Abstract
Introduction:
Immune checkpoint inhibitors (ICIs) are increasingly used to treat advanced cancer. Rheumatoid arthritis (RA) is associated with an increased risk for malignancies, however patients with RA have been excluded from ICI trials. In this study, we evaluated risk of toxicity after initiation of ICI treatment in RA patients.
Methods:
We conducted a single-institution, retrospective analysis to assess the incidence of immune-related adverse events (irAEs) and autoimmune disease (AID) flares among patients with autoimmune diseases treated with ICIs from 2011 to 2018. A subgroup analysis for RA patients was performed with frequencies of irAEs and AID flares reported.
Results:
22 patients with RA who were treated with ICI for malignancy were identified. At the time of ICI initiation, 86% had inactive RA disease activity. IrAEs occurred in 7 (32%) of patients, with 2 (9%) developing grade 3 (i.e., severe) irAEs. ICIs were temporarily discontinued due to irAEs in 5 (23%) patients, and permanently in one patient. RA flares occurred in 12 (55%) patients. Of those, 10 (83%) received oral corticosteroids with an adequate treatment response.
Conclusion:
Our analysis suggests that irAEs following ICI treatment are not increased among RA patients compared with other cancer patients. Heightened RA disease activity during ICI treatment is common, but most adverse events are manageable with oral corticosteroids and few require permanent ICI discontinuation. A close collaboration between the oncologist and rheumatologist is advisable when considering ICIs in patients with RA.
Keywords: Immune checkpoint inhibitor, rheumatoid arthritis, immune-related adverse events, autoimmune flare, PD-1, PD-L1, CTLA-4
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the therapeutic landscape of oncology. ICIs have been developed for three unique molecular targets – cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed-cell death protein 1 (PD-1), and programmed-death ligand 1 (PD-L1)–involved in the maintenance of immune tolerance. These agents have shown efficacy and been approved for use in a wide range of cancers, including non-small cell lung cancer (1), metastatic melanoma (2–4), and renal cell carcinoma (5), among others.
By blocking immunoregulatory pathways, ICIs also have the unintended but not unexpected side effect of autoimmune-like phenomena known as immune-related adverse events (irAEs)(6). The biology of irAEs has raised the concern that patients with pre-existing autoimmune diseases (AIDs) may experience more severe and potentially treatment-limiting irAEs than patients without autoimmune disease, or exacerbations of their underlying autoimmune illnesses. On this basis, patients with pre-existing AIDs have been largely excluded from clinical trials evaluating efficacy of immune checkpoint inhibitor therapy for treating malignancy (2, 6–9). While some clinicians nonetheless choose to administer ICIs in AID patients, the risks of irAE development and/or disease flare remain unclear.
Rheumatoid arthritis (RA) affects approximately 1% of the general population in the developed world (10). Importantly, RA is associated with more than double the risk of malignant lymphoma, and 60% increased risk of lung cancer, when compared to the general population (11). The CTLA-4 and PD-1/PD-L1 pathways have been implicated in the pathophysiology of RA (12–14), with several groups reporting that genetic polymorphisms in the PD-1 gene and CTLA-4 genes are associated with RA risk (15–17). Additionally, PD-1 expression levels have been shown to be increased on the surface of T lymphocytes from the blood and synovial fluid in RA patients with cells producing increased levels of IL-21. It is unclear, however, if increased PD-1 expression in RA is a compensatory mechanism to modulate disease via B cell T cell interactions (18). A recent study comparing the PD-1 pathway during different phases of RA progression also identified increased PD-1 transcription. Additionally, Matsuda et al showed increased PD-L1 transcription in T lymphocytes infiltrating synovium in RA patients with high disease activity, both suggesting a role for PD-1 and PD-L1 in RA pathogenesis.(12, 13). Furthermore, the CTLA-4 fusion protein abatacept is used to treat RA by promoting T cell inhibition, suggesting that native CTLA-4 on T cells may play an important role in down-modulating RA disease (19).
