ABSTRACT
Introduction
The diagnosis of SARS-CoV-2 infection is crucial for medical and public health reasons, to allow the best treatment of cases and the best control of the pandemic. Serology testing allows for the detection of asymptomatic infections and 19-COVID cases once the virus has been cleared. We analyzed the usefulness of the SARS-CoV-2 rapid test of Autobio and tried to correlate its pattern with the severity of COVID19 infection.
Material and methods
We analyzed the accuracy and clinical usefulness of a point-of-care IgM and/or IgG test for SARS-CoV-2 in 35 COVID-19 patients [12 (34.3%) mild-moderate and 23 (65.7%) severe-critical] admitted to a field hospital in Madrid, as well as in 5 controls.
Results
The mean time from the first day of symptoms to the antibody test was 28 days (SD: 8.7), similar according to the severity of the disease. All patients with SARS-CoV-2 PCR+ showed the corresponding IgG positivity, while these results were negative in all control individuals. A total of 26 (74%) cases also presented with positive IgM, 19 (83%) were severe-critical cases and 7 (58%) were mild-moderate cases. The IgM response lasted longer in the severe critical cases (mean: 29.7 days; SD: 8.4) compared to the moderate cases (mean: 21.2 days; SD: 2.0).
Conclusions
Rapid serology tests are useful for the diagnosis of patients with COVID-19 (mainly IgG detection) and may also be correlated with the severity of the infection (based on IgM detection).
Keywords: SARS-CoV-2, COVID-19 infection, Serologic rapid tests, Autobio, weak positive bands
RESUMEN
Introducción
El diagnóstico de la infección por SARSCoV-2 es crucial por razones médicas y de salud pública, para permitir el mejor tratamiento de los casos y el mejor control de la pandemia. Las pruebas de serología permiten la detección de infecciones asintomáticas y de casos de COVID-19 una vez que se ha logrado la eliminación del virus. El objetivo fue analizar la utilidad del test rápido SARS-CoV-2 de Autobio e intentar correlacionar su patrón con la gravedad de la infección por COVID19.
Material y métodos
Hemos analizado la precisión y la utilidad clínica de un test de IgM y/o IgG en el punto de atención para el SARS-CoV-2 en 35 pacientes COVID-19 [12 (34,3%) leves-moderados y 23 (65,7%) severos-críticos] ingresados en un hospital de campaña en Madrid, así como en 5 controles.
Resultados
El tiempo medio desde el primer día de síntomas hasta la prueba de anticuerpos fue de 28 días (DE: 8,7), similar según la gravedad de la enfermedad. Todos los pacientes con SARS-CoV-2 PCR+ mostraron la correspondiente positividad de IgG, mientras que estos resultados fueron negativos en todos los individuos de control. Un total de 26 (74%) casos también se presentaron con IgM positiva, 19 (83%) fueron casos severos-críticos y 7 (58%) fueron casos leves-moderados. La respuesta a la IgM duró más tiempo en los casos críticos severos (media: 29,7 días; DE: 8,4) en comparación con los casos moderados (media: 21,2 días; DE: 2,0).
Conclusiones
Las pruebas de serología rápida son de utilidad para el diagnóstico de los pacientes con COVID-19 (principalmente la detección de IgG) y también pueden estar correlacionadas con la gravedad de la infección (basada en la detección de IgM).
Palabras clave: SARS-CoV-2, infección por COVID-19, test serológicos rápidos, Autobio, bandas débilmente positivas
INTRODUCTION
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from the end of 2019 onwards [1, 2] has probably marked a new milestone in the history of Medicine, that of finding in the shortest time the best understanding of a new infection of unprecedented populational dimension. While in most instances the clinical picture resembles a common cold [3], around 15% of infections progress to severe pneumonitis and acute respiratory distress syndrome (ARDS) [4]; later this may lead to septic shock, and coagulation dysfunction [5]. Diagnosis of SARS-CoV-2 infection is crucial for medical and public health reasons, to allow best treatment of cases and best control of the pandemic. Availability of diagnostic tools is key to provide optimal treatment to infected individuals, to indicate isolation measures to cases and to monitor the efficacy of public health measures [6-8]. Direct detection of viral RNA by RT-PCR was soon developed, and mostly used for the diagnosis of SARS-CoV-2 infectious disease (COVID-19) and is the gold standard in symptomatic patients in medical facilities [9]. Among its limitations are false negatives from the first week, the need for technology, time and cost. Efficacy of social distancing measures during escalation and de-escalation periods needs close monitoring of novel COVID-19 cases. It is estimated that as high as 50 to 75% of infections may be asymptomatic [10], and these carriers have been described as effective SARS-CoV-2 shedders [11].
