Abstract
Purpose
In February 2020, the federal state of Tyrol in Austria has become one of the epicenters of the COVID-19 pandemic. Tyrol is known for numerous skiing areas. Thus, winter sport resorts became a starting point for COVID-19 infections spreading towards the rest of the state, Austria and other countries, leading to a mandatory quarantine for almost a million people, who were placed under a curfew and restrictions in daily life. Additionally, all ski resorts and hotels were closed. We aimed to analyze the influence of the COVID-19 quarantine on traumatic brain injury (TBI) cases in Tyrol.
Methods
We retrospectively compared demographical and injury characteristics from all TBI patients within the 2020 strict quarantine period with the respective time periods from 2016 to 2019. As our department is the only neurosurgical unit in Tyrol, all patients with moderate or severe TBI are transferred to our hospital.
Results
During 3 weeks of the full quarantine period, the weekly TBI cases load decreased significantly in comparison to the same time periods in the years 2016–2019. Furthermore, concomitant skull fractures decreased significantly (p < 0.016), probably reflecting different causative mechanisms. The other demographical and injury characteristics and particularly falls at home stayed relatively unchanged.
Conclusion
TBI remained an important contributor to the neurosurgical workflow during the COVID-19 pandemic. Strategies to ensure neurosurgical care also under pandemic-induced lockdown are important.
Keywords: Traumatic brain injury, COVID-19, Quarantine, Outcome
Introduction
Traumatic brain injury (TBI) remains the largest contributor to trauma-related mortality worldwide with considerable economic and social impact, particularly, for affected individuals, their families and socioeconomic systems [1]. Despite regional differences in incidence and severity across different areas of the world, TBI and its consequences result in a significant overall burden of disease [2]. Regional differences include patient characteristics, etiology and case management strategies [3, 4]. Severe TBI seems to occur with two incidence peaks in high-income countries. The first one in young adulthood (16–35 years) closely linked to active lifestyle and a second—rapidly progressing one—in the elderly who are often receiving polypharmacological therapy, especially oral anticoagulation [5–7]. A similar pattern has also been observed in Austria [8].
The state of Tyrol in Austria is internationally renowned for its popular winter sport resorts—especially skiing areas—and is hosting guests from all over the world with a seasonal peak during the Easter holidays. In 2018, around 5700 skiing accidents/year were observed in Tyrol [9]. TBI is the leading cause of death among skiers and snowboarders and accounts for 3–15% of winter sports-related injuries. In Austria, 5% of all TBIs are sports-related, whereas 50% are related to falls at home, especially in the elderly population [8, 10, 11]. Importantly, a significant proportion of sports-related TBI in Austria occurs to visitors with a seasonal peak in winter [12].
In December 2019, the first pneumonia cases of unknown origin were identified in Wuhan, China [13]. The identified pathogen, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rapidly emerged to become a global health concern also affecting acute surgical care [14–16]. In February 2020, Tyrol became increasingly affected by the coronavirus disease (COVID-19). A massive outbreak began in the district of Landeck, home to large and popular skiing areas. Due to the fast-increasing number of infections, the district of Landeck was put under quarantine on March 14th 2020, followed by the whole region of Tyrol on March 15th 2020. This resulted in the closure of all ski resorts, hotels and a curfew for all inhabitants, including a ban of outdoor sport activities. Furthermore, local regional and hospital task forces determined that all elective surgeries had to be postponed and only emergencies should be addressed in order to maintain ICU capacities for COVID-19 patients. These drastic measures to stem the pandemic also influenced the neurosurgical workflow, which tries to maximize the capability to provide neurosurgical care to patients while reducing the risk for health-care providers to a minimum [17, 18]. The aim of the present study was to evaluate the influence of the “COVID-19”—quarantine measures on neurosurgical caseload and injury pattern of TBI patients in the state of Tyrol during 3 weeks, in which the strictest quarantine rules were in place.
Methods
Setting
Strict quarantine sanctions in the federal state of Tyrol lasted from March 16th until April 6th, 2020. We retrospectively compared 3 weeks within the 2020 quarantine period with the respective time periods from 2016 to 2019. Importantly, March and April are within the peak season for winter sport tourism in Tyrol. As our department is the only neurosurgical unit in Tyrol, patients with moderate or severe TBI are transferred to our hospital, which is a level-1 trauma center by default. Mild TBI patients are presented teleradiologically by referring hospitals and/or are transferred if necessary.
