Abstract
Rationale & objective:
Soluble urokinase receptor (suPAR) is a novel biomarker associated with incident chronic kidney disease (CKD) and has been identified as an independent risk factor for CKD progression in children, although these findings remain preliminary, limited to a single point in time, and un-replicated in pediatric cohorts.
Study Design:
Prospective longitudinal cohort study.
Setting & Participants:
565 participants ages 1–16 years enrolled in the Chronic Kidney Disease in Children (CKiD) study.
Exposure:
Plasma suPAR levels, categorized by quartiles, measured at study entry and a six month follow-up interval.
Outcome:
CKD progression, defined as the initiation of kidney replacement therapy (KRT, dialysis or transplantation) or >50% decline in estimated glomerular filtrate rate (eGFR).
Analytic approach:
Associations between plasma suPAR quartiles and risk of CKD progression were estimated using lognormal survival models, adjusting for potential confounders.
Results:
Participants in the highest suPAR quartile experienced 54% faster progression compared to the lowest quartile after adjustment for demographic and traditional CKD risk factors (p<0.001). Addition of eGFR to the model attenuated the risk, although those in the highest quartile experienced a 33% faster progression compared to the lowest quartile (p=0.008). Plasma suPAR levels showed little change over 6 months.
Limitations:
Potential for residual confounding, reliance on observational data, relatively fewer patients with higher eGFRs for subgroup analysis.
Conclusions:
Higher suPAR levels are associated with a shorter time to RRT or halving of eGFR in children with CKD. This association is attenuated slightly with inclusion of eGFR in regression modeling but remains a significant association for participants with the highest suPAR levels.
Keywords: soluble urokinase plasminogen activator receptor (suPAR), chronic kidney disease (CKD), progression, end-stage renal disease (ESRD), renal failure, children, biomarker, pediatric, estimated glomerular filtration rate (eGFR), eGFR decline
Introduction
Children with chronic kidney disease (CKD) experience increased morbidity and mortality from co-morbidities unique to the pediatric population, resulting in a reduced life expectancy and health-related quality of life.1 Age-specific mortality rates for children treated by dialysis are more than 130-fold greater than the general US population,2 predominantly attributable to underlying cardiovascular disease.3 Traditional biomarkers of CKD and its progression in children include proteinuria, hypertension, and serum creatinine, with the latter the primary factor used in the estimation of glomerular filtration rate (eGFR). Limited epidemiological studies of CKD progression in children have shown renoprotective effects of renin-angiotensin-aldosterone system (RAAS) blockade for hypertensive and/or proteinuric CKD patients.4 However, approximately 60% of pediatric CKD is due to congenital anomalies of the kidney and urinary tract (CAKUT) which is less frequently accompanied by hypertension or proteinuria. Thus, while hypertension and proteinuria are important modifiable markers of CKD progression, they are not universal, and they tend to develop later in disease progression. The discovery of novel biomarkers that precede the development of these late clinical markers may lead to earlier intervention, risk stratification of high-risk individuals, and/or novel therapeutic targets, which may subsequently translate into improved patient outcomes.
Soluble urokinase-type plasminogen activator receptor (suPAR) is a circulating biomarker derived from the cleavage of the urokinase-type plasminogen activator receptor (uPAR) known to be expressed in podocytes, endothelial cells, and immune cells.5 SuPAR has been closely linked with inflammation in many different disease states,6 and has shown promise as an emerging CKD biomarker. Schaefer and colleagues recently performed a post hoc analysis of 898 subjects from two well-described European pediatric CKD cohorts, the ESCAPE Trial and the 4C Study, and found that serum suPAR was associated with an increased risk of CKD progression, especially in children with milder disease (in those with estimated glomerular filtration rate (eGFR) > 40 mL/min/1.73m2, the hazard ratio (HR) of 5.12 [95% CI, 1.56–16.7] was for each unit greater log-transformed suPAR).7 The association observed was independent of kidney disease etiology, important in children where the vast majority of CKD is due to non-glomerular disease. These intriguing results complement findings from adult studies8–11 and bear replication in additional cohorts. Furthermore, the reliability of suPAR over repeated measures remains to be established. We hypothesize that elevated plasma suPAR levels are associated with more rapid decline in kidney function in children with existing CKD even after adjustment for traditional CKD risk factors. We further hypothesize that suPAR levels will demonstrate acceptable reliability over longitudinal analysis.
Methods
This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement checklist for observational studies.12
Participants
The Chronic Kidney Disease in Children (CKiD) study is a prospective multicenter observational cohort study of children with mild to moderate CKD. The CKiD study design and methods have previously been published.13 Participants ages 1–16 years with an eGFR between 30 and 90 mL/min/1.73m2 were enrolled from 55 pediatric nephrology centers across North America. The study is registered with (ClinicalTrials.gov with identifier number NCT00327860. Informed consent was obtained at each study site through the Institutional Review Board (IRB), and the study design and conduct was overseen by an observational study monitoring board appointed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The Children’s Mercy Hospital Pediatric IRB approved this study. Participants underwent annual study visits that included demographic and clinical components, physical examination, and laboratory values; the majority of participants additionally consented to allow laboratory samples to be stored in a biorepository for future studies. Eligible individuals for this current analysis were participants with sufficient biorepository sample volumes available from both the baseline and follow-up visit six months later.