The rapid lateral-flow point-of-care antibody tests are simple, cheap, and fast. They do not require qualified personnel for interpretation and could be done in primary care. These tests may be optimal to study the prevalence of viral infections, as asymptomatic subjects and infections after viral RNA clearance may both be detected. Serology analysis can also correlate with the clinical severity of COVID-19 if neutralizing antibodies are assessed [12], although other immune pathways as T-cell response or cytokines may also be implicated in clinical recovery. The main objective of this study was to analyze the accuracy of a point-of-care SARS-CoV-2 IgM and/or IgG rapid test for the diagnosis of COVID-19, and to correlate this pattern of immune response with the severity of disease.
MATERIAL AND METHODS
Study setting, context and design. This was a transversal study carried out during the third week of confinement in Spain in a field-Hospital enabled in IFEMA (Ferial Institution of Madrid, Spain) with an occupation over 1,400 beds and full spectrum of COVID-19 severity. The project was approved by the Centre’s clinical research ethics committee.
Sample collection and testing. Anti-SARS-CoV-2 tests were performed to 35 randomly selected SARS-CoV-2 RT-PCR confirmed patients, admitted to IFEMA Field-Hospital between April 27th and April 29th, 2020. COVID-19 diagnosis had been established in all cases based on positive SARS-CoV-2 positive
RT-PCR for pharyngeal swabs. Additionally, 5 healthy volunteers with no history of COVID-19 symptoms and negative SARS-CoV-2 RT-PCR were enrolled as negative controls. Blood samples were obtained by peripheral venipuncture at the elbow flexure. The test was performed according to the manufacturer’s recommendations: firstly adding 10 μL of total blood in each well (IgM or IgG) from the EDTA blood tube and then adding 60 μL of the sample diluent to the corresponding well. A picture of every rapid test was taken at the manufacturer’s stablished time of reading. Test results were evaluated by two operators. In case of disagreement, a third operator was requested. According to the manufacturer instructions, IgG band reading rendered either negative or positive results. On the other hand, IgM band was classified as either negative, positive or weak positive depending on the intensity of the band staining. IgM positive, IgG positive and either IgM or IgG positive band staining were counted as positive results for the rapid test.
Severity disease categorization. Depending on the clinical features of SARS-CoV-2 diseases, the patients were categorized into mild, moderate, severe or critical [13]. Mild COVID-19: low grade fever, cough, malaise, rhinorrhea, sore throat with or without hemoptysis, nausea, vomiting, diarrhea, but without any radiological features of pneumonia and absence of mental changes. Moderate COVID-19: fever, respiratory symptoms including dry cough and shortness of breath that may emerge along with the radiological features. Severe COVID-19: dyspnea, respiratory frequency 30/minute, blood oxygen saturation 93%, PaO2/FiO2 ratio <300, and/or lung infiltrates >50% of the lung field within 24-48 h. Critical COVID-19: usually develops after 7 days in patients with mild/moderate/severe COVID-19 with features of acute respiratory distress syndrome (ARDS) requiring mechanical ventilation along with presence of multiorgan dysfunction failure, metabolic acidosis and coagulation dysfunction.
