1. Introduction
The European Neuromuscular Centre (ENMC) has always supported the use of ventilatory support in neuromuscular disorders (NMD's). There is a lack of provision of mouthpiece ventilation (MPV) and a lack of consensus with regard to how/when it can be delivered, The ENMC convened the 252nd ENMC International Workshop: Developing best practice guidelines for management of mouthpiece ventilation in Neuromuscular Disorders in Amsterdam, The Netherlands from the 6th to 8th March 2020. A total of 22 participants, 20 from Europe, one form the USA and one from Canada representing a total of 11 countries. The group included respiratory physicians, neurologists, nurses, respiratory physiotherapists all with specific experience in the provision of mouthpiece ventilation (MPV) in NMD, advocacy groups and patient representatives were also present, with the meeting program being available as an Online Supplement (1). We would like to highlight that the workshop took place at the start of the COVID-19 pandemic. Some institutions had issued a travel ban. In such cases some individuals contributed to the meeting via Skype. All talks and discussions led to the production of the best practice algorithm to initiate MPV in patients who require daytime ventilatory support (Fig. 1 ).
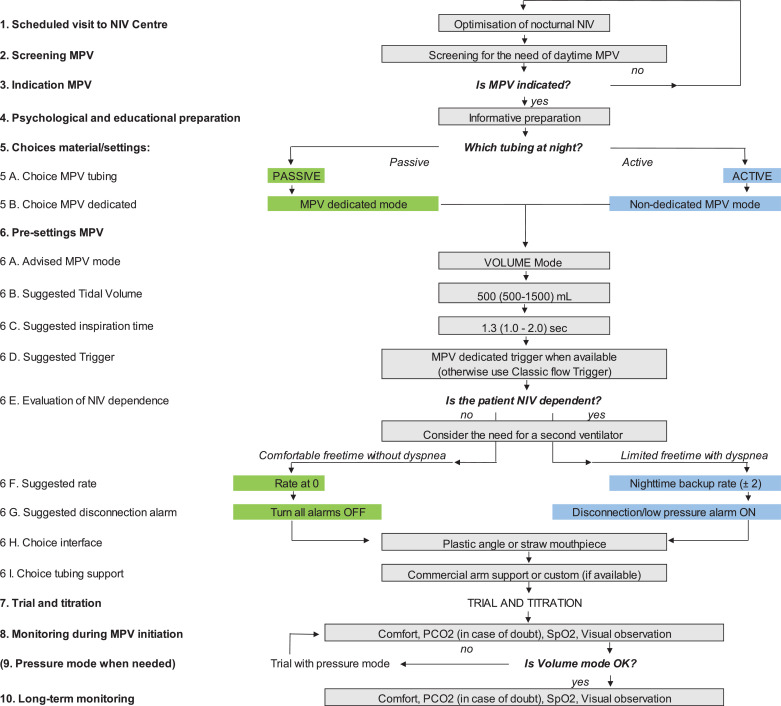
Fig. 1.
Fig. 1 shows the best practice algorithm for the initiation of mouthpiece ventilation (MPV) in the long-term setting. For each stage of the algorithm there is a description to assist in the provision of this service.