Laboratory
Biorepository specimens were stored at −80° C until assays were performed. The measurements of plasma suPAR were performed in duplicate using Quantikine ELISA immunoassay from R&D Systems, Minneapolis, MN, USA (Catalog Number DUP00). The intra-assay coefficient of variation (CV) ranged from 2.7–3.9% and inter-assay CV was 8.3–8.8%. Serum creatinine, urine protein and urine creatinine concentrations were analyzed in a central location using standard laboratory techniques. eGFR was calculated using the full CKiD estimating equation based on serum creatinine, cystatin C, and SUN concentrations (when available)14 and the bedside CKiD equation using height and serum creatinine15 if all analytes unavailable. Over the course of the CKiD study, the C-Reactive Protein (CRP) assay transitioned from wide range CRP (wr-CRP) to high sensitivity-CRP (hs-CRP) in November 2010; consequently, the hs-CRP method was used when available and wr-CRP used otherwise.
Covariates
Proteinuria was categorized based on the urinary protein-creatinine ratio (UPCR) as < 0.5 mg/mg, 0.5 to <2.0 mg/mg, or ≥2.0 mg/mg. Elevated blood pressure (BP) was defined as casual systolic or diastolic blood pressure at baseline greater than the 95th percentile for age and sex.16 Primary diagnoses of CKD were categorized as non-glomerular (obstruction/reflux, hypoplasia/dysplasia, cystic disease, pyelonephritis/interstitial nephritis, other non-glomerular disease) or glomerular (focal segmental glomerular sclerosis, familial nephritis, hemolytic uremic syndrome, other glomerular disease). Other covariates of interest and known traditional risk factors for CKD progression consisting of eGFR, body mass index (BMI), anemia, ACE inhibitor/ARB use, CRP, serum albumin, serum bicarbonate level, and LDL cholesterol were assessed. All covariates were measured at the baseline visit.
Statistical analysis
We summarized continuous variables as median [interquartile range (IQR)] as appropriate for skewed data, in the overall cohort and by plasma suPAR quartiles. We expressed categorical variables as frequencies and proportions. We compared median values and proportions of biochemical parameters between plasma suPAR quartiles using Kruskal-Wallis test for continuous variables and Chi-square test for categorical variables.
The primary endpoint of the study was a composite endpoint defined as the initiation of kidney replacement therapy (KRT, dialysis or transplantation) or > 50% decline in eGFR. Subjects who did not receive RRT (n=108) or experience a >50% decline in eGFR (n=77) were censored on the date of their last regularly attended follow-up visit (n=380). Kaplan-Meier renal survival curves stratified by suPAR quartiles were used to plot the empirical survival by quartile of plasma suPAR. Log-normal survival models were used to model relative time to composite event as a function of plasma suPAR quartiles. A log-normal regression approach was chosen because this parametric approach provides a clinically meaningful method with which to communicate results, as the strength of the association can be interpreted as the relative change in length of time until the average participant experiences the composite event.17 For example, a 0.5 relative time would indicate halving of the event time comparing exposed to unexposed. In addition prior CKiD investigations have found a lack of proportionality of the hazards with respect to important exposures including proteinuria for the outcome of RRT.18 Model 1 evaluated the association of suPAR quartiles with relative time to event, adjusted for baseline demographic factors of age, sex, race, and ethnicity. Model 2 further adjusted for covariates known to affect CKD progression, including body mass index, hypertension (systolic blood pressure age-sex specific percentiles), use of antihypertensive medications, proteinuria, and glomerular diagnosis. Model 3 additionally adjusted for eGFR. Similar secondary analyses were explored with an alternative endpoint of RRT or a 30% decline in eGFR, as well as using Cox proportional hazards to allow for comparison of effect estimates to prior studies. Finally, the six month change in plasma suPAR levels was examined to establish short-term variability of the biomarker. The Pearson correlation coefficient between the baseline and follow-up plasma suPAR levels was estimated and comparisons made using Bland Altman analysis.
Results
Demographic and clinical characteristics are presented in Table 1. Of the 565 children included, 60.4% were male, 20.9% were black, and 11.9% were of Hispanic ethnicity, with a median age of 12 years at enrollment. The median eGFR was 53.1 (IQR, 40.8–66.6) mL/min/1.73m2 at baseline. The median suPAR level at baseline was 3,204 (IQR, 2,627–3,785) pg/mL. Across all patients, 33% (N=185; 108 reaching RRT and 77 reaching >50% eGFR decline) experienced the composite endpoint after a median 4 (IQR, 2–6) years of observation (Table S1).
Table 1.