Patients
Demographic variables, patient history [cause of TBI, initial Glasgow Coma Score (GCS)], type of surgery, lesions, anticoagulation therapy and mortality were collected from routine medical data records, and imaging data were obtained from initial CT scans. All patients were treated according to the Brain Trauma Foundation (BTF) guidelines [19, 20]. Lesions included epidural hematomas, acute subdural hematoma, chronic subdural hematoma (if the causative fall occurred within the observation period), diffuse axonal injury, intracerebral contusions, hemorrhage, and bone injuries of the skull, skull base or midface.
The study was approved by the local ethics committee of the Medical University Innsbruck (Protocol number: AN2020-1050).
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics [version 21 (IBM Corp., Armonk, New York, USA)] unless otherwise stated. The distribution of categorical variables across the different years was assessed with a chi-square and Fisher exact test. The Shapiro–Wilk test was used to assess normal distribution of continuous data. For normally distributed data, a 1-way ANOVA followed by a Dunnett’s post hoc test was done to compare all other years to 2020, whereas a Kruskal–Wallis test with a Dunn’s multiple comparisons test (GraphPad Prism 8) was used for non-parametric data.
Results
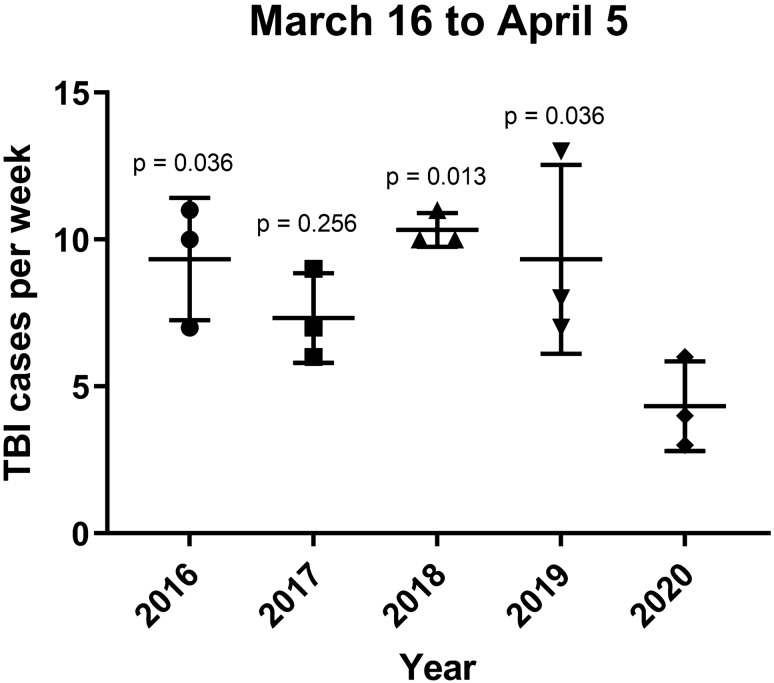
We identified 122 TBI patients (n = 73, 60% males) in the analyzed 3-week interval (March 16th–April 5th) within the 5 year period (2016–2020). Of those, 20 patients with mild TBI and minor injuries stayed in a peripheral hospital for clinical observation and repeated imaging examinations if indicated. During the strict quarantine in 2020, the weekly number of TBI patients was significantly decreased in comparison to 2016, 2018 and 2019 (Fig. 1).
Fig. 1.
Weekly number of TBI cases. In comparison to 2020 (mean 4.3, SD. 1.5), the number of weekly TBI cases in the study period was significantly lower in 2016 (mean 9.3, SD. 2.1, p < 0.036), 2018 (mean 10.3, SD. 0.6, p = 0.013), and 2019 (mean 9.3, SD. 3.2, p = 0.036), but not in 2017 (mean 7.3, SD. 1.5, p = 0.256). The previous years were compared to 2020 with a One-way ANOVA followed by a Dunnett’s post hoc test (p values are also shown in the graph)
The mean age at trauma was 58 years (range 0–96 years, IQR 43 years), with the oldest patient population presenting during the pandemic (only significantly different in comparison to 2016, p < 0.029, not the other years). In 86 patients, TBI was classified as mild (71%), in 8 patients as moderate (7%) and in 36 patients as severe (30%). 36 patients (30%) received anticoagulation therapy. The mortality rate was 11% (n = 13) among all patients (Table 1). In summary, there were hardly any statistically significant differences in the demographic categories between the years, but we did observe a significant decline in TBI cases per week within the quarantine period.