| 1. Scheduled visit to NIV centre | Timing to visit the reference centre between 3-12 months according to disease progression |
| 2. Screening MPV | The screening is first based on symptoms (dyspnea), then PaCO2 and SpO2, speed progression and severe drop in FVC |
| 3. Indication MPV | Timing for the choice of the ventilator (ideally with a dedicated MPV software) |
| 4. Psychological and educational preparation | This step includes the psychological preparation + the demonstration of the material + explanations on the impact of MPV: social impact, facilitating eating, speaking, protecting respiratory progression, etc.) |
| 5. Choices material/settings: | A similar tubing is desirable for night and daytime |
| 5A. Choice MPV tubing | A passive tubing include a single tubing, active tubing includes an exhalation valve |
| 5B. Choice MPV dedicated | A dedicated mode includes the availability of: PEEP at 0, high sensitive trigger via flow interruption, rate at 0, all alarms OFF |
| 6. Pre-settings MPV | Pre-settings occur before the first trial with MPV when patient is not connected |
| 6A. Advised MPV mode | Volume is 1st choice mode and allows: air-stacking, leaks NIV without untimely leak compensation |
| 6B. Suggested tidal volume | Volume is presented as “starting value (range)”. For a child, starting values can be 300 mL, for an adult DMD: 500-600mL, for an ALS patient: 1000mL |
| 6C. Suggested inspiration time | Inspiration time is presented as “starting value (range)” |
| 6D. Suggested trigger | MPV dedicated trigger is a high sensitive flow interruption trigger, classic flow trigger (high or medium sensitive) is less sensitive |
| 6E. Evaluation of NIV dependence | In many countries in Europe, NIV use >16/24 hours defines NIV-dependence |
| 6F. Suggested rate | Comfortable free time can be estimated as >4-6 consecutive hours without NIV |
| 6G. Suggested disconnection alarm | Apnoea alarms may be set in the very dependent patients (example: alarm after 5 minutes with apnoea) |
| 6H. Choice interface | Choice of the mouthpiece according to patient preference after trial with both pieces |
| 6I. Choice tubing support | Custom support on the shoulders is advised for dependent patients |
| 7. MPV trial | The trial & titration can be done in a 2 hour session |
| 8. Monitoring during MPV initiation | Monitoring at initiation is advised during a 20-30 minutes trial. Primary focus is comfort and the way they breath and less on CO2 and saturation |
| 9. Pressure mode | Pressure mode is not advised as the first choice Pressure mode is possible (ST or PSV) with dedicated mode solely. This mode is sometimes preferred by children Pressures can be set at 12-20cmH2O according to patient comfort Pressure mode is not adequate when the patient maintain MPV continuously in his mouth Lip seal around MPV is required. Air-stacking is not possible |
| 10. Long-term monitoring | Long-term monitoring is highly variable among countries and centres It is also recommended to monitor the MIC-VC difference this is to ensure a compliant thorax. When the MIC-VC difference decreases, education is essential to ensure patients optimise the MIC regularly to ensure stretching of the patients thorax to maintain maximal recruitable lung volumes |
Noninvasive ventilation (NIV), arterial carbon dioxide (PaCO2), oxygen saturation (SpO2), forced vital capacity (FVC), positive end expiratory pressure (PEEP), Duchenne muscular dystrophy, amyotrophic lateral sclerosis (ALS), spontaneous timed (ST), pressure support ventilation (PSV), maximum insufflation capacity (MIC).
NMDs may affect skeletal, heart and respiratory muscles. The level of respiratory muscle weakness depends on the severity and evolution of NMDs. Respiratory insufficiency and pneumonia are primary causes of mortality and morbidity in many NMDs. The introduction of ventilatory support will improve nocturnal hypoventilation. Ventilatory support is usually offered via non invasive techniques such as the use of a nasal mask during sleep. In some conditions, tracheostomy may be proposed when non-invasive ventilation (NIV) is no longer possible, due to the inability to use MPV, choice, bulbar impairment or the inability to manage secretions. Studies show mechanical ventilation improves symptoms, gas exchange, quality of life and survival in NMD's [1].
As disease progresses and ventilator dependency increases patients have a choice, either to continue with mask NIV or to have a tracheostomy inserted and continue with invasive mechanical ventilation. Whilst invasive ventilation (IV) negates the need for a mask on the face, complications from having direct access to the airway include tracheomalacia, pulmonary haemorrhage and loss of speech. Continuing with 24/7 mask NIV can lead to pressure sores, midfacial hypoplasia, lack of audibility of speech and swallowing difficulties [2].
An alternative to having a mask on the face is to receive NIV via a mouthpiece. This is known as open circuit MPV. MPV allows patients with NMDs to receive daytime support from a portable ventilator, which they can release at will, for example, for speaking, eating, swallowing, and coughing. MPV is not a new technique. In 1953, Dr Affeldt reported at a post-poliomyelitis conference, “respiratory equipment provided MPV via a simple mouthpiece used for pulmonary function testing attached to an intermittent positive pressure ventilation device” [3]. Bach and co-workers played an important role in disseminating MPV techniques around the world, making it increasingly popular [4].
2. Goals of the workshop
The overall aim of the workshop was to deliver the following:
-
•
Evaluate the pre workshop international survey on MPV and publish the results as a separate manuscript.