The implied role of PD-1 and CTLA-4 in modulating RA raises concerns that administration of ICIs might have particularly adverse implications for RA patients. For this reason, ICI treatment has not been formally studied in RA patients and ICI therapy is often either not offered or delayed due to concern for heightened toxicity (19). Available data has therefore been limited to case reports, small case series or reviews of case series (20–22). Here, we report the incidence of irAEs and RA flares after ICI treatment in a cohort of twenty-two patients with pre-existing rheumatoid arthritis. To our knowledge, this constitutes the largest retrospective analysis of ICI administration in RA patients.
Patients and Methods:
Using our institutional electronic medical record, we identified and collected clinicopathologic data of patients with pre-existing AIDs who had been treated for a malignancy with ICIs ipilimumab, pembrolizumab or nivolumab between April 1, 2011 and November 15, 2018. Eligible patients were over 18 years old, with documented AID diagnosis by ICD 9 and ICD 10 codes prior to initiation of ICI. Patients with asthma or hypothyroidism without evidence of autoimmune thyroiditis were excluded. Cancer diagnoses included melanoma 7 (32%), non-small cell 7 (32%), as well as gynecologic, urologic, renal and blood malignancies (Table1). In total, we identified 246 patients who received ICIs and carried a pre-existing AID diagnosis. After excluding patients who were diagnosed with autoimmune disease processes after ICI initiation, as well as those with non-autoimmune thyroiditis and gout, 84 patients met eligibility criteria with AIDs including rheumatologic 40 (48%), dermatologic 24 (29%), endocrine 16 (19%), gastrointestinal 14 (17%), neurologic 5 (6%) and hematologic diseases 1(1%). Of those, we identified 22 patients with a documented diagnosis of RA. The electronic record was reviewed for documentation of an RA diagnosis in accordance with the American College of Rheumatology (ACR) 2010 criteria when such information was available (Figure 1). In cases where no ACR criteria data was available, the patient was categorized as having RA if a diagnosis was documented by a rheumatologist or an oncologist who was in communication with rheumatologist and was coded with an ICD 9 or 10 code.
Table 1.
Rheumatoid arthritis patient characteristics: demographics, malignancy, immune checkpoint inhibitor therapy (ICI)
| N=22 (%) | |
|---|---|
| Age at cancer diagnosis, Median, years | 67 |
| Male | 6 (27) |
| Female | 16 (73) |
| RA disease duration prior to start of ICI, years | |
| >5 years | 10 (45) |
| <5 years | 2 (9) |
| Duration unknown | 10 (45) |
| Other autoimmune disease (AID) present | 7 (32) |
| Polymyalgia Rheumatica | 2 (9) |
| Chronic Inflammatory Demyelinating Polyneuropathy | 1 (5) |
| Psoriasis | 1 (5) |
| Sjogrens/Sicca^ | 3 (14) |
| Ulcerative Colitis | 1 (5) |
| Sarcoidosis^ | 1 (5) |
| Psoriatic Arthritis^ | 1 (5) |
| Disease Activity | |
| Inactive | 19 (86) |
| Active | 3 (14) |
| Immunomodulatory therapy at start of ICI* | |
| No treatment for AID | 6 (27) |
| Systemic corticosteroids | 12 (55) |
| Hydroxychloroquine | 3 (14) |
| Methotrexate | 7 (32) |
| Etanercept | 1 (5) |
| Sulfasalazine | 2 (9) |
| IVIG | 1 (1) |
| Prednisone equivalent dose (mg/day) | |
| >11–20 | 2 (9) |
| 5–10 | 7 (32) |
| <5 | 3 (14) |
| Malignancy Type | |
| Melanoma | 7 (32) |
| Lung Adenocarcinoma | 4 (18) |
| Squamous Cell Carcinoma of Lung | 3 (14) |
| Merkel Cell Carcinoma | 2 (9) |
| Ovarian Adenocarcinoma | 1 (5) |
| Squamous Cell Carcinoma of Head and Neck | 2 (9) |
| Urothelial Carcinoma | 1 (5) |
| Renal Cell Carcinoma | 1 (5) |
| Hodgkin Lymphoma | 1 (5) |
| Malignancy disease duration prior to ICI, median, months | 18 |
| Melanoma disease duration prior to ICI, median, months | 34 |
| ICI used | |
| Ipilimumab | 5 (23) |
| Nivolumab | 9 (41) |
| Pembrolizumab | 13 (59) |
One patient had inflammatory arthritis, diagnosed as seronegative RA/Psoriatic arthritis overlap, Sicca syndrome and Sarcoidosis
7 patients received more than one immunomodulatory agent simultaneously (i.e. methotrexate/hydroxychloroquine; prednisone/methotrexate)
Figure 1.