Table 1.
Baseline characteristics
| Year (n) | Age, years [median (IQR)] |
Male sex [n(%)] |
Initial GCS [median (IQR)] |
TBI grade I [n(%)] |
TBI grade II [n(%)] |
TBI grade III [n(%)] |
Oral anticoagulation [n(%)] |
Mortality [n(%)] |
|---|---|---|---|---|---|---|---|---|
| 2016 (28) | 47 (37)* | 20 (71) | 14 (6) | 17 (61) | 5 (18) | 6 (21) | 4 (14) | 5 (18) |
| 2017 (22) | 69 (49) | 11 (50) | 15 (2) | 18 (82) | 1 (5) | 3 (14) | 8 (36) | 1 (5) |
| 2018 (31) | 64 (49) | 18 (58) | 15 (6) | 21 (68) | 1 (3) | 9 (29) | 12 (39) | 3 (10) |
| 2019 (28) | 57 (33) | 18 (64) | 15 (3) | 21 (75) | 1 (4) | 6 (21) | 8 (31) | 2 (7) |
| 2020 (13) | 79 (33) | 6 (46) | 15 (2) | 9 (69) | 0 (0) | 4 (31) | 4 (31) | 2 (15) |
| Total (122) | 58 (43) | 73 (60) | 15 (2) | 86 (71) | 8 (7) | 28 (23) | 36 (30) | 13 (11) |
Baseline characteristics of TBI patients in the study period from March 16th–April 5th of all assessed years (2016–2020). The median age was significantly lower in 2016 compared to 2020 (*p < 0.029), apart from that there were no significant differences between the years
GCS Glasgow Coma Scale
Causes of TBI are demonstrated in Fig. 2. Notably, we observed a relative increase in low-impact falls at home, which occur predominately in the elderly, and no alpine sports-related TBI during the full lockdown period. The reduction in the weekly rate of sport injury-induced TBI was only significant in comparison to 2019 (p < 0.012) and not to the other years. Different TBI-related lesions and injuries as well as the need for operative management are shown in Table 2. During the COVID-19 pandemic, a significantly lower number of TBI patients had concomitant skull fractures.
Fig. 2.
Causes of TBI cases. Causes of TBI in the period from March 16th–April 5th of all assessed years (2016–2020) shown as percent of total cases per study period of each year. There were no significant differences between the years
Table 2.
Diagnosis and management
| Year (n) | EDH [n(%)] | Chronic SDH [n(%)] | Acute SDH [n(%)] | Contusion [n(%)] | IVH [n(%)] | Diffuse brain injury [n(%)] |
Skull fracture* [n(%)] | ICP monitoring [n(%)] | Surgery [n(%)] |
|---|---|---|---|---|---|---|---|---|---|
| 2016 (28) | 3 (11) | 2 (7) | 9 (32) | 1 (4) | 2 (7) | 3 (11) | 12 (43) | 2 (7) | 8 (30) |
| 2017 (22) | 4 (18) | 1 (5) | 14 (64) | 0 (0) | 0 (0) | 3 (14) | 6 (27) | 2 (9) | 8 (36) |
| 2018 (31) | 4 (13) | 3 (10) | 11 (36) | 0 (0) | 1 (3) | 5 (16) | 20 (65) | 3 (10) | 9 (29) |
| 2019 (28) | 1 (4) | 6 (21) | 8 (29) | 0 (0) | 2 (7) | 2 (7) | 13 (46) | 1 (4) | 9 (32) |
| 2020 (13) | 0 (0) | 1 (8) | 5 (39) | 0 (0) | 0 (0) | 2 (15) | 2 (15) | 0 (0) | 0 (0) |
| Total (122) | 12 (10) | 13 (11) | 47 (39) | 1 (1) | 5 (4) | 15 (12) | 53 (43) | 8 (7) | 34 (28) |
Diagnosis and management of TBI patients in the study period of the years 2016–2020. Surgeries include EVDs for ICP monitoring, burr holes, craniotomies, and craniectomies. There was a significant difference in the distribution of skull fractures across the years (*p < 0.016). All other variables did not differ significantly between the years
EDH epidural hemorrhage, SDH subdural hemorrhage, IVH intraventricular hemorrhage, ICP intracranial pressure, EVD external ventricular drains
Discussion
Traumatic brain injury remains a global health issue due to a variety of reasons. However, regional differences exist, which are strongly influenced by preventative measures and age distribution among the population. In the Western civilization, we are witnessing a strong increase in TBI occurring in the elderly, but the incidence of TBI is also influenced by our leisure behavior. Our study provides first insights into the influence of the “COVID-19”-quarantine on the neurosurgical TBI caseload in a highly affected region during a strict quarantine period. Since our institution is the only neurosurgical department treating TBI in the federal state of Tyrol, we are able to analyze comprehensive data surveying the lockdown period and compare it to previous years.