-
•
Produce a best practice guideline on the provision of MPV in NMD's by reviewing and recommending the most appropriate monitoring, mode, settings and equipment required for MPV.
-
•
Produce a best practice algorithm on MPV for daytime ventilatory support (see Fig. 1).
-
•
Define optimal outcome measures for future studies in MPV.
3. Introductory talks
Following a welcome from Alexandra Breukel, ENMC director, and the chairpersons of the workshop, Michelle Chatwin, Michel Toussaint, Miguel Gonçalves and Jesus Gonzalez-Bermejo. Michelle Chatwin and Michel Toussaint gave an overview of the meeting program and workshop objectives and Jesus Gonzalez-Bermejo began the session with when to start more than night time ventilation. Night time ventilation is required as a result of respiratory muscle weakness. This leads to ventilatory insufficiency initially during rapid eye movement sleep due to selective inhibition of the accessory muscles of breathing. As the weakness progresses abnormalities in ventilation will occur during all stages of sleep and subsequently progress to daytime hypercapnic respiratory failure. Treatment with NIV at night will correct gas exchange and normalise blood gases [5]. Some patients will develop daytime hypercapnia and subsequently require daytime ventilatory support. Prior to commencing daytime ventilatory support it is best practice to check that the patient is optimally ventilated overnight and using their device for the full period when they are asleep. It is recommended that patients are monitored over night with carbon dioxide (CO2) (measured by arterial blood gas, end tidal CO2 (EtCO2) or transcutaneous CO2 (TcCO2), oximetry and where possible evaluation of the device software via download.
Adjustments in settings should be carried out in the event of hypercapnia. If on ventilator download the patient is not using the device for long enough, they should be reviewed as to why and a strategy put in place to increase ventilator time. If the patient is normocapnic over-night then daytime ventilatory support would be commenced when indicated (Box 1 ). However, it was discussed that daytime hypercapnia is a late sign for the commencement of daytime ventilatory support. Box 1 shows the criteria that would indicate the provision of daytime ventilation.
Box 1.
Indications for a trial of MPV.
|
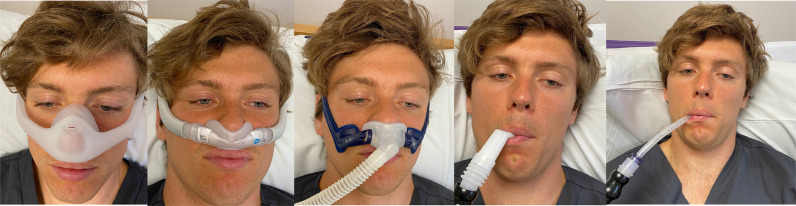
Peter Wijkstra and Laura Verweij, highlighted the physiological differences for the patient between night-time and daytime ventilatory support. Patients are asleep when using nigh-time ventilation and lying down. During sleep there is decreased ventilator drive and their ventilatory support needs to have an appropriate back up respiratory rate to support their nocturnal ventilatory requirment. In the day patients are awake, moving around, eating, drinking, socialising and coughing, leading to a different ventilation requirement highlighted by different daytime and night-time settings. The daytime ventilator needs to be portable and easily adapted to fit on to the wheelchair with good battery provision. Traditionally, daytime ventilation would utilize a trachesostomy as an interface. But with technological advances in masks a nasal mask or pillows is used, leaving the mouth uncovered, (as in Fig. 2 ) is recommended. This will help to facilitate speech, swallowing and social interaction.
Fig. 2.
Fig. 2 shows five photographs, the left hand shows a patient with a nasal mask (Dreamwear, Phillips Respironics, Murrysville, PA, USA). The second left photograph shows the patient using a nasal mask (AirFit N30i ResMed, San Diego, CA, USA) The centre photograph shows the patient using a nasal pillows interface (Swift Lt, ResMed, San Diego, CA, USA). The two right hand photographs show the same patient using mouthpiece ventilation with a mouthpiece (left) and straw (right) (Phillips Respironics, Murrysville, PA, USA).
