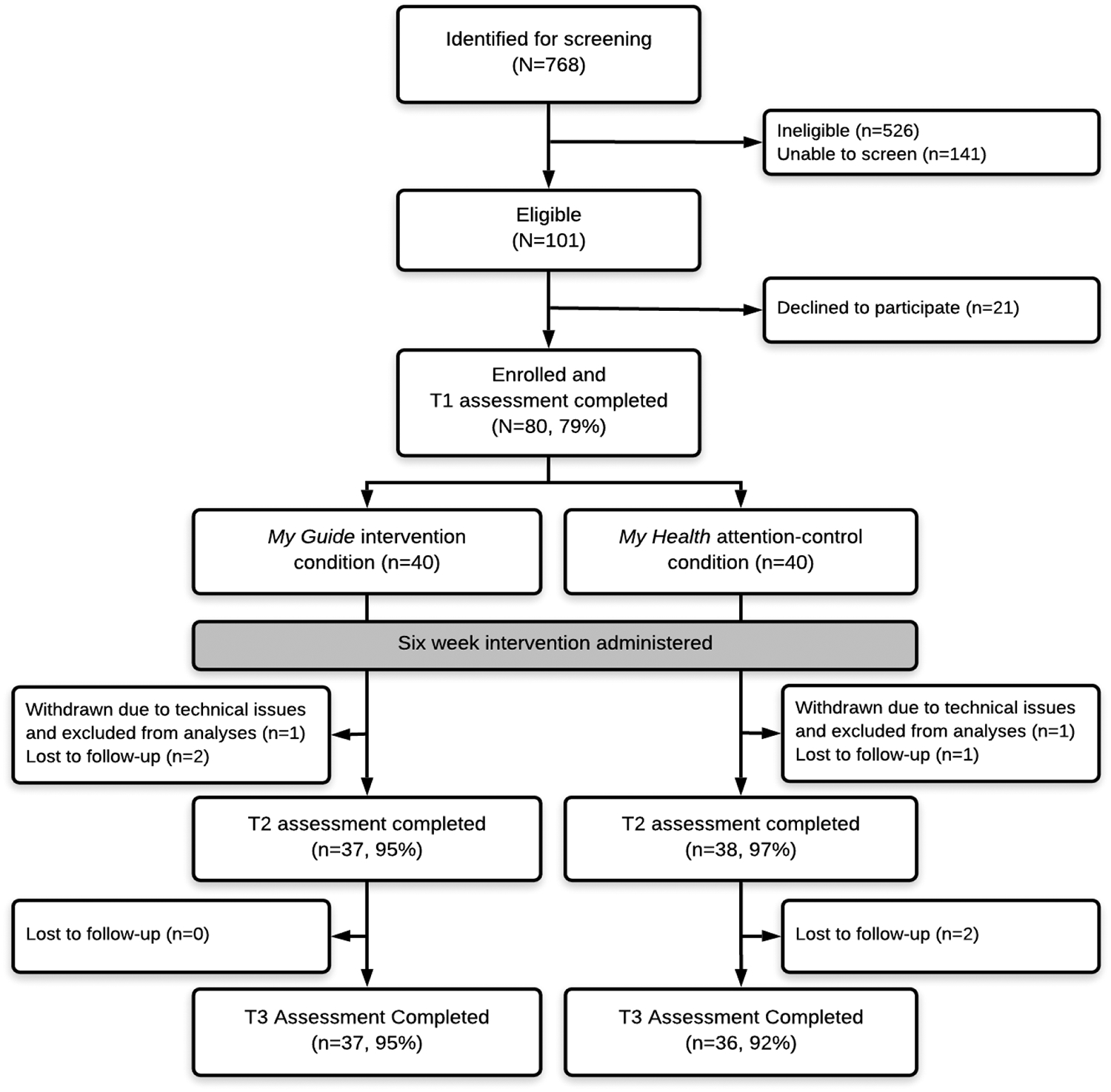
Participant flow and retention by study condition.
Table 3.