Hospital/Medical resources were focused to ensure available intensive care capacities for patients with severe coronavirus infections. As in most other neurosurgical institutions from affected regions, elective surgeries were canceled. However, all emergent cases were still taken care of. This remains crucial as several neurosurgical conditions—especially TBI—most often require prompt surgical care and delaying treatment may negatively affect outcome [17]. In the hospital, we had COVID-19 and non-COVID-19 areas for inpatients and patients needing intensive care. All emergency cases were screened for a potential infection. During the height of the pandemic, acute patients were considered as positive with all related consequences until proven otherwise.
Additionally, we have to keep in mind that that prior coronavirus-related pandemics have reported high numbers of neurological sequelae such as large vessel stroke and infectious complications [21]. The influence of COVID-19 on TBI patients is currently unknown. Notably, both conditions lead to a coagulopathy [22, 23]. However, pneumonia during the acute setting leads most likely to worse outcome and increased length of stay [24, 25]. Furthermore, early tracheostomy might be beneficial for TBI patients [26]. However, this seems unlikely for patients with COVID-19 infections.
The implemented quarantine measures, such as curfews and the closure of skiing areas, aimed to reduce the spread of COVID-19 [27]. These restrictions most likely led to the statistically significant decrease in the average TBI case load per week observed in our study (Fig. 1). No TBI patient met the clinical criteria for polytrauma during the COVID-19 quarantine period. Possible explanations include less risky activities during quarantine and mild TBI patients avoiding presentation to the hospital for fear of infection. Although the mean age was the highest during the pandemic, this was only significant in comparison to 2016. One could speculate, that this would have changed if we would have investigated a longer time period or a larger cohort. However, on April 6th, 2020, full quarantine measures in the federal state of Tyrol ended and the same, less stringent, rules for social distancing as applied for the rest of Austria were implemented. With this first study, we wanted to analyze the impact of strict quarantine measures on the TBI landscape.
Visitors contribute to 9.2% of all TBI cases in Austria, with a seasonal peak in winter, and 20% of all skiing or snowboarding accidents are TBIs (which are also the most common cause for hospitalization) [12, 28]. Expectedly, we did not see a single patient with alpine sports-related injuries and subsequent TBI within the lockdown period. Interestingly, concomitant skull fractures significantly decreased during the pandemic as well. This probably reflects different injury causes.
Falls are the major cause for TBI worldwide, mostly affecting the elderly and being the leading cause of death for persons 65 years of age or older [11, 29, 30]. Corresponding to our findings, the risk of ground level falls resulting in TBI is known to be rising with age [31, 32]. Reason for this are mostly trivial falls (from furniture or from tripping/stumbling) due to multifactorial unsteady gait or polypharmacological therapy [7, 33]. But despite a massive increase in people staying at home and reduction of daily activities, we did find a relatively unchanged incidence in low-impact falls, despite age of TBI being the highest during the quarantine period.
Several limitations need to be acknowledged in our study, being not only retrospective the most obvious one, but also inevitable under these specific conditions. This study also contains low case numbers because of the limited time period, in which the full restrictions were in place. We cannot guarantee, that all patients were referred to our department. As being the only neurosurgical department treating moderate and severe TBI cases in the state, this is less likely the case in this cohort. However, mild TBIs are often underreported and have been referred as the “silent epidemic” even before the current COVID-19 pandemic [5, 34]. We believe, that the number of mild TBIs not seen by primary care or in hospitals was probably even more likely to be underreported during the quarantine period.