Michel Toussaint and Miguel Goncalves led the discussion session on tracheostomy versus non-invasive support for daytime ventilation. The group would like to point out that sometimes the driver for a tracheostomy maybe dependant on what the centre has done historically and whether it was inserted due to an acute episode. In some countries having a tracheostomy qualifies the patient for funded, round the clock care and in some instances this maybe by a nurse rather than a career. In other countries the insertion of a tracheostomy may lead to living in a care institution rather than independent living. Sometimes the driver for a tracheostomy is when secretions are troublesome and cannot be cleared with effective proximal airway clearance techniques (ACT's) or for the face to be free of a mask. MPV is an alternative to an interface secured on to the face. There have been no randomised studies showing that either technique is superior in terms of survival. The group concluded for best practice that patients should be given a trial of MPV prior to consideration of tracheostomy. In the first instance nocturnal ventilatory support should be optimised along with proximal ACT’s [6,7]. If a long-term tracheostomy is indicated then it should be the choice of the patient, having understood all the implications, not the doctor.
Peter Wijkstra, presented the mask options, should MPV not be possible. He highlighted that a nasal mask and nasal pillows system can be used and that these are smaller options than the full face mask (see Fig. 2). There is also the possibility to use negative pressure ventilators or a pneumobelt [8].
Miguel Goncalves and Michelle Chatwin presented the preliminary results from the MPV survey that was carried out. The results showed that there was a wide variation in the provision of MPV within countries and internationally. Where there was a predominant indications and method of introduction of MPV, we have incorporated this into the workshop algorithm (Fig. 1) for the suggested initiation of MPV in patients with NMD's. The workshop lectures and discussions in the next three topics also contributed to the production of Fig. 1.
4. Topic 1: technicalities of MPV
This topic consisted a discussion about the modes and settings of MPV led by Michel Toussaint and Miguel Gonçalves. Only some centres offer MPV. It is typically delivered via a volume cycled mode of ventilation [9], set to deliver a tidal volume of greater than 700mls and up to 1500mls [10,11] without positive expiratory pressure (PEEP), via a mouthpiece or straw supported by a flexible arm/support [10,12]. However, patients may only have ventilators that have a pressure support or pressure control mode so there may be an indication to use a pressure cycle mode in this instance. There was also debate around whether pressure [9,13] or volume [11,14] modes are best and whether PEEP adds benefit. It was identified that some clinicians maybe concerned about using volume cycled ventilation as it may cause the patient to have a set volume delivered at a high pressure, especially in children. The group highlighted that pneumothorax is rare, and that any risk is far outweighed by benefit. A pnumothorax is likely multifactorial and can be as a result of any device providing positive pressure (ventilator or mechanical insufflation-exsufflation device (MI-E). Indeed if MPV is delivered in volume mode and the breath feels too large for the patient, they can simply open their mouth and let the air leak out. Some clinicians will set the device to deliver a large volume to enable the patient to take as much air as they want. The aim of a super-inspiration is to rest the patient so they are able to breathe without support for some breaths prior to requiring another super-inspiration via MPV. Others provided a tidal volume which is comfortable and sufficient but also allows the patient to achieve a MIC in three breaths for utility/convenience. This means that they use MPV to support every breath rather than, intermittently. Depending on the settings and the aim of MPV there is the potential for the patient to be under ventilated when disconnected from the circuit [15] and therefore patients need to undergo daytime monitoring and re-evaluation of settings. However, the group concluded it is possible to deliver MPV in pressure or volume mode but, pressure is the mode of choice only in young children. The preference being to start MPV with volume cycled ventilation and this recommendation for best practice has been inserted into Fig. 1.
Doug McKim and Michel Toussaint reported there is no consensus around which type of mouthpiece is best or why certain patients prefer one type over another. Some centres advocate that custom made supports for the mouthpiece are best [16], whilst others are limited to commercially available arms. The key to a successful arm support is that it should move with the patient. This means that it is always in the correct position when they need to have a breath. To facilitate the use of MPV the group recommended that centres without the ability to provide customisation, use commercially available supports. Patients should trial a straw or mouthpiece and be allowed to decide what is best for them. They should be able to successfully ventilate, relieve any dyspnea and be able to breath stack with the mouthpiece. If required further support should be requested from a specialist centre.