Serology rapid test. The anti-SARS-CoV-2 lateral flow qualitative immunochromatography rapid tests (Autobio Diagnostics Co. Zhengzhou, China) is based on a one-step capture method. The Cassette contains membranes which are pre-coated with two murine anti-human monoclonal antibodies (anti-IgG and anti-IgM) on two separated test lines. SARSCoV-2 recombinant spike protein antigen reagents which can specifically bind to SARS-CoV-2 antibodies (IgM and/or IgG), are bound to colloidal gold and sprayed on conjugation pads. When the sample is applied to the test wells, antibody and labeled antigen complexes are formed and travel up the strip. The labeled gold colorimetric reagent is used to form a visible red/ pink line. The presence of anti-SARS-CoV-2 IgM and/or IgG will be indicated by a visible red/pink test line (T) in the IgM and IgG result windows. Anti-SARS-CoV2 IgM antibodies are bound on the IgM line, and anti-SARS-CoV-2 IgG antibodies are bound to the IgG line. The control (C) line appears in each result window when sample has flowed through the strip. The manufacturer has performed cross-reactivity tests using serum samples containing antibodies to other pathogens, including endemic human CoV, with no IgM or IgG false positive results observed. Across studies, positive and negative percent agreement for the test were 85-88% and 99%, respectively (https://www.fda.gov/media/137367/download).
RESULTS
A total of 40 individuals were subjected to the anti-SARSCoV-2 rapid test. The sample included 17 (42.5%) men and 23 (57.5%) women, with a mean age of 56.8 years (SD: 10.5). Of the 40 individuals studied, 35 (87.5%) suffered from COVID-19, confirmed by means of a SARS-CoV-2 positive PCR. In every case, the lateral flow test was able to detect an IgG positive band. As expected, the lateral flow test produced a negative result for both IgM and IgG bands in all 5 control cases.
In 31 (88.6%) of the COVID-19 cases studied, bilateral pneumonia was diagnosed by means of chest radiology and/ or CT Scan. A total of 12 (34.3%) COVID-19 cases were categorized as mild or moderate, whereas 23 (65.7%) were categorized as severe or critical. The average time from the first day of reported symptoms to the lateral flow test were 28 days (SD: 8.7). The ranges were similar between the mild-moderate cases (minimum: 17 days; maximum: 45 days) and the severe-critical (minimum: 16 days; maximum: 48 days).
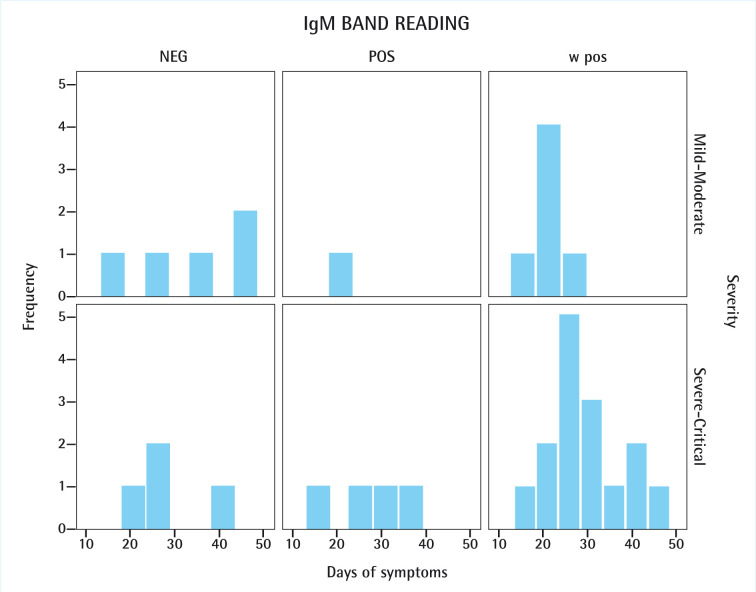
Among the 35 COVID-19 cases registered, 5 (14.3%) were clearly positive for both IgM and IgG bands, 21 (60%) were weakly positive for IgM and positive for IgG, and none were negative for both IgG and IgM. No cases of a positive IgM band together with a negative IgG band were observed (figure 1). A remaining total of 9 (25.7%) COVID-19 cases presented a positive IgG band in the lateral flow test with a negative IgM band result, regardless of them being mild-moderate (5; 14.3%) or severe-critical (4; 11.4%) ones.
Figure 1.
IgM band readings observed in COVID-19 patients regarding severity of the disease and number of days since first symptom was reported.
NEG: negative; POS: positive; w pos: weak positive.