Conclusion
In summary, our study demonstrates a change of the TBI landscape in Tyrol during the COVID-19 pandemic. However, TBI patients particularly after falls at home remain a relevant cohort which require time-sensitive treatment also under strict quarantine conditions. Therefore, appropriate strategies to prioritize and rationing neurosurgical care are required also during a pandemic [17, 35].
Acknowledgements
We thank all colleagues and health care workers involved in patient care throughout all years but especially during the COVID-19 pandemic.
Author contributions
DP: literature search, study design, data collection, data analysis, data interpretation, writing. BK: data analysis, data interpretation, critical revision. CT: data interpretation, writing, critical revision. LG: literature search, study design, data collection, data analysis, data interpretation, writing.
Funding
No funding was received for this study.
Data availability
Anonymous data can be made available upon request.
Compliance with ethical standards
Conflict of interest
All authors report no conflict of interest concerning the content presented within this manuscript.
Ethical approval
The local ethical committee approved this study as indicated in the methods section.
References
- 1.Rubiano AM, Carney N, Chesnut R, Puyana JC. Global neurotrauma research challenges and opportunities. Nature. 2015;527(7578):S193–S197. doi: 10.1038/nature16035. [DOI] [PubMed] [Google Scholar]
- 2.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018 doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 3.Hukkelhoven CW, Steyerberg EW, Farace E, Habbema JD, Marshall LF, Maas AI. Regional differences in patient characteristics, case management, and outcomes in traumatic brain injury: experience from the tirilazad trials. J Neurosurg. 2002;97(3):549–557. doi: 10.3171/jns.2002.97.3.0549. [DOI] [PubMed] [Google Scholar]
- 4.Rickels E. Focus on traumatic brain injury. Eur J Trauma Emerg Surg. 2017;43(6):729–730. doi: 10.1007/s00068-017-0866-7. [DOI] [PubMed] [Google Scholar]
- 5.Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci. 2018;62(5):535–541. doi: 10.23736/S0390-5616.18.04532-0. [DOI] [PubMed] [Google Scholar]
- 6.Rusticali B, Villani R, Working G. Treatment of minor and severe traumatic brain injury. National reference guidelines. Minerva Anestesiol. 2008;74(10):583–616. [PubMed] [Google Scholar]
- 7.Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23(7):753–758. doi: 10.1002/pds.3624. [DOI] [PubMed] [Google Scholar]
- 8.Mauritz W, Brazinova A, Majdan M, Leitgeb J. Epidemiology of traumatic brain injury in Austria. Wien Klin Wochenschr. 2014;126(1–2):42–52. doi: 10.1007/s00508-013-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schöppl IJA, Bauer R. Sport- Kuratorium für Verkehrssicherheit: Freizeit- und Haushaltsunfälle in Tirol. Analyse und Präventionsansätze; 2019. [Google Scholar]
- 10.Ackery A, Hagel BE, Provvidenza C, Tator CH. An international review of head and spinal cord injuries in alpine skiing and snowboarding. Injury Prevention. 2007;13(6):368–375. doi: 10.1136/ip.2007.017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015;157(10):1683–1696. doi: 10.1007/s00701-015-2512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauritz W, Brazinova A, Majdan M, Leitgeb J. Hospital admissions for traumatic brain injury of Austrian residents vs of visitors to Austria. Brain Inj. 2014;28(10):1295–1300. doi: 10.3109/02699052.2014.916418. [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. The N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurihara H, Bisagni P, Faccincani R, Zago M. Covid-19 outbreak in Northern Italy: viewpoint of the Milan area surgical community. J Trauma Acute Care Surg. 2020 doi: 10.1097/TA.0000000000002695. [DOI] [PubMed] [Google Scholar]