Johan Chaulet and Wendy Hughes provided insight to what healthcare professional need to know from the patient and carers perspective. Patients need any anxieties addressed when starting MPV and psychological support may be required. This should include referral to psychology services, other users and advocacy groups as a source of independent encouragement and practical troubleshooting. Regular reviews by the prescriber helps patients to resolve problems before they escalate into a psychosocial barrier. Shared patient/clinician decision-making around the device and accessories is essential. Timing for intervention of MPV should be planned early to help lessen the burden. If a local centre cannot provide the required support, patients may appreciate referral to a home mechanical ventilation centre, where available.
Hélène Prigent and Adam Ogna provided the evidence form bench studies with regard to ventilator, modes for MPV. Home mechanical ventilators are primarily designed to provide ventilation support to patients in stable respiratory conditions, where the respiratory demand of patients, the workload of the ventilator and the resistances of the circuit remain almost stable or change gradually over time [17]. MPV represents a difficult task for home ventilators, because of the irregular breathing pattern and the rapidly changing load conditions resulting from intermittent disconnection of from the circuit [15]. This has been highlighted in studies showing a high burden of alarms during MPV [12].
The latest home ventilators have a pneumatic system based on a turbine and perform volumetric ventilation using an algorithm to adjust the working pressure based on the previous cycles, corresponding to a volume-targeted pressure ventilation. During volumetric MPV, the feedback loop is long, resulting in slow reactivity of the ventilator to changing loads, tidal volume overshoot during circuit disconnection, followed by a down-regulation of the ventilator's working pressure and a lower-than-expected delivered tidal volume in the subsequent cycles [13]. Similar changes can be observed between controlled and assisted ventilatory cycles even without circuit disconnection, with the ventilator adapting slowly to the changing muscular effort performed by the patient. This phenomenon varies significantly between the available ventilators, needing 2–6 cycles to stabilize tidal volume, and is particularly evident when a backup respiratory frequency is set [14]. Furthermore, intentional leaks, which are frequent in MPV, decrease the accuracy of ventilators to estimate the delivered tidal volume [18], [19], [20] potentially magnifying this phenomenon. The use of pressure cycled MPV results in an even larger volume overshoot during circuit disconnection, but reduces the drop in delivered tidal volume at the moment of the circuit reconnection [13]. This can be explained by a shorter feed-back loop, allowing the adjustment of the ventilator working pressure during the ongoing respiratory cycle. Since the feed-back loop does not include the circuit and the patient, this ventilation mode doesn't guarantee a stable tidal volume in the case of changing resistance or compliance of the respiratory system (e.g. in case of airways obstruction due to secretions), potentially exposing dependant patients to a risk of insufficient ventilatory support.
Currently available ventilators show differences in their capacity to deal with the rapidly changing respiratory load features that characterize MPV. Therefore, the choice of the ventilator to be used for MPV in a specific patient should contemplate the advantages and limitations of each machine, which also depend on the planned ventilator mode.
Alarms on ventilators can be a problem. Alarms going off unnecessarily may stop an individual using this technique. Annalisa Carlucci led on this topic. An important issue is to distinguish between intermittent or continuous use of ventilation.
In the non-ventilator dependant patient MPV may be used “on demand” with a respiratory rate set to zero. In this case alarms are useless. Recently, the availability of MPV mode on ventilators, allows one to set the disconnection and apnoea alarm to off and low-pressure alarm from 1 to 5 mbar as well as back-up respiratory rate to zero. If a MPV “dedicated” mode is not available or not applicable, a correct combination between tidal volume, inspiratory time and the choice of the mouthpiece (in term of resistance) may avoid disconnection and low-pressure alarms settings.
For more highly ventilator-dependant patients, different alarm settings may be considered. In this case disconnection and apnoea alarms may be useful as well as a back-up in very weak ventilator dependant patients (Fig. 1). A rebreathing alarm is useful in the ventilator dependant patient. In some ventilators it is on by default and in others can be set to “on”. If a rebreathing alarm is not available it is recommended that a single limb circuit with a exhalation valve is used.