Demographic and clinical characteristics of the baseline study population, by plasma suPAR quartiles.
| Characteristic | Entire Cohort | Baseline plasma SuPAR quartile (pg/mL) | ||||
|---|---|---|---|---|---|---|
| Quartile 1 (<2,627) | Quartile 2 (2,627-3,204) | Quartile 3 (3,204–3,785) | Quartile 4 (>3,785) | p | ||
| Age (years) | 12 [8, 15] | 15 [11, 16] | 11 [8, 14] | 11 [7, 13] | 10 [7, 13] | <.001 |
| Male sex | 341 (60.4%) | 97 (68.8%) | 90 (63.8%) | 83 (58.9%) | 71 (50%) | 0.01 |
| Black race | 118 (20.9%) | 40 (28.4%) | 23 (16.3%) | 31 (22.0%) | 24 (16.9%) | 0.05 |
| Hispanic ethnicity | 67 (11.9%) | 9 (6.4%) | 14 (9.9%) | 19 (13.5%) | 25 (17.6%) | 0.03 |
| BMI (kg/m2) | 19 [16, 22] | 20 [18, 24] | 18 [16, 22] | 18 [16, 21] | 18 [16, 23] | 0.001 |
| Height SDS < −2 | 60 (10.6) | 14 (9.9) | 10 (7.1) | 12 (8.5) | 24 (16.9) | 0.04 |
| Elevated BPa | 179 (31.7%) | 36 (25.5%) | 44 (31.2%) | 44 (31.2%) | 55 (38.7%) | 0.09 |
| Antihypertensive use | 364 (64.4%) | 89 (63.1%) | 86 (61.0%) | 89 (63.1%) | 100 (70.4%) | 0.4 |
| Proteinuria | 0.002 | |||||
| <0.5 mg/mg | 320 (56.6%) | 94 (66.7%) | 89 (63.1%) | 76 (53.9%) | 61 (43.0%) | |
| 0.5–2 mg/mg | 164 (29.0%) | 34 (24.1%) | 30 (21.3%) | 49 (34.8%) | 51 (35.9%) | |
| ≥2 mg/mg | 61 (10.8%) | 10 (7.1%) | 16 (11.3%) | 14 (9.9%) | 21 (14.8%) | |
| Glomerular diagnosis | 173 (30.6%) | 60 (42.6%) | 44 (31.2%) | 38 (27.0%) | 31 (21.8%) | 0.001 |
| eGFR (mL/min per 1.73m2) | 53.1 [40.8, 66.6] | 64.4 [52.8, 80.2] | 57.8 [47.5, 70.4] | 50.0 [40.3, 60.4] | 40.4 [33.3, 52.5] | <.001 |
| Anemia b | 176 (31.2%) | 37 (26.2%) | 36 (25.5%) | 46 (32.6%) | 57 (40.1%) | 0.03 |
| Elevated CRP (>3 mg/L) c | 96 (17.0%) | 16 (11.3%) | 17 (12.1%) | 29 (20.6%) | 34 (23.9%) | 0.007 |
| LDL cholesterol | 96 [76, 115] | 91 [73, 112] | 95 [77, 117] | 98 [81, 117] | 99 [73, 115] | 0.3 |
| Acidosis (CO2 < 22 mmol/L) | 291 (51.5%) | 47 (33.3%) | 78 (55.3%) | 79 (56%) | 87 (61.3%) | <.001 |
| Hypoalbuminemia (<3.8 g/dL) | 47 (8.3%) | 11 (7.8%) | 14 (9.9%) | 9 (6.4%) | 13 (9.2%) | 0.7 |
Elevated blood pressure defined as casual systolic or diastolic blood pressure > 95th percentile for age and sex.
Anemia defined as hemoglobin < 5th percentile for age and sex or erythropoietin stimulating agent (ESA) medication use.
hsCRP used for n=232 and wrCRP used for n=314
BP, blood pressure
Figure 1 depicts the unadjusted nonparametric Kaplan-Meier curve of renal survival, stratified by plasma suPAR quartile. Across all patients, 8-year renal survival was 55% (95% CI, 50%−61%). Participants in the highest suPAR quartile (quartile 4) demonstrated more rapid progression of CKD over time, with a renal survival of only 48% after 8 years of observation, as compared to a renal survival of 66% in the lowest suPAR quartile (quartile 1, p=0.002). Put another way, an individual in the highest suPAR quartile will reach the study endpoint 6.1 (95% CI, 2.6–9.7) years earlier than an individual with suPAR values in the lowest quartile. Curves were similar when stratified by CKD diagnosis into glomerular and non-glomerular diagnoses (Figure S1).
Figure 1.
Unadjusted nonparametric Kaplan-Meier curves of progression to the composite event of either kidney replacement therapy or >50% eGFR decline, by baseline plasma suPAR quartiles. Solid line represents Quartile 1 (< 2,627 pg/mL); dashed line represents Quartile 2 (2,267 to <3,204 pg/mL); dot-dashed line represents Quartile 3 (3,204 to <3,785 pg/mL; and two-dashed lines represents Quartile 4 (≥3,785 pg/mL). The lognormal fit is shown as an overlay (gray lines).