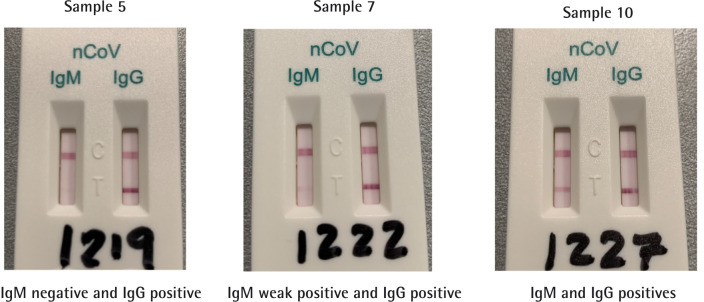
Regarding the IgM band staining, the positive result was observed more frequently among the severe-critical cases (4; 11.4%) than in the mild-moderate ones (1; 2.9%). Among the 21 patients with weak IgM positive results 15 were severe-critical cases (42.9%) and 6 were mild-moderate cases (17.1%). IgM response was more durable in severe-critical cases (mean: 29.7 days; SD: 8.4) as compared with moderate cases (mean: 21.2 days; SD: 2.0). Moreover, in mild-moderate cases, weak positive IgM result was not observed beyond the 25th day of symptoms. In the case of the severe-critical cases, the weak positive IgM result appeared throughout the whole range of symptomatic days. For mild-moderate COVID-19 cases, positive IgG band appeared mostly around day 20 of symptoms (mean: 26.3 days; SD: 9.6). In the severe-critical cases, positive IgG band spanned more uniformly (mean: 28.9 days; SD: 8.3). All results are summarized in tables 1 and 2 and graphically depicted in figures 1 and 2. Figure 3 shows three Rapid Tests displaying an example of each possible positive result observed in our study.
Table 1.
Main characteristics of the sample.
| N | Sex | Age | RT-PCR | IgM | IgG | Severity of disease | Days of symptoms |
|---|---|---|---|---|---|---|---|
| 1 | Female | 53 | POS | w pos | POS | Severe | 24 |
| 2 | Female | 54 | POS | w pos | POS | Critical | 27 |
| 3 | Female | 73 | POS | NEG | POS | Severe | 26 |
| 4 | Female | 49 | POS | NEG | POS | Moderate | 17 |
| 5 | Female | 54 | POS | NEG | POS | Severe | 41 |
| 6 | Female | 74 | POS | w pos | POS | Severe | 40 |
| 7 | Female | 40 | POS | w pos | POS | Moderate | 18 |
| 8 | Female | 60 | POS | w pos | POS | Severe | 37 |
| 9 | Male | 71 | POS | POS | POS | Moderate | 23 |
| 10 | Male | 65 | POS | POS | POS | Severe | 37 |
| 11 | Male | 77 | POS | w pos | POS | Severe | 31 |
| 12 | Male | 47 | POS | w pos | POS | Severe | 23 |
| 13 | Male | 57 | POS | w pos | POS | Severe | 30 |
| 14 | Male | 51 | POS | w pos | POS | Severe | 18 |
| 15 | Male | 68 | POS | w pos | POS | Severe | 28 |
| 16 | Male | 57 | POS | w pos | POS | Moderate | 20 |
| 17 | Female | 70 | POS | w pos | POS | Severe | 19 |
| 18 | Female | 56 | POS | NEG | POS | Moderate | 24 |
| 19 | Female | 61 | POS | w pos | POS | Severe | 29 |
| 20 | Female | 66 | POS | NEG | POS | Severe | 19 |
| 21 | Female | 54 | POS | POS | POS | Severe | 25 |
| 22 | Female | 59 | POS | w pos | POS | Moderate | 22 |
| 23 | Female | 62 | POS | w pos | POS | Moderate | 22 |
| 24 | Female | 67 | POS | NEG | POS | Severe | 26 |
| 25 | Male | 52 | POS | POS | POS | Severe | 16 |
| 26 | Male | 74 | POS | w pos | POS | Severe | 48 |
| 27 | Male | 58 | POS | w pos | POS | Severe | 40 |
| 28 | Female | 42 | POS | w pos | POS | Mild | 24 |
| 29 | Male | 60 | POS | w pos | POS | Critical | 26 |
| 30 | Male | 59 | POS | w pos | POS | Moderate | 21 |
| 31 | Female | 54 | POS | POS | POS | Severe | 30 |
| 32 | Female | 45 | POS | w pos | POS | Severe | 25 |
| 33 | Female | 58 | POS | NEG | POS | Moderate | 35 |
| 34 | Female | 45 | POS | NEG | POS | Mild | 44 |
| 35 | Male | 45 | POS | NEG | POS | Mild | 45 |
| 36 | Female | 30 | NEG | NEG | NEG | Negative control | |
| 37 | Male | 54 | NEG | NEG | NEG | Negative control | |
| 38 | Male | 61 | NEG | NEG | NEG | Negative control | |
| 39 | Female | 41 | NEG | NEG | NEG | Negative control | |
| 40 | Male | 49 | NEG | NEG | NEG | Negative control |
RT-PCR: real time-polimerase chain reaction; POS: positive; NEG: negative; w pos: weak positive.