5. Topic 2: conditions for success of MPV
MPV needs to be initiated at the right time in order to maximise success. Josh Benditt presented on the timing of implementation of MPV. Medical society statements about when to commence daytime ventilatory support state, when the patient experiences daytime hypercapnia [21]. MPV is recognised as a treatment choice for daytime ventilation. Clinical experts suggest when ventilatory support is greater than 12 h per day with or without daytime hypercapnia [16], MPV should be introduced in those that wish to peruse non-invasive daytime ventilatory support and can maintain a lip seal [22] and or in those with symptoms of dyspnoea [23] and tachypnoea [23], [24], [25].
The group concluded that the best practice timing for the initiation of MPV should be:
-
•
Using mask ventilation for ≥ 12 h/day
-
•
Daytime hypercapnia with nocturnal normocapnia
-
•
When dyspnoea is relieved by ventilatory support
-
•
To increase voice volume
-
•
To improve cough strength outside the home (i.e. when a mechanical insufflation-exsufflation device is unavailable)
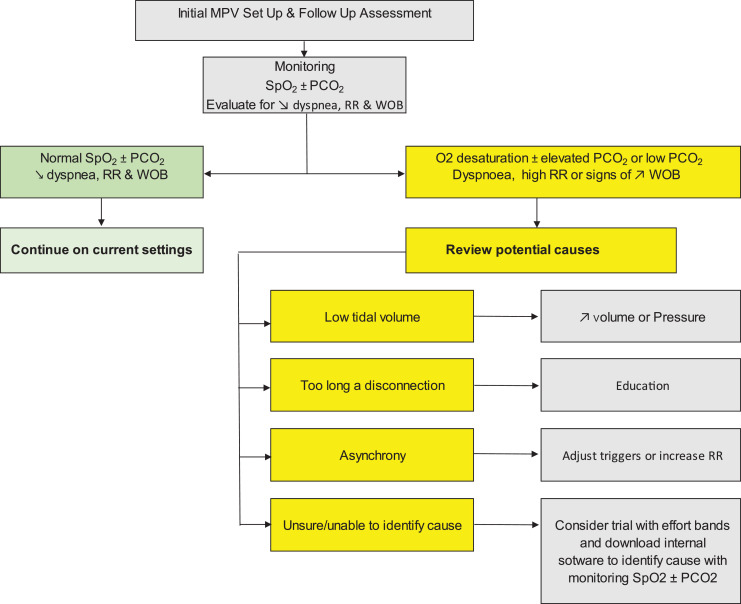
Miguel Gonçalves and Jesus Gonzalez-Bermejo presented on the topic of monitoring for initiation of MPV and long-term use of MPV. Nardi et al., [15] using daytime polygraphy at home showed patients may unknowingly under ventilate themselves, despite not having any symptoms. Not only is hypoventilation an issue with MPV but also hyperventilation can occur. See Fig. 3 for a suggested algorithm for monitoring patients with MPV.
Fig. 3.
Fig. 3 shows the suggested algorithm for monitoring during the initial and follow up of patients with mouthpiece ventilation. Depending on the identified problem is a suggested solution.
Carbon dioxide (CO2), oxygen saturation (SpO2), respiratory rate (RR), work of breathing (WOB).
In terms of safety-risk management potential issues were highlighted by Jesus Gonzalez-Bermejo and Wendy Hughes. This session was followed by Johan Chaulet and Laura Verweij-van den Oudenrijn highlighting the education, training, needs for the MPV and carers. Barriers to the provison of MPV have been identified as a lack of knowledge and training [9,10]. At present there are no protocols, it has been suggested that individualized patient care is needed to successfully apply MPV [26,27]. This causes issues for centres not familiar in the provision of MPV. Based on these sessions and discussion three documents were formulated to support the ongoing training needs of the MPV user and their team along with a check list for a clinical team providing MPV and a prescription and equipment information sheet (see online supplement, S1, S2 and S3). The group highlighted that it is essential for the ventilator dependent patient to always carry a resuscitation bag. This can be used in the event of the ventilator failing.
Secretions can affect the success of NIV. Barbara Garabelli provided a presentation on ACT, with a specific focus on MPV. MPV can be used as a proximal ACT [6,7]. Breath-stacking is typically performed with an resuscitation bag [25] or volume-cycling ventilator [25]. MPV can be set in volume or pressure assist-control mode [25]. Patients can be taught either to have a deep breath in if MPV is in a pressure mode [28] but, breath stacking in volume mode is preferential as it will provide a greater volume of air. The group concluded that when implementing MPV it is essential to teach how to assist cough by a single deep breath in or breath stacking with MPV in volume mode.