A total of 521 participants were available for lognormal regression models, with a total of 44 participants omitted due to missing ethnicity (n=5), systolic blood pressure percentile (n=18), BMI (n=1), and proteinuria (n=2). In multivariable analysis (Table 2), higher quartiles of plasma suPAR were consistently associated with faster times to event as compared to the first quartile. Specifically, in Model 1 adjusted for demographic factors, participants within the highest suPAR quartile (quartile 4) reached the composite outcome at a 63% faster rate than participants in suPAR quartile 1 (relative time (RT), 0.37; 95% CI, 0.27–0.51). Age and male sex were also significantly associated with the outcome (Model 1). Although Hispanic ethnicity demonstrated a univariate trend, with a higher prevalence of Hispanic ethnicity in the highest suPAR quartile, this lost significance during adjustment for other demographic factors in Model 1 (p=0.6). Model 2 further adjusted for traditional risk factors (BMI, elevated BP, and proteinuria) for CKD progression. Quartiles of plasma suPAR remained at similar magnitudes of association compared to the minimally adjusted model (RT, 0.46 [95% CI, 0.34–0.61] for quartile 4 versus quartile 1). In Model 3, the addition of eGFR was noted to attenuate the association between plasma suPAR and development of the composite event, with only a 33% faster progression in patients within suPAR quartile 4 compared to quartile 1, although the association remained statistically significant (RT, 0.67; 95% CI, 0.50–0.90). A sensitivity analysis with inclusion of CRP as a covariate due to the potential confounding from underlying inflammation did not yield substantially different results. Similar results were observed when an alternate endpoint of RRT or a 30% decline in eGFR was used (Table S2). For comparison to previous studies, results from Cox proportional hazards models are shown in Table S3. Results provide a similar inference to the main analysis, with increasing hazard of the event with each higher quartile of suPAR, with a hazard ratio of 2.09 (95% CI, 1.04–4.21) observed in quartile 4 compared to quartile 1. The analysis using continuous suPAR did not show statistically significant associations, although effect estimates indicated a 45% increased risk with each unit change in log-transformed suPAR concentration. The Kaplan Meier curves suggested modest non-proportionality; adding interaction terms with time to the models as a method to address non-proportionality revealed improvement in some, but not all, models.
Table 2.
Relative time to composite event of kidney replacement therapy or >50% decline in eGFR
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| RT (95% CI) | p | RT (95% CI) | p | RT (95% CI) | p | |
| SuPAR | <0.001 | <0.001 | 0.05 | |||
| Quartile 2 vs 1 | 0.60 (0.44, 0.82) | 0.001 | 0.66 (0.50, 0.88) | 0.004 | 0.75 (0.57, 1.00) | 0.05 |
| Quartile 3 vs. 1 | 0.47 (0.35, 0.64) | <0.001 | 0.56 (0.42, 0.74) | <0.001 | 0.71 (0.54, 0.94) | 0.02 |
| Quartile 4 vs. 1 | 0.37 (0.27, 0.51) | <0.001 | 0.46 (0.34, 0.61) | <0.001 | 0.67 (0.50, 0.90) | 0.008 |
| Age (per 1 y older) | 0.92 (0.89, 0.94) | <.001 | 0.92 (0.89, 0.94) | <.001 | 0.93 (0.91, 0.96) | <.001 |
| Male sex (v. female) | 0.79 (0.64, 0.97) | 0.02 | 0.80 (0.67, 0.96) | 0.02 | 0.77 (0.64, 0.93) | 0.005 |
| Black race (vs nonblack) | 0.80 (0.64, 1.01) | 0.06 | 0.81 (0.66, 1.01) | 0.06 | 0.75 (0.60, 0.93) | 0.008 |
| Hispanic (v. nonHispanic) | 0.93 (0.69 1.25) | 0.6 | 1.09 (0.83, 1.43) | 0.5 | 1.08 (0.83, 1.41) | 0.6 |
| BMI (per 1 kg/m2 increase) | 1.03 (1.01, 1.04) | 0.002 | 1.01 (0.99, 1.03) | 0.2 | ||
| SBP | 0.05 | 1 | 0.06 | |||
| ≤ 50th percentile | 1.00 (reference) | -- | 1.00 (reference) | -- | ||
| 50th-90th percentile | 0.94 (0.76, 1.15) | 0.5 | 0.98 (0.79, 1.20) | 0.8 | ||
| > 90th percentile | 0.75 (0.59, 0.95) | 0.02 | 0.77 (0.60, 0.98) | 0.03 | ||
| Antihypertensive use | 0.85 (0.70, 1.04) | 0.1 | 0.89 (0.73, 1.09) | 0.3 | ||
| Proteinuria | <0.001 | <0.001 | ||||
| < 0.5 mg/mg | 1.00 (reference) | -- | 1.00 (reference) | -- | ||
| 0.5–<2.0 mg/mg | 0.65 (0.54, 0.80) | <0.001 | 0.71 (0.58, 0.86) | 0.001 | ||
| ≥2 mg/mg | 0.37 (0.29, 0.49) | <0.001 | 0.42 (0.32, 0.54) | <0.001 | ||
| Glomerular diagnosis (v. non-glomerular) | 0.89 (0.72, 1.12) | 0.3 | 0.76 (0.60, 0.95) | 0.02 | ||
| eGFR (per 10 mL/min/1.73m2 lower) | 0.81 (0.76, 0.86) | <.001 | ||||
based on lognormal parametric survival models among 521 participants contributing 171 events.