Sample characteristics at time of study enrollment.
| Full Sample (N=78) | My Guide (n=39) | My Health (n=39) | |
|---|---|---|---|
| Age; M (SD) | 52.54 (11.36) | 53.52 (11.25) | 51.55 (11.53) |
| Spanish-language preference; n (%) | 50 (64) | 25 (64) | 25 (64) |
| Born in United States; n (%) | 23 (30) | 14 (36) | 9 (23) |
| Mexican ancestry; n (%) | 50 (64) | 25 (65) | 25 (64) |
| High school education or less; n (%) | 42 (54) | 23 (59) | 19 (49) |
| Employed; n (%) | 34 (44) | 17 (44) | 17 (44) |
| Annual household income < $25,000; n (%) | 41 (53) | 23 (59) | 18 (46) |
| Married or partnered; n (%) | 50 (64) | 23 (59) | 27 (69) |
| Stage of disease; n (%) | |||
| 0 | 3 (4) | 2 (5) | 1 (3) |
| I | 28 (36) | 14 (36) | 14 (36) |
| II | 32 (41) | 16 (41) | 16 (41) |
| III | 11 (14) | 5 (13) | 6 (15) |
| Did not report | 4 (5) | 2 (5) | 2 (5) |
| Time since diagnosis (months); M (SD)* | 15.50 (7.04) | 16.18 (7.88) | 14.78 (6.07) |
| Received radiation therapy; n (%) | 55 (71) | 28 (72) | 27 (69) |
| Received chemotherapy; n (%) | 45 (58) | 19 (49) | 26 (67) |
Note.
Time since diagnosis was calculated as months between the date of baseline assessment and diagnosis date confirmed by medical chart review or patient-report if medical chart confirmation was not possible; M, mean; n, frequency; SD, standard deviation.
Nutrition
Table 4 displays the estimated marginal means and standard errors for daily fat sources and daily servings of fruits/vegetables over time adjusted for language preference.
Table 4.
Descriptive statistics of study outcomes across time adjusted for language preference.
| T1 | T2 | T3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| My Guide (n=39) | My Health (n=39) | p-value | My Guide (n=37) | My Health (n=38) | p-value | My Guide (n=37) | My Health (n=36) | p-value | |
| Daily nutrition intake | |||||||||
| Fat sources; EMM (SE) | 2.38 (0.21) | 2.86 (0.21) | 0.106 | 2.42 (0.22) | 2.38 (0.21) | 0.892 | 2.36 (0.22) | 2.20 (0.22) | 0.607 |
| Fruit/vegetable servings; EMM (SE) | 3.48 (0.27) | 3.80 (0.27) | 0.397 | 3.41 (0.28) | 3.53 (0.28) | 0.883 | 3.26 (0.28) | 3.59 (0.28) | 0.399 |
| Weekly physical activity in MET minutes | |||||||||
| Walking; GM [95% CI] | 161 [116, 304] | 144 [76, 271] | 0.775 | 301 [218, 567] | 281 [149, 529] | 0.883 | 262 [190, 494] | 203 [108, 383] | 0.572 |
| Moderate PA; GM [95% CI] | 9 [5, 25] | 5 [1, 15] | 0.515 | 12 [7, 35] | 7 [2, 21] | 0.521 | 11 [6, 32] | 6 [1, 17] | 0.415 |
| Vigorous PA; GM [95% CI] | 4 [2, 11] | 3 [1, 10] | 0.888 | 2 [1, 7] | 4 [1, 12] | 0.403 | 5 [3, 15] | 2 [0, 6] | 0.225 |
| Weekly physical activity in minutes | |||||||||
| Walking; GM [95% CI] | 49 [35, 92] | 44 [23, 82] | 0.807 | 91 [66, 172] | 85 [45, 160] | 0.858 | 79 [58, 150] | 62 [33, 116] | 0.571 |
| Moderate PA; GM [95% CI] | 2 [1, 6] | 1 [0, 4] | 0.542 | 3 [2, 9] | 2 [1, 5] | 0.510 | 3 [2, 8] | 2 [0, 4] | 0.406 |
| Vigorous PA; GM [95% CI] | 1 [0, 1] | 0 [0, 1] | 0.857 | 0 [0, 1] | 1 [0, 2] | 0.396 | 1 [0, 2] | 0 [0, 1] | 0.248 |
| Daily sitting in simple minutes; EMM (SE) | 232 (25) | 240 (25) | 0.809 | 215 (26) | 212 (26) | 0.939 | 208 (26) | 224 (26) | 0.657 |
| Physical activity categorization | 0.898 | 0.914 | 0.591 | ||||||
| Minimally active; n (%) | 20 (51) | 22 (56) | 20 (54) | 19 (50) | 20 (56) | 21 (58) | |||
| Moderately active; n (%) | 12 (31) | 11 (28) | 11 (30) | 13 (34) | 14 (39) | 11 (31) | |||
| Highly active; n (%) | 7 (18) | 6 (15) | 6 (16) | 6 (16) | 2 (6) | 4 (11) | |||
Note. Cl, confidence interval; EMM, estimated marginal mean; GM, geometric mean; MET, metabolic equivalent; n, frequency; PA, physical activity; SE, standard error; T1, study baseline; T2, immediately post-intervention; T3, two weeks post-T2. p-values reflect comparisons between study conditions at each time point. Means for physical activity were back-transformed from log(x+1) values.