- 17.Eichberg DG, Shah AH, Luther EM, Menendez I, Jimenez A, Perez-Dickens M, et al. Letter: academic neurosurgery department response to COVID-19 pandemic: the university of Miami/Jackson memorial hospital model. Neurosurgery. 2020 doi: 10.1093/neuros/nyaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnaout O, Patel A, Carter B, Chiocca EA. Letter: adaptation under fire: two harvard neurosurgical services during the COVID-19 pandemic. Neurosurgery. 2020 doi: 10.1093/neuros/nyaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury. Fourth Edition Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 20.Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Guidelines for the Surgical Management of Traumatic Brain Injury Author Group. Neurosurgery. 2006;58(Suplement):S2-1–S2-3. doi: 10.1227/01.neu.0000210361.83548.d0. [DOI] [Google Scholar]
- 21.Daou BJ, Koduri S, Palmateer G, Thompson BG, Chaudhary N, Gemmete JJ, et al. Letter: neurological implications of COVID-19 and lessons learned from prior epidemics and pandemics. Neurosurgery. 2020 doi: 10.1093/neuros/nyaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuels JM, Moore EE, Silliman CC, Banerjee A, Cohen MJ, Ghasabyan A, et al. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J Trauma Acute Care Surg. 2019;86(4):686–693. doi: 10.1097/TA.0000000000002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar RG, Kesinger MR, Juengst SB, Brooks MM, Fabio A, Dams-O'Connor K, et al. Effects of hospital-acquired pneumonia on long-term recovery and hospital resource utilization following moderate to severe traumatic brain injury. J Trauma Acute Care Surg. 2020;88(4):491–500. doi: 10.1097/TA.0000000000002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofman M, Andruszkow H, Kobbe P, Poeze M, Hildebrand F. Incidence of post-traumatic pneumonia in poly-traumatized patients: identifying the role of traumatic brain injury and chest trauma. Eur J Trauma Emerg Surg. 2020;46(1):11–19. doi: 10.1007/s00068-019-01179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin C, Darcy D, Park C, Lane CJ, Mack WJ, Bliss DW, et al. Timing of tracheostomy placement among children with severe traumatic brain injury: a propensity-matched analysis. J Trauma Acute Care Surg. 2019;87(4):818–826. doi: 10.1097/TA.0000000000002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocklov J, Sjodin H. High population densities catalyze the spread of COVID-19. J Travel Med. 2020 doi: 10.1093/jtm/taaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun H, Samra NS, Kalakoti P, Sharma K, Patra DP, Dossani RH, et al. Impact of prehospital transportation on survival in skiers and snowboarders with traumatic brain injury. World Neurosurg. 2017;104(909–18):e8. doi: 10.1016/j.wneu.2017.05.108. [DOI] [PubMed] [Google Scholar]
- 29.Kirkman MA, Jenks T, Bouamra O, Edwards A, Yates D, Wilson MH. Increased mortality associated with cerebral contusions following trauma in the elderly: bad patients or bad management? J Neurotrauma. 2013;30(16):1385–1390. doi: 10.1089/neu.2013.2881. [DOI] [PubMed] [Google Scholar]
- 30.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartholt KA, Van Lieshout EM, Polinder S, Panneman MJ, Van der Cammen TJ, Patka P. Rapid increase in hospitalizations resulting from fall-related traumatic head injury in older adults in The Netherlands 1986–2008. J Neurotrauma. 2011;28(5):739–744. doi: 10.1089/neu.2010.1488. [DOI] [PubMed] [Google Scholar]
- 32.Poyry T, Luoto TM, Kataja A, Brander A, Tenovuo O, Iverson GL, et al. Acute assessment of brain injuries in ground-level falls. J Head Trauma Rehabil. 2013;28(2):89–97. doi: 10.1097/HTR.0b013e318250eadd. [DOI] [PubMed] [Google Scholar]
- 33.Bossers SM, Pol KM, Oude Ophuis EPA, Jacobs B, Visser MC, Loer SA, et al. Discrepancy between the initial assessment of injury severity and post hoc determination of injury severity in patients with apparently mild traumatic brain injury: a retrospective multicenter cohort analysis. Eur J Trauma Emerg Surg. 2018;44(6):889–896. doi: 10.1007/s00068-017-0861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, et al. The International incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(6):774–785. doi: 10.1017/cjn.2016.290. [DOI] [PubMed] [Google Scholar]
- 35.Kondziolka D, Couldwell WT, Rutka JT. Introduction on pandemics: the impact of COVID-19 on the practice of neurosurgery. J Neurosurg. 2020 doi: 10.3171/2020.3.JNS201007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymous data can be made available upon request.