Model 1: Minimally adjusted: age, sex, race
Model 2: Model 1 plus BMI, hypertension (systolic BP percentiles), antihypertensive use, proteinuria, glomerular diagnosis
Model 3: Model 2 plus eGFR
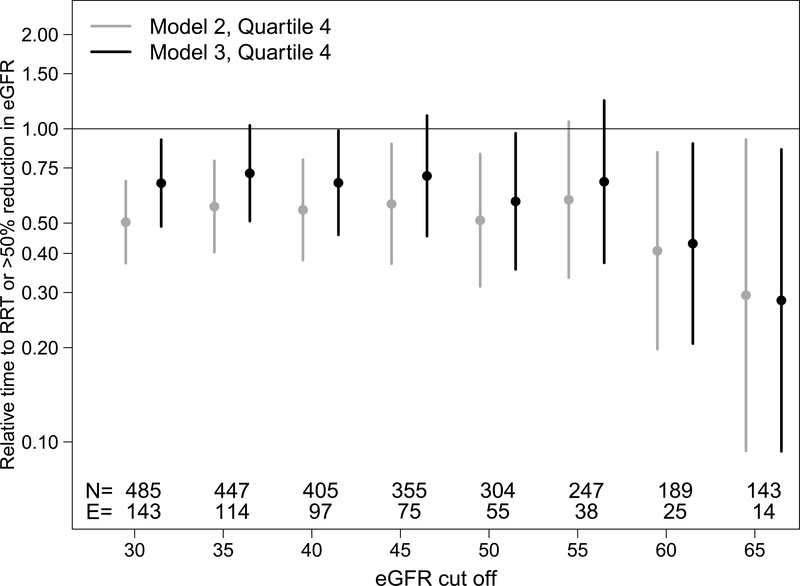
To further explore the nature of the association between suPAR and progression as a function of baseline eGFR, a descriptive analysis examined the trend in effect size magnitude across 5 mL/min/1.73m2 increments of eGFR. As shown in Figure 2, effect estimates comparing Quartile 4 to Quartile 1 of suPAR remained stable and modest as eGFR increased until approximately a eGFR of 60 mL/min/1.73m2. Adjustment for eGFR (model 3) tended to attenuate suPAR effect estimates. Beyond 60 mL/min/1.73m2, the strength of association between suPAR and progression strengthened and the addition of eGFR to the model no longer substantially attenuated estimates. This analysis suggested that suPAR may be most meaningful for progression risk prediction when eGFR is above 60 mL/min/1.73m2. A test for heterogeneity was performed with adding an interaction term of suPAR x eGFR and was not significant.
Figure 2.
Relative times (95% confidence intervals) of SuPAR quartiles to composite event of kidney replacement therapy or >50% decline in eGFR. Each grouping of relative times are from lognormal parametric survivals models among participants who have eGFR > the cut off displayed on the x-axis. The N’s and number of events (E) in each eGFR cut off group are shown at the bottom of the figure. Circles represent relative times of SuPAR Quartile 4 compared to Quartile 1. Gray symbols are adjusted relative times from model 2 (adjusting for age, sex, race, ethnicity, hypertension (systolic BP percentiles), antihypertensive use, BMI, proteinuria, glomerular diagnosis) and black symbols are the adjusted relative times of SuPAR from model 3 (also adjusting for eGFR).
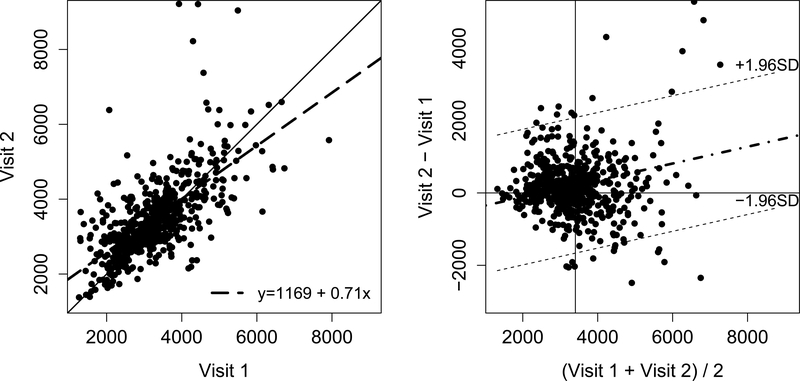
Finally, we examined the short-term variability of suPAR as a CKD biomarker by comparing repeated measurements taken over a period of six months. The median plasma suPAR was 3204.4 pg/mL at visit 1 and 3335.3 pg/mL at visit 2, with a Pearson correlation of 0.589 indicating a moderate positive correlation. A Bland-Altman plot shows relatively good agreement of plasma suPAR levels between visits 1 and 2 (Figure 3). A comparison of the standard deviations indicated no expanding or compressing of the distribution.