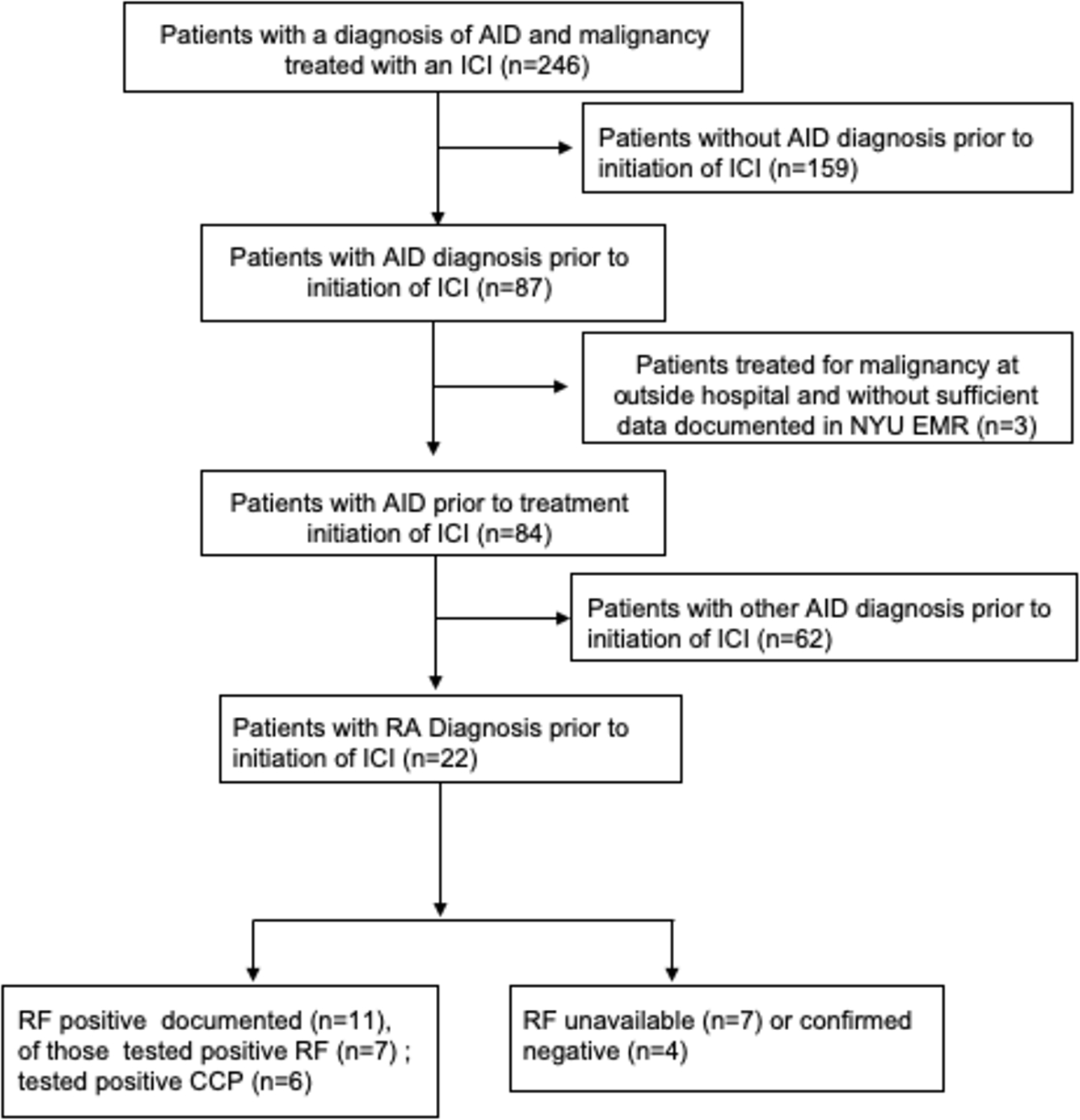
Sample recruitment and serologic characteristics
Abbreviations: AID - autoimmune disease, RA- rheumatoid arthritis, RF - rheumatoid factor antibody; CCP- cyclic citrullinated peptide antibodies; ICI - immune check-point inhibitors
Chart review was conducted for each patient including outpatient and inpatient records, medication prescriptions and documentation by treating oncologists and rheumatologists. Patients’ RA was classified as active at the time of ICI initiation if, 1) prior to receiving ICI, the patient exhibited symptoms of RA (joint pain, swelling, stiffness, and tenderness) and 2) the medication dose for RA was increased or new medications to control RA were prescribed within 1 month prior to ICI initiation. Medications for RA were categorized as immunomodulatory if they were systemic corticosteroids, methotrexate, biologics (TNF-α inhibitors, abatacept), hydroxychloroquine, sulfasalazine and JAK inhibitors. Medications categorized as supportive (i.e., not immunosuppressive) included topical steroids, lotions, thyroid hormone replacement therapy, NSAIDs, and anti-diarrheal medicines.