Table 2.
RT-PCR and rapid test results in studied sample, including sensitivity and specificity calculated for the Rapid Test.
| RT-PCR positive | RT-PCR negative | |
|---|---|---|
| IgM negative/ IgG negative | 0 | 5 |
| IgM positive/ IgG negative | 0 | 0 |
| IgM weak positive/ IgG positive | 21 | 0 |
| IgM positive/ IgG positive | 5 | 0 |
| IgM negative/ IgG positive | 9 | 0 |
| Total | 35 | 5 |
| Sensitivity | 100% | |
| Specificity | 100% |
RT-PCR: real time-polimerase chain reaction.
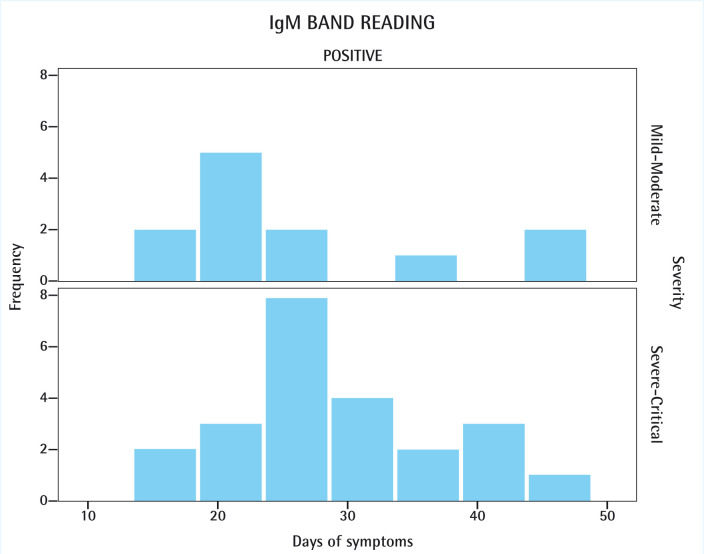
Figure 2.
IgG band readings observed in COVID-19 patients regarding severity of the disease and number of days since first symptom was reported.
POS: positive.
Figure 3.
Example of results in anti-SARS-CoV-2 lateral flow qualitative immunochromatography rapid tests Autobio Diagnostics Co. Zhengzhou, China
DISCUSSION
The usefulness of rapid tests for the diagnosis of active COVID-19 has been questioned regarding their inferiority when compared to the RT-PCR testing. Some authors think it should not be used for triage, diagnosis, individual risk assessment or public health decisions [14]. In that context, suboptimal levels of rapid test’s sensitivity have been attributed by some authors to delayed humoral responses. Studies showed that, normally, seroconversion occurred sequentially for IgM and then IgG with a median time of 11 and 14 days, respectively [15]. Another limitation of serological tests may come from the differences in individual antibody production, that could render false-negative results [16]. It has been shown that during the early stages of the disease, the SARS-CoV-2 heavily proliferates at the nasopharyngeal area, rendering the RT-PCR a better option for detection. But, at more advanced stages, the lower respiratory or intestinal tract may be infected, representing the epicenter of viral replication. Thus, the nasopharyngeal swab may not be the best sampling method for all the stages of the disease [17-20].