Figure 3.
Scatterplot and Bland-Altman plot comparing the plasma SuPAR concentration distributions at baseline visit and the second visit, six months later. There are 2 points in the visit 2 distribution >13000 pg/mL that are not shown. In the first panel, the solid line indicates the identity line and the dashed line indicates the regression line between SuPAR concentration at baseline visit and the second visit. In the second panel, the dashed-dotted lines indicates the regression line while the dashed lines represent the 95% confidence limits (+/− 1.96 standard deviations).
Discussion
In this study, we characterized the association between plasma suPAR and CKD progression in a prospective cohort of pediatric patients with mild to moderate chronic kidney disease. We demonstrated that individuals in the highest baseline plasma suPAR quartile experienced 33% faster CKD progression as compared to the individuals in the lowest quartile even after adjustment for known CKD progression risk factors. This is one of the first studies to analyze the association of suPAR with CKD progression in children, as well as report on reliability measurements through the use of repeated biomarker measures.
There has been increasing awareness of suPAR’s association with kidney disease, with initial interest in its potential role as the underlying permeability factor in the pathogenesis of focal segmental glomerulosclerosis.5,19 More recent attention has focused on its association with incident CKD and CKD progression in adult8,9 and pediatric7 cohorts, as well as with higher mortality and adverse cardiovascular outcomes in adult hemodialysis patients.20 Our results are generally consistent with these prior investigations. Prior studies have shown significant heterogeneity in the association between suPAR and progression across a wide range of GFR, but disagree as to the best breakpoint. Hayek et. al. found the strongest effect for individuals with eGFR > 90 mL/min/1.73m2, whereas Schaefer and colleagues noted the most robust associations for eGFR > 40 mL/min/1.73m2. In our study, an interaction term of suPAR × eGFR was not statistically significant, although this is unsurprising as the interaction assumes a linear incremental effect of eGFR on the relationship and by visual inspection the associations across the eGFR spectrum do not appear linear. The relationship between suPAR and CKD progression is somewhat modest and strongly attenuated by eGFR adjustment until around a GFR of 60 mL/min/1.73m2, at which point the relationship actually strengthens and is much less affected by eGFR adjustment. Our findings generally agree with prior studies that plasma suPAR levels may be most useful as a biomarker in patients with reasonable preservation of their eGFR, perhaps due to its role in subclinical inflammatory activity.21 Inferences were similar using Cox models for categorical suPAR, with an approximate doubling of the hazard for the highest suPAR quartile, although were not statistical significant for the continuous suPAR, likely due to lack of proportionality which was suggested with the Kaplan Meier curves and perhaps also due non-linearity.
Novel biomarkers with the ability to identify patients at high risk of CKD progression are urgently needed, particularly in children in whom the existing traditional biomarkers of elevated serum creatinine, hypertension, and proteinuria are observed relatively late in the disease process. SuPAR is a potential biomarker with biological plausibility due to its role as an inflammatory mediator6 which is known to interact in several key signaling pathways involved in renal fibrosis.22 There is accumulating evidence that suPAR may play an important role as a signaling molecule in the pathogenesis of kidney disease through its activation of podocyte β(3) integrin.23–27 There exists great interest in stimulation of novel therapeutics that target these underlying pathophysiological processes and may one day be used to alleviate CKD progression.28,29 Although rigorous validation of potential biomarkers is necessary, they remain woefully understudied, especially in the pediatric population. A recent review of emerging biomarkers in pediatric CKD discovered only 17 studies in children, all of which were cross-sectional, and only one of which had a sample size greater than 100.30
The median value noted in the CKiD population of 3,204 pg/mL is lower than the value of 5,658 pg/mL reported by Schaefer et. al. in the ESCAPE and 4C Study cohorts. We attribute the difference mostly likely to the higher baseline eGFR of 53 mL/min/1.73m2 in our patients as compared to 34 mL/min/1.73m2 in ESCAPE and 4C the Study. Advancing CKD leads to declining filtration capacity and impaired clearance of multiple biomarkers, presumably including plasma suPAR, which may lead to an inverse relationship between suPAR and eGFR. An alternate explanation could be due to different assays used. The suPAR values we observed are slightly higher than those observed in other healthy pediatric populations31–33 although comparable to levels seen in high-risk adult patients who later developed incident CKD.8 We postulate that the relatively small increase in suPAR levels over time may be explained by the slow decline in GFR experienced in the cohort, with average annual GFR changes of −1.5 and −4.3 mL/min/1.73m2 for non-glomerular and glomerular etiologies, respectively.18,34 Although other serum biomarkers of low-level inflammation have been validated as reliable, with good to excellent intra-class correlation coefficients over multiple time points,35 there remains a dearth of evidence regarding the reliability of suPAR as a biomarker with repeat measures over time. Prior work has shown suPAR to be stable in plasma over several days with little to no circadian rhythm,35 and levels remain stable despite multiple freeze-thaw cycles.36 We are reassured at the reproducibility of suPAR in our study over time which enhances further validity to its potential use as a CKD biomarker. Additional measures of plasma suPAR over several months to years of observation may be worth examining in future studies of CKiD and other similar cohorts.