Primary outcomes included incidence of irAEs, and incidence of RA flares after ICI initiation, determined based on documentation by the treating oncologist and when available, by rheumatologist. The adverse reaction was categorized as an RA flare if there was documentation of synovitis by rheumatologist that was deemed to be a flare, or if the patient reported worsening pain and swelling in RA affected joints documented by the treating oncologist. An adverse event was categorized as an irAE if the patient experienced de novo symptoms not consistent with their pre-existing RA presentation (i.e. diarrhea, rash, other). IrAEs were classified by grade according to Common Terminology Criteria for Adverse events v5.0, with severe qualifying as grade 3 and above. If an irAE (eg. dermatitis) recurred in the same patient it was counted as a new event. The number of events for common irAEs was reported as frequencies. Incidence could not be determined for these patients as this was a retrospective analysis and time of exposure was not always clear. The secondary outcome was overall survival (OS), defined as number of months from initiation of ICI treatment to date of death or most recent follow up with a physician if date of death was unavailable. Descriptive statistics were used to summarize characteristics of our cohort which were reported using percentages for categorical variables and medians for continuous variables with interquartile range (IQR) reported for AID flares and the most common irAEs. Data was analyzed using STATA software. Our study was approved by the institutional review board of New York University School of Medicine.
Results:
Of 84 patients with pre-existing autoimmunity who developed malignancy and were treated with ICIs, 22 patients had RA. Of those, 11 had documented positive rheumatoid factor (RF) and 5 had a positive anti-citrullinated protein antibody (ACPA) (Figure 1). Among RA patients, 16 (73%) were female and 6 (27%) were male. Median age was 67 years. Seven (32%) patients carried another autoimmune diagnosis in addition to RA, including 3 with Sjogren’s syndrome (14%), 2 with polymyalgia rheumatica (9%) and 5 others (23%). One patient carried a diagnosis of inflammatory arthritis with a differential of seronegative RA, psoriatic arthritis, sarcoidosis and sicca syndrome (Table 1). Ten patients (45%) were diagnosed with RA more than 5 years prior to ICI start, two patients (9%) had RA disease duration of less than 5 years, while the rest of patients had no documentation of duration of their RA.
Most RA patients (86%) had no evidence of clinically active disease at the time of ICI initiation, as documented by their treating physician. In the majority of patients (96%) inflammatory markers were not checked at start of ICI therapy. Sixteen (73%) patients were nonetheless receiving RA immunomodulatory therapy at the start of ICI, with 12 (55%) patients receiving systemic corticosteroids and 7 (32%) patients on methotrexate. Of those receiving corticosteroids only 2 (9%) were on intermediate prednisone equivalent dose of 20 mg/daily, 4 (18%) patients received doses of 7.5mg-10 mg/daily, and 6 (27%) were on a very low dose of less than 5 mg/daily. Three patients were on methotrexate 25 mg weekly and 1 patient was receiving methotrexate 15 mg weekly and no corticosteroids. Two patients were receiving both methotrexate (10 mg and 12 mg weekly) and low dose prednisone (less than 5 mg daily). In 2 patients who were on methotrexate and low dose steroids prior to initiation of ICI, methotrexate was discontinued within 8 weeks prior to the start of ICI and they were continued on their oral corticosteroids only. All patients who were on corticosteroids for RA continued their current dose.