Although, strictly, lateral flow tests can only provide qualitative (positive/negative) results, considering the weak positive result on the IgM band, could render an extra information useful when combined with the disease severity. This division may correlate with the three different types of antibody responses (strong, weak and non-response) identified by other authors in COVID-19 patients by means of ELISA assays [21], or it may represent a form of prozone effect when high titers of IgM are present. As a matter of fact, studies on lateral flow immunochromatographic strip tests have showed that the prozone effect may be the cause for weak/faint readings on this kind of tests, especially in undiluted samples such as those used in our work [22, 23]. Finding in our series that also weak IgM bands were more frequently present in patients with more severe COVID-19 supports this hypothesis, although further studies are needed. In that sense, although serological ELISA assays still represent a superior alternative to the quantitative analysis of antibody titers, they are less available and more complex to perform as a point-of-care or community-based diagnostic strategy.
In comparison with RT-PCR, lateral flow immunoassays detecting IgM and IgG against SARS-CoV-2 can detect patients at different infection stages. In fact, some authors believe rapid tests based on IgM-IgG detection could provide valuable information on the COVID-19 time-course [16]. Nevertheless, infection time point based on how long each patient was infected or for how long each patient had symptoms is fundamental to correctly outline the infection time course for a single patient. In our study, we registered the time from the first day of reported symptoms until the rapid test was performed. In this context we observed a more durable IgM response in more severe COVID-19 cases, while mild-moderate cases did not show positive IgM results beyond day 25 of symptoms. This finding could be related to faster viral clearance in patients with benign outcome, or with IgM persistence indicative of more intense inflammatory response leading to respiratory distress.
Despite extensive clinical experience suggests that mild and moderate COVID-19 cases may have shorter symptomatic periods than severe or critical ones, in our sample we observed similar ranges between both groups (17-45 days vs. 16-48 days). The fact that we did not include any patient with early (<7 days) or intermediate (8-15 days) disease stage may account for the high sensitivity and specificity observed in our study. In fact, several studies have already reported low positive rates for both IgM and IgG during the first 7 days of illness. Those rates dramatically increased 15 days after onset of symptoms [15, 18].
The quality and quantity of antibodies produced against a specific virus may condition the final immunological response [24]. For example, Tan et al. [21] have reported an association between more severe forms of COVID-19 and higher titers of IgM. This same study has also showed that IgM levels remained positive for longer periods in severe COVID-19 cases. Similarly, we found that patients with more severe respiratory compromise had longer anti-S IgM production, as detected with the rapid test. Neutralizing antibodies can block viral entry, fusion or egress, therefore easing the course of the infection. However, a phenomenon known as antibody-dependent enhancement (ADE), may boost inflammation and tissue damage by activating phagocytes via Fc region receptors. This phenomenon has been documented for SARS-CoV and may be suggested in SARS-CoV-2 infection according to our observations. Furthermore, in vitro studies suggest that ADE occurs in phagocytes expressing Fc receptors when antibodies remain at a low concentration, whereas higher antibody titers effectively block viral entry [25]. Other studies suggest that high-affinity antibodies exert better neutralization and protection. Apparently, ADE is induced when the antibody-antigen interaction strength is below the threshold for neutralization [26]. It is important to recall that IgM is a strong pro-inflammatory immunoglobulin that efficiently activates complement. Some authors have described strong IgM responses as independent factors associated with disease severity [21]. Relevantly, ADE in macrophages leads to an intense production of TNF and IL-6 [27]. Those cytokines have been held responsible for the potent immune inflammatory response elapsed by SARS-CoV-2 in the lung, and inflammation have been closely related to severity of COVID-19 [28]. Therefore, several therapeutic approaches currently used to mitigate COVID-19, such as tocilizumab or infliximab, are based on their blockade [29, 30].
Regarding SARS-CoV, studies reported association between severe forms of the disease and a more robust IgG response, namely by and earlier seroconversion and higher antibody titers [31]. Recent studies in patients with COVID-19 have identified potentially detrimental effects of certain antibody responses in some patients. Jiang et al. [32] found a correlation between IgG response and COVID-19 severity. Nevertheless, that correlation was not directly stablished, but by means of its subordination to LDH levels. Moreover, their work stablished a correlation between female gender and younger ages with stronger IgG responses, even though higher mortality rates have been identified in aged male patients [33]. In our study, a positive IgG band appeared mostly around day 20 of symptoms for mild-moderate cases, whereas it spanned more uniformly in the severe-critical ones. Two likely explanations may account for that observation. First, mild and moderate cases tend to display shorter clinical courses and, therefore, is logical that seroconversion would have occurred sooner. Second, the fact that in our sample the severe and critical cases predominated, may have smoothed the distribution of the data. Probably if more mild-moderate cases would have been included, both distributions would look rather similar (figure 2).