Daily fat sources.
There was a significant interaction of time and condition on daily fat sources (F[2,145]=4.05, p=0.019; Figure 2a). From T1 to T2, there was a more negative slope in My Health compared to My Guide (b=−0.53, SE=0.24, p=0.030, Cohen’s d=0.30) which was maintained at T3 (b=−0.65, SE=0,24, p=0.009, Cohen’s d=0.47). Pairwise comparisons showed that for My Health participants, daily fat sources were significantly lower at T2 compared to T1 (p=0,015) and this was maintained at T3 (p=0.001). For My Guide participants, daily fat sources did not change from T1 to T2 (p=0.991) or from T1 to T3 (p=0.999). However, average daily fat sources did not differ between conditions at any time point (T1 p=0.106; T2 p=0.892; T3 p=0.607) and exceeded two fat sources per day across both conditions (range 2.20–2.86).
Figure 2.
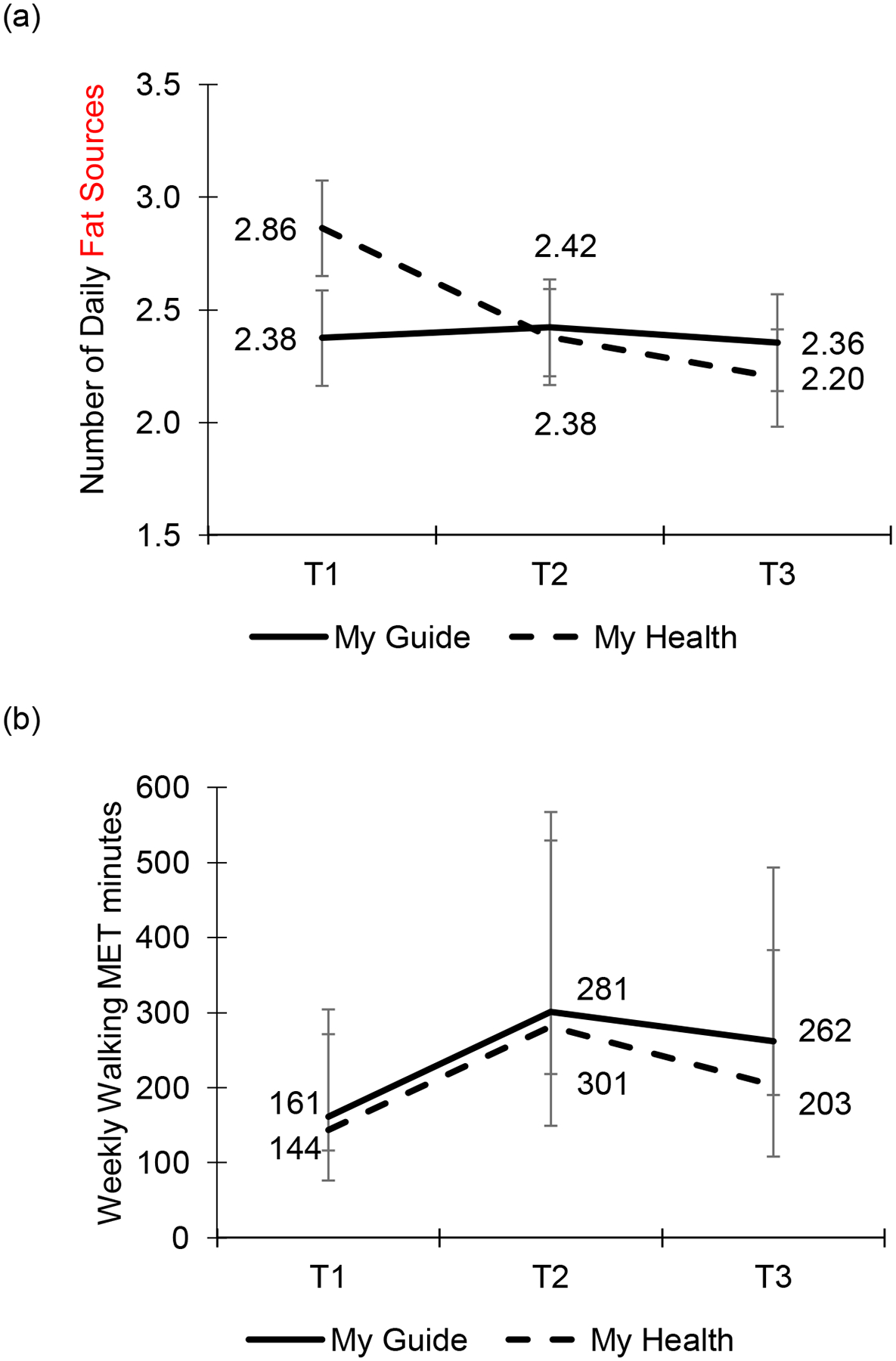
(a) Change in daily fat sources was significantly more negative for My Health from T1 to T2 and T3 than it was for My Guide after adjusting for language preference. Error bars reflect standard errors of estimated marginal means, (b) Weekly walking MET minutes significantly increased from T1 to T2 for both conditions after adjusting for language preference. Error bars reflect 95% confidence intervals of geometric means.
Daily servings of fruits and vegetables.
There was no interaction of time and condition on daily servings of fruits and vegetables (F[2,146]=0.45, p=0.641), and there were no main effects of time (F[2,148]=0.88, p=0.419) or condition (F[1,76]=0.50, p=0.480). Average daily servings of fruits and vegetables exceeded three (range 3.26–3.80) for women in both My Health and My Guide.
Physical Activity
Table 4 displays the back-transformed geometric means and 95% confidence intervals of each physical activity intensity level within each condition across time adjusted for language preference in both MET minutes per week and minutes per week. Table 4 also displays the proportion of women categorized as minimally, moderately, and highly physically active.
Walking.