The most common cancer diagnoses were metastatic melanoma in 32% of patients and non-small cell lung cancer (NSCLC) in 32% of patients. At time of ICI initiation, all malignancies were Stage IV (86%) or III (14%) with median disease duration of 18 months. Melanoma patients had a median disease duration of 34 months, while for lung cancer median disease duration was 14 months. Twelve patients underwent surgical resection and 8 patients received chemotherapy within 3 months prior to ICI. Only three patients received ICI as first-line therapy. Thirteen patients (59%) were treated with pembrolizumab, 9 (41%) with nivolumab and 4 (18%) with ipilimumab. One patient received combination ICI therapy with ipilimumab and nivolumab, followed by monotherapy with pembrolizumab; 2 patients received sequential therapy of ipilimumab and either nivolimumab or pembrolizumab; and 1 patient received monotherapy with nivolumab and subsequently was switched to pembrolizumab (Table 1).
De novo irAEs occurred in 7 (32%) RA patients, with only 2 (9%) developing grade 3 irAEs. The most common toxicities were dermatitis in 4 (18%) patients and colitis in 3 (14%) patients. Median time to development of irAEs was 1 month (IQR=3.5); some patients developed irAEs as long as 9 months after ICI initiation. Four patients (57%) were treated for irAEs using oral corticosteroids (at doses between 20 to 60 mg/day) with slow taper. There were no irAE recurrences reported. Immunotherapy was temporarily discontinued due to irAEs in 5 (23%) patients. Only 1 patient required permanent ICI discontinuation after irAEs.
Frank RA flares occurred in 12 (55%) patients. Median time to flare was 1 month (IQR=1.75). Of those, who had an RA flare, 10 (83%) patients received oral corticosteroids for flare (8 – oral corticosteroids only, 2 - in combination with methotrexate or sulfasalazine). Two patients were continued on current immunosuppressive regimen with addition of supportive care with NSAIDs for their flare. Median prednisone equivalent dose for flare was 20 mg/daily. Four patients (33%) received prednisone equivalent dose of 40 mg/daily or higher, 4 patients (33%) received dose of less than 10 mg/daily, and 2 patients (17%) were on prednisone equivalent dose of 20 mg/daily. In 2 patients corticosteroids were stopped after resolution of flare, however most (8 patients) were slowly tapered over weeks to a lower dose or continued on the current dose (2 patients) due to persistent, albeit tolerable arthritis. Three patients had recurrent RA flare after improvement in initial symptoms, necessitating increase in corticosteroids dose or addition of a DMARD (sulfsalazine or methotrexate). All patients had close (weekly/biweekly) follow up with their oncologist and/or rheumatologist for symptomatic monitoring if active RA flare was present. ICIs were permanently discontinued due to flare in two patients (9%); however the majority continued to receive ICI therapy despite flare (Table 2). Inflammatory markers (ESR and CRP) were checked only in one patient at time of flare and were significantly elevated at 79 and 66 respectively. Overall, either RA flare, de novo irAE or both occurred in 16 (73%) patients. Median OS for patients with RA after start of ICI was 10.5 months.
Table 2.