Lymphopenia has been widely reported as a key laboratory finding in COVID-19 cases [34-36]. It may be the result of direct T-cell apoptosis induction by the SARS-CoV-2 [37] or induced by the pro-inflammatory cytokines released in the context of a “cytokine storm” [38]. Specifically, CD4+ and CD8+ T-cell depletion have been more frequently observed in the more severe cases [34, 39]. CD4+ T-cells stimulate B-cells to produce antibodies, and CD8+ T-cells directly eliminate virus-infected cells [37]. Moreover, CD8+ T-cells have been reported to function as T follicular helper (Tfh) cells in the germinal center of the B-cell follicle in the context of infection. By expressing B-cell co-stimulatory proteins they may have a role in promoting B-cell differentiation and antibody isotype class switching [40]. Those findings suggest that an incomplete or lagged isotype change from IgM to IgG may be more frequent among severe-critical COVID-19 cases, which is again in line with the results of the present study. More studies regarding the different patterns of isotype switching according to the severity of the disease would be of great use to shed some light on the immunological fingerprint of COVID-19. The selection of targeted therapies or the development of new immunomodulating agents could benefit from this approach up to an extent that we still cannot foresee.
Limitations of our work included a potential source of recall bias, since the first day of reported symptoms ultimately relies on patient recalling after RT-PCR confirmation. That could have altered the accurate application of the already described IgM/IgG dynamics to our particular sample. Another obvious limitation of our study is the reduced sample size. We couldn’t dilute the samples for testing either, because we didn’t have any more tests. Finally, there is a selection bias in the study, as it is difficult to find patients admitted with mild diseases, however we analyzed them together with patients with moderate clinical behavior, in which respiratory involvement could appear with or without radiological findings. By associating them, two populations were obtained that were more easily comparable to severe and critical patients, who also contributed with parainfectious phenomena such as distress and thrombosis. Even though, such limited size already gave us adequate exploratory information to plan for more ambitious studies in order to confirm the hypothesis raised.
As a conclusion, in late stage (after more than 15 days of symptoms) COVID-19 cases, lateral flow immunochromatography Rapid Tests (such as Autobio Diagnostics Co.) may be useful for diagnosis and clinical management. The weak positive staining for IgM could represent a prozone effect that would act as a surrogate marker for a stronger IgM response. That fact, together with a broader period of detection in patients with more severe COVID-19, could account for a delay in the immunoglobulin isotype switching. Further quantitative studies would be necessary to allow us to correlate the immunoglobulin kinetics with pathocrony and severity of COVID-19 disease.
FUNDING
None to declare
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel corona-virus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382:727–733. DOI: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneu-monia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579:270-273. DOI: 10.1038/s41586-020- 2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Wu Q, Xu W-Z, Qiao B, Wang J, Zheng H, et al. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. Preprint at medRxiv. 2020. 10.1101/2020.02.12.20.022327. [DOI] [Google Scholar]
- 4.Chen N-S, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiolog-ical and clinical characteristics of 99 cases of 2019 novel coronavi-rus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395 (10223): 507-513. DOI: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F, Kruip M, van der Meer N, Arbous M, Gommers D, Kant K, et al. Confirmation of the high cumulative incidence of throm-botic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020; 3848: 30157-30162. DOI: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003; 361:1319–1325. DOI: 10.1016/s0140-6736(03)13077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev 2007; 20:660–694. DOI: 10.1128/CMR.00023-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavi-rus causing SARS-like disease. Clin Microbiol Rev. 2015; 28: 465–522. DOI: 10.1128/CMR.00102-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; 25:1-8. DOI: 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 2020; 368:1165 DOI: 10.1136/bmj.m1165 [DOI] [PubMed] [Google Scholar]