There was no interaction of time and condition on weekly walking MET minutes (F[2,146]=0.07, p=0.934). However, there was a significant main effect of time (F[2,148]=3.11, p=0.048; Figure 2b), such that weekly walking MET minutes increased from T1 to T2 in both study conditions (b=0.28, SE=0.11, p=0.015, Cohen’s c d=0.31) with significantly more weekly walking MET minutes at T2 compared to T1 (p=0.045), though this improvement was not maintained at T3 (b=0.18, SE=0.12, p=0.116). There was no main effect of condition on weekly walking MET minutes (F[1,75]=0.20, p=0.654). The median values for walking were at least 90 minutes per week at each time point for both My Health (T1=90; T2=120; T3=105) and My Guide (T1=100; T2=100; T3=100).
Moderate physical activity.
There was no interaction of time and condition on weekly moderate MET minutes (F[2,147]=0.01, p=0.987), and there were no main effects of time (F[2,149]=0.24, p=0.790) or condition (F[1,74]=1.17, p=0.282). The majority of women reported no moderate physical activity at each time point in both My Health (T1=72%; T2=64%; T3=64%) and My Guide (T1=62%; T2=56%; T3=56%). The median values for moderate physical activity were 0 minutes per week at all time points for women in both conditions.
Vigorous physical activity.
There was no interaction of time and condition on weekly vigorous MET minutes (F[2,145]=1.72, p=0.182), and there were no main effects of time (F[2,147]=0.05, p=0.949) or condition (F[1,74]=0.04, p=0.834). The majority of women reported no vigorous physical activity at each time point in both My Health (T1=80%; T2=74%; T3=80%) and My Guide (T1=77%; T2=80%; T3=67%). The median values for vigorous physical activity were 0 minutes per week at all time points for women in both conditions.
Sitting.
There was no interaction of time and condition on daily sitting minutes (F[2,143]=0.12, p=0.890), and there were no main effects of time (F[2,145]=0.79, p=0.457) or condition (F[1,74]=0.07, p=0.795). Median time spent sitting each day exceeded 3 hours (180 minutes) for women in both My Health and My Guide (see Table 4).
Physical activity categorization.
The proportion of women categorized as minimally, moderately, and highly active did not differ between My Health and My Guide at any time point (T1 χ2[2]=0.22, p=0.898; T2 χ2[2]=0.18, p=0.914; T3 χ2[2]=1.05, p=0.591). The majority of women in both My Health and My Guide were categorized as minimally active at each time point (range 50–58%).