Tiago Pinto and Nicolas Audag presented on the technicalities of the wheelchair, ventilator and external batteries. The wheelchair must be able to accommodate a strong and safe system for the ventilator and a fastening solution to ensure that the tubing/circuit/arm and the mouthpiece will be held in place. The main objectives are comfort, safety, battery life and manoeuvrability for the patients and their caregivers.
Recently, ventilator companies developed a dedicated MPV mode with dedicated arms and circuits without expiratory valve allowing the patient to exhale outside the mouthpiece [29]. Another advantage of this arms and circuits is the practicality to support and hold the mouthpiece in perfect position and easiest way to adapt it to a wheelchair. Nowadays the ventilator manufacturers provide us life support ventilators with an 8 hour battery, and the possibility of external batteries increasing battery life for up to 16 h.
When deciding what ventilator is appropriate for MPV you need to assess the ventilator with the chair. Ask yourself if it is able to still tilt appropriately with the ventilator and equipment. Does the mouthpiece fix on to the chair or the patient and does the support move with the patient? Is there any risk of clamping or loosening or the tubing. Are you able to connect the ventilator to the wheelchair circuitry and other accessories? We also need to remember that the external battery weight increases the ventilator weight. A ventilator backpack also exits for ambulatory patients to help facilitate mobility.
6. Topic 3: the experience (and effects) of MPV in NMD's, acute care and exercise
Hélène Prigent and Johan Chaulet presented on MPV in Duchenne muscular dystrophy (DMD). MPV has been proposed as an alternative NIV method in DMD since the 90′s [30]. This technique has been increasingly used over the years to improve NIV tolerance and lengthen NIV duration therefore allowing NIV use for ventilator-dependant patient. This technique has become an integral part of the DMD patient's respiratory management in order to delay, if not completely avoid, tracheostomy ventilation. Patients report a positive effect of the technique on dyspnoea and fatigue but they also highlight their preference for this NIV technique as it improves speech, swallowing and communication [25]. One of the main challenges in the DMD population for MPV use is, to ensure a proper and secured access to MPV interface as these patients present with severe motor function impairment and cannot secure their mouthpiece with anything but their lips and teeth.
Doug McKim and Jesus Sancho prepared a session on MPV in amyotrophic lateral sclerosis (ALS). Like many NMD's the ALS patients suffer from progressive respiratory muscle weakness, leading to ventilatory failure and ineffective coughing. However, this is at a significantly faster rate. NIV is recognized as being able to prolong survival, relieve symptoms, avoid hospitalizations and improve quality of life [22]. Like other NMD's, NIV is used during sleep hours, but as respiratory muscles progressively weaken, the requirement for ventilatory support increases. In some patients with ALS, MPV is an option. MPV once started can prolong tracheostomy-free survival, up to 9.5 months [22]. However, only 19% ALS patients are able to successfully use MPV [22]. There are two factors that determine the success of the MPV. Firstly the presence of an effective assisted cough peak flow (CPF) capable of clearing secretions. Secondly the presence of sufficient bulbar function in order to; retain a mouthpiece, and achieve an adequate seal and manage to attain a maximum insufflation capacity (MIC). As bulbar function worsens over time, MPV becomes ineffective and can be assessed by the MIC being greater than their vital capacity (MIC-VC difference). Patients with ALS should be assessed to see whether they will benefit from MPV rather than limiting choice to tracheostomy or 24 hour mask ventilation.
Brit Hov prepared a presentation on MPV in spinal muscular atrophy (SMA). With the introduction of emerging therapies (gene therapy and Nusinersen) the natural history of the disease is changing and whilst MPV may have been used in SMA type II [31], it may be possible in SMA type I (if sufficient bulbar function). In children who require daytime ventilatory support the advantage of a MPV over having a nasal mask include; prevention of mid facial hypoplasia, prevention or improvement in pectus excavatum. There may also be an impact on self-image with an increased ability to speak and participate, for example in class and social activities. It maybe that children are better started in pressure support mode. This may be because of concerns around barotrauma with volumes. However, volumes may be needed to assist in improving pectus excavatum. In the experience of the group, MPV is possible in children over the age of 8 years old. However, if a clinician wanted to start in children younger MPV can be trialled. MPV In SMA has also been reported to normalise blood gases [31].