It bears mentioning that, although multiple commercially available suPAR assays exist, current use remains limited to the research realm and suPAR measurements are not currently used in routine clinical practice. There is extremely limited data regarding comparability regarding various biomarker assays, although one investigation in the sepsis literature comparing a Luminex (8-plex) assay and suPAR ELISA showed a correlation coefficient of 0.95 with a 95% limit of agreement between 99–140%.37 Further validation work and direct comparison of assays should be pursued in future studies to ensure consistency across multiple assays.
A number of limitations must be acknowledged. We report only on observed associations and cannot infer conclusions related to causality. Despite careful adjustment for known confounders of CKD progression, we cannot rule out the possibility for residual confounding. We attempted to control for the potential of underlying inflammation with the use of adjustment for CRP, although acknowledge that the transition from the use of wrCRP to hsCRP over the course of the CKiD study posed an additional challenge. Prior studies indicate excellent agreement between hsCRP and wrCRP with a bias of only −0.11 ± 0.17 mg/L.38 Inclusion of CRP into our multivariate analysis did not substantially change our results, so it was excluded for simplicity. A relatively small percentage of missing data regarding ethnicity, blood pressure, BMI, or proteinuria was noted (in 8% of the cohort) which resulted in a smaller sample size of 521 participants available for lognormal regression models. Finally, our findings were observed in a patient cohort with established CKD, and although we note tantalizing findings with regards to utility in the higher eGFR range we cannot generalize our results to healthy children with normal eGFR. The main strengths of our study are related to long-term prospective follow-up, large sample size of one of the largest prospective cohorts of children with CKD with an ethnically diverse cohort, use of repeated measurements, and the relatively high incidence of the composite endpoint of the study.
In summary, we found that elevated concentrations of plasma suPAR are independently associated with CKD progression in children with mild to moderate CKD. Although the strength of the association observed was strongly dependent on baseline eGFR, the association remains significant for the highest quartile of plasma suPAR after adjustment for traditional CKD risk factors. Plasma suPAR levels show little change over six months of observation in CKD patients. Our study adds to the growing body of evidence supporting a strong association between suPAR and CKD progression in children, although future studies are necessary to determine whether suPAR may one day become an important clinical biomarker to predict future risk of CKD progression, particularly at the earliest stages of GFR decline, when traditional biomarkers may have their greatest limitations.
Supplementary Material
Figure S1. Unadjusted nonparametric Kaplan-Meier curves of CKD progression, by glomerular vs nonglomerular diagnosis.
Table S1. Follow-up duration and number of events in overall cohort and stratified by suPAR quartiles.
Table S2. Relative time to composite event of KRT or >30% decline in eGFR based on log-normal parametric survival models.
Table S3. Cox proportional hazards for progression to KRT or 50% decline in eGFR, overall and stratified by eGFR <40 and >40 mL/min/1.73 m2.
Acknowledgements:
We are grateful to Tarak Srivastava of the Children’s Mercy Research Laboratory for performing plasma suPAR assays; additionally, we want to acknowledge the substantial contribution of all of the investigators and coordinators in CKiD, in addition to all of the participating patients and their families.
Support: Direct funding for the study was provided by the Marion Merrell Dow Scholarship Award of the Children’s Mercy Hospital to Dr. Weidemann. The funding source had no role in the study design, collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication. The Chronic Kidney Disease in Children Cohort Study (CKiD) was conducted by the CKiD Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), with additional funding from the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). The data and samples from the CKiD study reported here were supplied by the NIDDK Central Repositories.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial disclosures.
Disclaimer: This article does not necessarily reflect the opinions or views of the CKiD study, the NIDDK Central Repositories, or the NIDDK.