Immune related adverse events (irAEs) and rheumatoid arthritis (RA) flares among RA patients receiving Immune checkpoint inhibitor therapy (ICI)
| Characteristic | N=22 (%) |
|---|---|
| Developed irAEs | 7 (32) |
| Did not develop irAEs | 15 (68) |
| Types of irAE | |
| Dermatitis | 4 (18) |
| Colitis | 3 (14) |
| Hepatitis | 1 (5) |
| Median time to irAE, months (IQR) | |
| Colitis | 3 (3.5) |
| Dermatitis | 1 (8.5) |
| All irAEs | 1 (3.5) |
| Severity of irAEs | |
| Grade 1–2 irAEs | 5 (23) |
| Grade 3 irAEs* | 2 (9) |
| irAE management | N=7 |
| Systemic corticosteroids | 4 (57) |
| Topical steroids/Supportive Treatment | 4 (57) |
| ICI use at time of irAE | |
| Permanently discontinued | 1 (5) |
| Temporarily discontinued | 5 (23) |
| ICI continued after irAE | 1 (5) |
| RA Flares | |
| Developed a flare | 12 (55) |
| Did not develop a flare | 10 (45) |
| Median time to flare, months (IQR) | 1 (1.75) |
| Flare treatment | N=12 |
| Oral prednisone | 10 (83) |
| NSAIDs | 2 (17) |
| Methotrexate^ | 1 (8) |
| Sulfasalazine^ | 1 (8) |
| Prednisone equivalent dose for flare (mg/day) | |
| >40 mg | 4 (33) |
| <20 mg | 6 (50) |
| ICI use at time of flare | |
| Permanently discontinued | 2 (9) |
| Temporarily discontinued | 1 (5) |
| ICI continued | 9 (41) |
no grade 4 or 5 irAEs occurred
Used in combination with oral prednisone
Abbreviations: IQR-interquartile range
Discussion:
In our cohort, a large number of RA patients (73%) experienced either a flare, irAE or both, which is consistent with retrospective reports of adverse events in patients with RA who received ICIs (20, 23, 24). Approximately 55% of RA patients experienced RA disease flare, and one-third of the patients had an irAE (not including RA flare) after initiation of ICI, with 9% accounting for grade 3/4 irAEs also consistent with current literature on RA patients (20, 24). In clinical trials, patients without pre-existing autoimmune disease experienced an adverse event of any grade at a rate of 60–90%, and grade 3 – 4 irAEs at a rate of 14–34% with higher severity reported in ipilimumab treated patients (25). It is worth noting that in our cohort a few patients had their RA immunosuppressive regimen de-escalated prior to ICI, which could lead to exacerbation of RA symptoms independent of immune activation of ICI therapy.
Also consistent with previously published retrospective reports, the majority (83%) of RA flares were treated using corticosteroids, with 80% responding well to treatment (20, 24). In our cohort, only 32% of patients required temporary discontinuation of ICI therapy and only 14% required permanent discontinuation of ICI therapy for either RA flare or irAE, which is lower than the reported rates of permanent ICI discontinuation in RA patients (20–35%) (3, 20, 25). One potential explanation is that as oncologists become increasingly experienced in the management of irAEs, many choose to continue ICI after an irAE to provide survival benefit in patients suffering from life-threatening malignant conditions. It should be noted that patients were followed closely by treating physicians and the majority used a slow steroid taper as their strategy for management of both flares and irAEs, with many patients continued on low dose steroids or maintenance DMARDs.
Our study has a number of limitations. Because of the retrospective nature of our data collection, ICI toxicities may not have been thoroughly documented when compared to prospective and randomized controlled trials. Most patients had inactive RA disease at entry, which might be associated with a lower incidence of flares and irAEs when compared to patients with active symptoms. As such, our data is reported as frequency because incidence rate could not be calculated without clear exposure risk data for all patients. Admittedly one of the challenging aspects of clinical practice is to differentiate worsening arthritis of underlying RA from development of a new irAE. Given inconsistent and limited involvement of rheumatologists in prior care of oncologic patients with AID, there is insufficient data about how treating physicians determined the etiology of arthritis. However, physicians’ documentation provided impression of the cause whether due to AID flare or de novo irAE. In addition, several patients received systemic chemotherapy within 3 months prior to ICI treatment and developed toxicities such as colitis, dermatitis and hepatitis soon after ICI. Given the lack of pathologic specimens in these cases, the possibility of delayed chemical toxicities in response to these earlier chemotherapies cannot be formally excluded, although the timing makes this possibility unlikely. These cases underscore the importance of a multidisciplinary approach to treat patients with malignancy and concomitant AIDs, involving rheumatologists, dermatologists and other specialists in early discussions of ICI therapy in patients with autoimmunity. Furthermore, despite including the largest group of RA patients reported to date, our cohort was small, and our patients were predominantly treated with anti-PD-1 agents, both of which may limit generalizability of these findings.