- 11.Chan J, Yuan S, Kok K, To K, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020; 395: 514–523. DOI: 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered pa-tient cohort and their implications. Preprint at medRxiv. 2020. doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 13.Singh A, Shaikh A, Singh R, Singh AK. COVID-19: From bench to bed side. Diabetes Metab Syndr. 2020;14: 277-281. DOI: 10.1016/j.dsx.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassaniti I, Novazzi F, Giardina F, Salinaro F, Sachs M, Perlini S, et al. : Members of the San Matteo Pavia COVID-19 Task Force. Per-formance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J Med Virol. 2020; March 30; 10.1002/jmv.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody respons-es to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020; March 28;ciaa344. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020; February 27; 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dy-namics in viral shedding and transmissibility of COVID-19. Nat Med. 2020; February 27; doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, et al. Serological immuno-chromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020; April 10;S0163-4453(20)30175-4. doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020; May 6. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implica-tion of multiple shedding routes. Emerg Microbes Infect. 2020; 9: 386-389. DOI: 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan W, Lu Y, Zhang J, Wang J, Dan Y, Tan Z, et al. Viral Kinetics and Antibody Responses in Patients with COVID-19. Preprint at medRx-iv. 2020. 10.1101/2020.03.24.20042382. [DOI] [Google Scholar]
- 22.Gillet P., Mori M., Van Esbroeck M, Van der Ende J, Jacobs J. As-sessment of the prozone effect in malaria rapid diagnostic tests. Malar J. 2009; 8: 271 DOI: 10.1186/1475-2875-8-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee GH, Arthur I, Leung M. False-negative serum cryptococcal later-al flow assay result due to the prozone phenomenon. J Clin Micro-biol. 2018; 56 (4): e01878-17. DOI: 10.1128/JCM.01878-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki A, Yang Y. The potential danger of suboptimal antibody re-sponses in COVID-19. Nat Rev Immunol. 2020;1-3 doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaume M, Yip MS, Cheung CY, Leung HL, Li PH, Kien F, et al. An-ti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH-and cysteine protease-independent Fc-R pathway. J Virol. 2011; 85(20): 10582-97. DOI: 10.1128/JVI.00671-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of fla-vivirus infection: implications for vaccine development. Cell Host Microbe. 2008; 4(3): 229-38. DOI: 10.1016/j.chom.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, Shen CW, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208-14. DOI: 10.1016/j.bbrc.2014.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong J, Dong H, Xia SQ, Huang YZ, Wang D, Zhao Y, et al. Corre-lation analysis between disease severity and inflammation-relat-ed parameters in patients with COVID-19 pneumonia. Preprint at medRxiv. 2020. 10.1101/2020.02.25.20025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): an-ti-inflammatory strategies. J Biol Regul Homeost Agents. 2020. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 30.Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395(10234):1407-1409. DOI: 10.1016/S0140-6736(20)30858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee N, Chan PK, Ip M, Wong E, Ho J, Ho C, et al. Anti-SARS-CoV IgG response in relation to disease severity of severe acute res-piratory syndrome. J Clin Virol. 2006;35(2):179-84. DOI: 10.1016/j.jcv.2005.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang HW, Li Y, Zhang HN, Wang W, Men D, Yang X, et al. Global profiling of SARS-CoV-2 specific IgG/IgM responses of convales-cents using a proteome microarray. Preprint at medRxiv. 2020. 10.1101/2020.03.20.20039495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe dis-ease and death. BMJ. 2020;368:m1198 DOI: 10.1136/bmj.m1198 [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. DOI: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with Differ-ent Severity: A Multi-center Study of Clinical Features. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siordia JA Jr. Epidemiology and clinical features of COVID-19: A review of current literature. J Clin Virol. 2020;127:104357 DOI: 10.1016/j.jcv.2020.104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16(10):1753–1766. DOI: 10.7150/ijbs.45134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cron R.Q., Chatham W.W. The Rheumatologist’s Role in Covid-19. J. Rheumatol. 2020. doi: 10.3899/jrheum.200334. [DOI] [PubMed] [Google Scholar]
- 39.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. : Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19). Preprint at medRxiv. 2020. 10.1101/2020.02.18.20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentine KM, Davini D, Lawrence TJ, Mullins GN, Manansala M, Al-Kuhlani M, et al. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. J Immunol. 2018;201(1):31-40. DOI: 10.4049/jimmunol.1701079 [DOI] [PMC free article] [PubMed] [Google Scholar]