Peer Review: Received _______. Evaluated by 3 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form November 4, 2019. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(3S1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141(2):191–197. [DOI] [PubMed] [Google Scholar]
- 3.Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol. 2012;23(4):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group ET, Wuhl E, Trivelli A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–1650. [DOI] [PubMed] [Google Scholar]
- 5.Zeier M, Reiser J. suPAR and chronic kidney disease-a podocyte story. Pflugers Arch. 2017;469(7–8):1017–1020. [DOI] [PubMed] [Google Scholar]
- 6.Backes Y, van der Sluijs KF, Mackie DP, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38(9):1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer F, Trachtman H, Wuhl E, et al. Association of Serum Soluble Urokinase Receptor Levels With Progression of Kidney Disease in Children. JAMA Pediatr. 2017;171(11):e172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayek SS, Sever S, Ko YA, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015;373(20):1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz CA, Persson M, Christensson A, et al. Soluble Urokinase-type Plasminogen Activator Receptor (suPAR) and Impaired Kidney Function in the Population-based Malmo Diet and Cancer Study. Kidney Int Rep. 2017;2(2):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo S, Coresh J, Tin A, et al. Soluble Urokinase-Type Plasminogen Activator Receptor in Black Americans with CKD. Clin J Am Soc Nephrol. 2018;13(7):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park MY, Herrmann SM, Saad A, et al. Biomarkers of kidney injury and klotho in patients with atherosclerotic renovascular disease. Clin J Am Soc Nephrol. 2015;10(3):443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. [DOI] [PubMed] [Google Scholar]
- 13.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 17.Cox C, Chu H, Schneider MF, Munoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26(23):4352–4374. [DOI] [PubMed] [Google Scholar]
- 18.Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis. 2015;65(6):878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei C, Trachtman H, Li J, et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23(12):2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drechsler C, Hayek SS, Wei C, et al. Soluble Urokinase Plasminogen Activator Receptor and Outcomes in Patients with Diabetes on Hemodialysis. Clin J Am Soc Nephrol. 2017;12(8):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijers B, Sprangers B. The hype cycle for soluble urokinase receptor in FSGS: passing the trough of disillusionment? Clin J Am Soc Nephrol. 2014;9(11):1835–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv W, Booz GW, Wang Y, Fan F, Roman RJ. Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. [DOI] [PubMed] [Google Scholar]
- 24.Wei C, Li J, Adair BD, et al. uPAR isoform 2 forms a dimer and induces severe kidney disease in mice. J Clin Invest. 2019;130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayek SS, Koh KH, Grams ME, et al. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23(8):945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leth JM, Leth-Espensen KZ, Kristensen KK, et al. Evolution and Medical Significance of LU Domain-Containing Proteins. Int J Mol Sci. 2019;20(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17(8):952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machowska A, Carrero JJ, Lindholm B, Stenvinkel P. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl Res. 2016;167(1):204–213. [DOI] [PubMed] [Google Scholar]
- 29.Mihai S, Codrici E, Popescu ID, et al. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J Immunol Res. 2018;2018:2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg JH, Kakajiwala A, Parikh CR, Furth S. Emerging biomarkers of chronic kidney disease in children. Pediatr Nephrol. 2018;33(6):925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirinoglu M, Soysal A, Karaaslan A, et al. The diagnostic value of soluble urokinase plasminogen activator receptor (suPAR) compared to C-reactive protein (CRP) and procalcitonin (PCT) in children with systemic inflammatory response syndrome (SIRS). J Infect Chemother. 2017;23(1):17–22. [DOI] [PubMed] [Google Scholar]
- 32.Wrotek A, Jackowska T, Pawlik K. Soluble urokinase plasminogen activator receptor: an indicator of pneumonia severity in children. Adv Exp Med Biol. 2015;835:1–7. [DOI] [PubMed] [Google Scholar]
- 33.Kosecik M, Dervisoglu P, Koroglu M, et al. Usefulness of soluble urokinase plasminogen activator receptor (suPAR) as an inflammatory biomarker in obese children. Int J Cardiol. 2017;228:158–161. [DOI] [PubMed] [Google Scholar]
- 34.Fathallah-Shaykh SA, Flynn JT, Pierce CB, et al. Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol. 2015;10(4):571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen O, Eugen-Olsen J, Kofoed K, Iversen J, Haugaard SB. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol. 2008;80(2):209–216. [DOI] [PubMed] [Google Scholar]
- 36.Riisbro R, Christensen IJ, Hogdall C, Brunner N, Hogdall E. Soluble urokinase plasminogen activator receptor measurements: influence of sample handling. Int J Biol Markers. 2001;16(4):233–239. [DOI] [PubMed] [Google Scholar]
- 37.Kofoed K, Schneider UV, Scheel T, Andersen O, Eugen-Olsen J. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem. 2006;52(7):1284–1293. [DOI] [PubMed] [Google Scholar]
- 38.Monneret D, Mestari F, Djiavoudine S, et al. Wide-range CRP versus high-sensitivity CRP on Roche analyzers: focus on low-grade inflammation ranges and high-sensitivity cardiac troponin T levels. Scand J Clin Lab Invest. 2018;78(5):346–351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Unadjusted nonparametric Kaplan-Meier curves of CKD progression, by glomerular vs nonglomerular diagnosis.
Table S1. Follow-up duration and number of events in overall cohort and stratified by suPAR quartiles.
Table S2. Relative time to composite event of KRT or >30% decline in eGFR based on log-normal parametric survival models.
Table S3. Cox proportional hazards for progression to KRT or 50% decline in eGFR, overall and stratified by eGFR <40 and >40 mL/min/1.73 m2.