Discussion
Breast cancer is the most common form of cancer among Latina women, and Latina women are at higher risk for breast cancer recurrence than white women (Lara-Medina et al.,2011). Lifestyle factors, such as consuming a nutritious diet and engaging in regular physical activity, are protective against breast cancer recurrence (Ibrahim & Al-Homaidh, 2011; Lahart et al., 2015; Spei et al., 2019). Overall, Latina women tend to consume diets high in fat and low in fruits, vegetables, and whole grains (Chlebowski et al., 1991). Further, they engage in lower levels of physical activity than is recommended by expert guidelines (Ortiz et al., 2018). Previous studies have developed and tested lifestyle interventions for Latina BCS, however, these have been in-person interventions and limited by low participation rates (Greenlee et al., 2015; Mama et al., 2017). No studies to date have developed a smartphone delivered intervention to address lifestyle factors among Latina BCS. The purpose of the current study was to compare two Smartphone delivered interventions, My Health and My Guide, on dietary and physical activity outcomes.
In terms of dietary intake, participants receiving the My Health intervention reported a greater reduction in daily fat sources over time than participants in My Guide. This finding is particularly salient given that fat intake has been linked to increased cancer recurrence and mortality (Borugian et al., 2004; Chlebowski et al., 2018; Zhang et al., 1995). However, it is important to note that there have also been studies that did not report a relationship between fat intake and breast cancer survival (Chlebowski et al., 2006; Pierce, Stefanick et al., 2007). Moreover, despite the greater reduction in daily fat sources among participants in My Health, average daily fat sources did not significantly differ from participants in My Guide at any time point. It is possible that stronger patterns of change and/or greater differences between groups could emerge in studies with larger sample sizes and longer follow-up. Thus, these findings require replication. Both My Health and My Guide contained some content on reducing fat intake, but My Health included more comprehensive material focused on nutrition in cancer survivorship, along with culturally tailored healthy recipes that might appeal to Latina women. Regarding daily servings of fruits and vegetables, there were no statistically significant differences in consumption between the two groups at any time points. Consuming diets low in fat and high and fruits and vegetables are consistent with lower overall disease risk (Daviglus et al., 2012), and are important to consider within this already vulnerable population. Future research should determine the best way to deliver evidence-based interventions that promote consumption of fruits and vegetables for Latina BCS.
Regarding physical activity, there were no between group differences for walking, moderate or vigorous physical activity, or sitting. However, there was a main effect for time for walking, meaning that walking MET minutes increased across time for both groups. These findings are surprising given that previous research has found that breast cancer survivors are generally less active after treatment (Sabiston et al., 2014). Despite the increase in walking across groups, the overall rates of walking are low compared to the ACS guidelines. Although the My Health intervention did include content on the importance of physical activity and strategies to increase physical activity, the intervention was largely educational and did not include many intensive behavioral lifestyle components (Diabetes Prevention Program Research Group, 2002). The relatively short 6-week study period and lack of evidence-based strategies may have limited the intervention’s impact on other forms of physical activity. Of note, the majority of women across groups were categorized as “minimally active” and engaged in almost no moderate or vigorous physical activity. This is concerning given the importance of physical activity on cancer recurrence (Holmes et al., 2005) and general overall health. Future interventions should target more intensive forms of physical activity outside of walking and have a longer time frame.
The findings of this study should be interpreted within the context of its limitations. Given that My Health served as a comparison intervention in this study and lifestyle behaviors were not our primary outcomes, we did not utilize gold standard measures for diet and physical activity due to participant burden, budgetary restrictions, and risk for drop out. Although both measures have been validated in Latina samples, they are not objective or comprehensive. Further, the servings of fruits and vegetables subscale of the Brief Dietary Assessment Tool for Hispanics (Wakimoto et al., 2006) and the walking subscale of the IPAQ-SF (Craig et al., 2003; Lee et al., 2011) did not have acceptable internal consistency reliability at T2 or T3, which may have affected our outcomes. Future studies should utilize 24-hour dietary recalls to measure dietary intake and actigraphy to measure physical activity. A second limitation is that although My Health included more comprehensive content on lifestyle factors, both interventions included content on diet and physical activity. This may have limited our ability to detect differences between groups. Additionally, our ability to detect differences in our outcomes may have been influenced by our relatively small sample and short intervention length and follow up time frame. Further, it is possible that some women lacked access to safe spaces for outdoor exercise. Future research should measure built environment factors that might have affected physical activity. Finally, the intervention was largely educational in nature, and did not include many intensive behavioral lifestyle components (Holmes et al., 2005). Future mobile interventions should incorporate evidence-based strategies for health behavior change such as selfmonitoring of diet and physical activity, problem solving, goal setting, and self-reward.