In conclusion, our data suggest that patients with RA do not experience more frequent or severe irAEs when treated with ICIs than patients without autoimmune disease. In addition, most irAEs and RA flares can be readily managed. Prospective studies are planned ( NCT03816345, NCT03656627) to determine whether ICIs are safe and effective for patients with RA as agents when used for an FDA approved malignancy indication as well as to ascertain clear incidence rates of de novo irAEs. Close collaboration between the oncologist and rheumatologist is advisable when considering ICIs in patients with rheumatic diseases (25).
Acknowledgements:
We would like to thank Michael H. Pillinger, MD for his assistance with review and editing of the manuscript.
Footnotes
Conflicts of Interest:
JW consults for and has received less than $10,000 dollars per annum from Merck, Genentech, Astra Zeneca, GSK, Novartis, Nektar, Medivation, Celldex, Incyte and EMD Serono and $10–25,000 dollars from BMS for membership on Advisory Boards, holds equity in CytoMx, Biond and Altor, is on a scientific adcisory board for Celldex, CytoMx, Incyte, Biond, Protean, CV6 and Sellas and was named on a patent from Moffitt Cancer Center on an ipilimumab biomarker and a PD-1 patent from Biodesix.
References:
- 1.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. New Engl J Med. 2018;379(24):2342–50. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016;2(2):234–40. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. [DOI] [PubMed] [Google Scholar]
- 11.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo YX, Walsh AM, Canavan M, Wechalekar MD, Cole S, Yin XF, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. Plos One. 2018;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda K, Miyoshi H, Hiraoka K, Hamada T, Yoshida S, Ishibashi Y, et al. Clinicopathological value of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) expression in synovium of patients with rheumatoid arthritis. Clin Exp Med. 2018;18(4):487–94. [DOI] [PubMed] [Google Scholar]
- 14.Raptopoulou AP, Bertsias G, Makrygiannakis D, Verginis P, Kritikos I, Tzardi M, et al. The Programmed Death 1/Programmed Death Ligand 1 Inhibitory Pathway Is Up-Regulated in Rheumatoid Synovium and Regulates Peripheral T Cell Responses in Human and Murine Arthritis. Arthritis Rheum-Us. 2010;62(7):1870–80. [DOI] [PubMed] [Google Scholar]
- 15.Westra HJ, Martinez-Bonet M, Onengut-Gumuscu S, Lee A, Luo Y, Teslovich N, et al. Fine-mapping and functional studies highlight potential causal variants for rheumatoid arthritis and type 1 diabetes. Nat Genet. 2018;50(10):1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karami J, Aslani S, Jamshidi A, Garshasbi M, Mahmoudi M. Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene. 2019;702:8–16. [DOI] [PubMed] [Google Scholar]
- 17.Tseng CC, Lin YZ, Lin CH, Li RN, Tsai WC, Ou TT, et al. Genetic and Epigenetic Alteration of the Programmed Cell Death 1 (PDCD1) in Rheumatoid Arthritis. Eur J Clin Invest. 2019:e13094. [DOI] [PubMed] [Google Scholar]
- 18.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter MD, Pinkston O, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Brief Report: Cancer Immunotherapy in Patients With Preexisting Rheumatic Disease: The Mayo Clinic Experience. Arthritis Rheumatol. 2018;70(3):356–60. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease RESPONSE. Ann Intern Med. 2018;169(2):133–4. [DOI] [PubMed] [Google Scholar]
- 21.Kyi C, Carvajal RD, Wolchok JD, Postow MA. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer. 2014;2(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B, Wong A, Kee D, Neeson P, Shackleton M, McArthur G, et al. The use of ipilimumab in patients with rheumatoid arthritis and metastatic melanoma. Ann Oncol. 2016;27(6):1174–7. [DOI] [PubMed] [Google Scholar]
- 23.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–76. [DOI] [PubMed] [Google Scholar]
- 24.Tison A, Quere G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients with Cancer and Preexisting Autoimmune Disease: A Nationwide Multicenter Cohort Study. Arthritis Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- 25.Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639–48. [DOI] [PubMed] [Google Scholar]