To the best of our knowledge, this is the first study to demonstrate the efficacy of a smartphone delivered lifestyle intervention for Latina BCS. Specifically, participants receiving the My Health intervention consumed fewer daily fat sources than the My Guide group at all time points, and weekly walking MET minutes increased across time points in both groups. These preliminary findings suggest that mHealth interventions aimed at improving lifestyle factors may favorably impact nutritional intake and physical activity among Latina BCS. Future research should utilize more comprehensive and objective measures of diet and physical activity, and incorporate more behavioral lifestyle components into the intervention in larger samples with a longer follow-up period.
Acknowledgements
Research reported in this publication was supported in part by the National Institutes of Health National Cancer Institute grants U54-CA-202995, U54-CA-202997, and U54-CA-203000. Authors LBO and SHB were supported by the National Cancer Institute training grant T32-CA-193193. SMP was supported by K07CA196840. The content reported here is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- American Cancer Society. (2018). Cancer Facts & Figures for Hispanics/Latinos 2018–2020. [Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, & Caggiano V (2007). Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population- based study from the California cancer Registry. Cancer, 109(9), 1721–1728. doi: 10.1002/cncr.22618 [DOI] [PubMed] [Google Scholar]
- Borugian MJ, Sheps SB, Kim-Sing C,Van Patten C, Potter JD, Dunn B, … Hislop TG (2004). Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev, 13(7), 1163–1172. [PubMed] [Google Scholar]
- Chi F, Wu R, Zeng YC, Xing R, Liu Y, & Xu ZG (2013). Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev, 14(4), 2407–2412. doi: 10.7314/apjcp.2013.14.4.2407 [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Aragaki AK, Anderson GL, Simon MS, Manson JE, Neuhouser ML, … Prentice RL (2018). Association of Low-Fat Dietary Pattern With Breast Cancer Overall Survival: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trial. JAMA Oncol, 4(10), e181212. doi: 10.1001/jamaoncol.2018.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, … Elashoff RM (2006). Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst, 98(24), 1767–1776. doi: 10.1093/jnci/djj494 [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Rose D, Buzzard IM, Blackburn GL, Insull W, Grosvenor M, … Wynder EL (1991). Adjuvant dietary fat intake reduction in postmenopausal breast cancer patient management. Breast Cancer Res Treat, 20(2), 73–84. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2 ed). Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, … Oja P (2003). International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc, 35(8), 1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, … Stamler J (2012). Prevalence of Major Cardiovascular Risk Factors and Cardiovascular Diseases Among Hispanic/Latino Individuals of Diverse Backgrounds in the United States. Jama-Journai of the American Medical Association, 308(17), 1775–1784. doi: 10.1001/jama.2012.14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis C, Siegel R, Bandi P, & Jemal A (2011). Breast cancer statistics, 2011. CA Cancer J Clin, 61(6), 409–418. doi: 10.3322/caac.20134 [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. (2002). The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care, 25(12), 2165–2171. doi: 10.2337/diacare.25.12.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde C Scoring the International Physical Activity Questionnaire (IPAQ). Retrieved from https://uac.futurelearn.com/uploads/files/bc/c5/bcc53b14-ec1e-4d90-88e3-1568682f32ae/IPAQ_PDF.pdf
- Greenlee H, Gaffney AO, Aycinena AC, Koch P, Contento I, Karmally W, … Crew K (2015). ¡ Cocinar Para Su Salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. Journal of the Academy of Nutrition and Dietetics, 115(5), 709–723. e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, & Colditz GA (2005). Physical activity and survival after breast cancer diagnosis. JAMA, 293(20), 2479–2486. doi: 10.1001/jama.293.20.2479 [DOI] [PubMed] [Google Scholar]
- Ibrahim EM, & Al-Homaidh A (2011). Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol, 28(3), 753–765. doi: 10.1007/s12032-010-9536-x [DOI] [PubMed] [Google Scholar]
- Lafourcade A, His M, Baglietto L, Boutron-Ruault MC, Dossus L, & Rondeau V (2018). Factors associated with breast cancer recurrences or mortality and dynamic prediction of death using history of cancer recurrences: the French E3N cohort. BMC Cancer, 18(1), 171. doi:ARTN171 10.1186/s12885-018-4076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahart IM, Metsios GS, Nevill AM, & Carmichael AR (2015). Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol, 54(5), 635–654. doi: 10.3109/0284186X.2014.998275 [DOI] [PubMed] [Google Scholar]
- Lara-Medina F, Perez-Sanchez V, Saavedra-Perez D, Blake-Cerda M, Arce C, Motola- Kuba D, … Arrieta O (2011). Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer, 117(16), 3658–3669. doi: 10.1002/cncr.25961 [DOI] [PubMed] [Google Scholar]
- Lee PH, Macfarlane DJ, Lam TH, & Stewart SM (2011). Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act, 8,115. doi: 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MH, Gonzalez-Barrera A, & Patten E (2013). Closing the Digital Divide: Latinos and Technology Adoption. Retrieved from http://www.pewhispanic.org/2013/03/07/closing-the-diqital-divide-latinos-and-technoloqy-adoption/
- Mama SK, Song J, Ortiz A, Tirado-Gomez M, Palacios C, Hughes DC, & Basen- Engquist K (2017). Longitudinal social cognitive influences on physical activity and sedentary time in Hispanic breast cancer survivors. Psychooncology, 26(2), 214–221. doi: 10.1002/pon.4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VH, Krumholz HM, & Gross CP (2004). Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA, 291(22), 2720–2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- Ortiz A, Tirado M, Hughes DC, Gonzalez V, Song J, Mama SK, & Basen-Engquist K (2018). Relationship between physical activity, disability, and physical fitness profile in sedentary Latina breast cancer survivors. Physiotherapy Theory and Practice, 34(10), 783–794. doi: 10.1080/09593985.2018.1424978 [DOI] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, … Stefanick ML (2007). Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer - The Women’s Healthy Eating and Living (WHEL) Randomized Trial. Jama-Journal of the American Medical Association, 298(3), 289–298. doi:DOI 10.1001/jama.298.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Stefanick ML, Flatt SW, et al. (2007). Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncolology, 25(17), 2345–2351. DOI: 10.1200/JC0.2006.08.6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Coughlin SS, & Lyons EJ (2017). Social Media and Mobile Technology for Cancer Prevention and Treatment. Am Soc Clin Oncol Educ Book, 37, 128–137. doi: 10.14694/EDBK_173841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabiston CM, Brunet J, Vallance JK, & Meterissian S (2014). Prospective examination of objectively assessed physical activity and sedentary time after breast cancer treatment: sitting on the crest of the teachable moment. Cancer Epidemiol Biomarkers Prev, 23(7),1324–1330. doi: 10.1158/1055-9965.EPI-13-1179 [DOI] [PubMed] [Google Scholar]
- Spei ME, Samoli E, Bravi F, La Vecchia, C, Bamia C, & Benetou V (2019). Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast, 44,144–152. doi: 10.1016/j.breast.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Wakimoto P, Block G, Mandel S, & Medina N (2006). Development and reliability of brief dietary assessment tools for Hispanics. Prev Chronic Dis, 3(3), A95. [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, … Emanuel E (2006). Are racial and ethnic minorities less willing to participate in health research? PLoS Med, 3(2), e19. doi: 10.1371/journal.pmed.0030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RR (1993). Some results on a Winsorized correlation coefficient. Bf J Math Stat Psychol, 46(2), 339–349. [Google Scholar]
- Yanez BR, Buitrago D, Buscemi J, lacobelli F, Adler RF, Corden ME, … Penedo FJ (2018). Study design and protocol for My Guide: An e-health intervention to improve patient-centered outcomes among Hispanic breast cancer survivors. Contemp Clin Trials, 65, 61–68. doi: 10.1016/j.cct.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez B Oswald LB, Baik SH, Buitrago D, lacobelli F, Perez-Tamayo A, … Buscemi J (2019). Brief culturally informed smartphone interventions decrease breast cancer symptom burden among Latina breast cancer survivors Psycho-Oncology. Advance online publication. doi: 10.1002/pon.5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Folsom AR, Sellers TA, Kushi LH, & Potter JD (1995). Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women’s Health Study. Cancer, 76(2), 275–283. doi: [DOI] [PubMed] [Google Scholar]