Michelle Chatwin and Wendy Hughes presented on MPV in other NMD's. When looking to the literature there is very little evidence for the use of MPV in other NMDs. Studies have shown MPV to be used in acid maltase deficiency [32], myotonic dystrophy [32] and limb girdle muscular dystrophy [32], fascioscapular muscular dystrophy [9], congenital myopathy [9], Becker muscular dystrophy [9], metabolic myopathy [9], post poliomyelitis [15], primary adhalinopathy, congenital dystrophies [29], Pompe's disease [15] and other neuromuscular diseases [9]. Based on the MPV survey we conducted, use of MPV in other NMD's is common in congenital myopathies (n = 60), cervical spine injury (n = 46), myotonic dystrophy (n = 30). It was less commonly but, used in congenital muscular dystrophies and Pompe's disease. The group believes that all patients should be assessed on an individual basis and not be excluded from a trial of MPV due to the inadequacy of the litrature in this patient group.
Josh Benditt and Johan Chaulet presented on the effects of MPV on swallowing. In normal individuals, the swallowing process is highly synchronized and the initiation of breathing immediately after swallowing occurs at a higher lung volume and is immediately followed by exhalation. This results in a positive subglottic pressure that is thought to be protective against aspiration [33]. In individuals with diffuse NMD the careful sequencing of swallowing can be lost, and even in those without bulbar dysfunction, respiratory muscle weakness can lead to swallowing dysfunction, likely through inability to develop a positive subglottic pressure [34] and may be one of the reasons aspiration may be more frequent in patients with trachostomies. Discoordination of pharyngeal and laryngeal swallowing sequences can occur, as well as inhalation rather than exhalation occurring immediately after swallowing which increases the risk for aspiration.
Garguilo et al., showed that nasal NIV improved swallowing function in DMD by increasing the number of swallows followed by expiration, reducing swallowing fragmentation and improved dyspnoea during swallow [35]. In a qualitative study of swallowing, subjects reported that MPV improved swallowing compared to spontaneous breathing and that nasal NIV was more difficult that MPV to use during eating [36].
Clinically those using MPV are able to speak more with greater volume and with longer uninterrupted sentences. However, little evidence supports clinical impressions. A recent qualitative study showed that speech volume and duration was improved in patients. However, the need to take a breath was felt to interfere some with fluidity of speech and also posed some challenges when using voice recognition software [37].
For best practice, when initiating MPV, we should educate patients how to swallow with MPV. In patients where aspiration is a concern an increase in the volume delivered prior to deglutition gives when more time to eat and swallow. This may mean that patients need two MPV settings one for eating and one for breathing.
Miguel Gonçalves presented on MPV in acute care. Whilst there is little evidence established for the use of MPV in acute care, MPV can be used to prevent intubation in chronic lung disease [38]. MPV can be used in the post extubation phase in a protocolised approach, which also used MPV to assist cough [39]. The group strongly recommends that in the critical care environment MPV should be not taken away from any patients who normally use it long term. A trial of MPV post extubation may prevent the need for tracheostomy in patients with very low vital capacities.
Miguel Gonçalves also highlighted that MPV is capable of supporting exercise (walking) in NMDs. The benefit of NIV has been well documented in exercise. MPV has also been used as a tool to increase exercise tolerance in patients with diapragm paralysis [40]. MPV is also possible during walking but the equipment needs to be adapted so that it can be mounted on a walker, wheelchair, trolley or fitted into a back pack. One of the main problems is portability, as the ventilator can be quite heavy. One other option is to use a resuscitation bag. Patients also may need to use nose clips to ensure that there is no nasal passage leak.
Joan Escarrabill presented on the patient reported experience with MPV. Survival, health related quality of life, physical activity or hospital admissions are examples of classical tools which primarily evaluate safety and effectiveness. Satisfaction is a subjective construct that is closely linked to expectations. The patient experience goes far beyond satisfaction. Some available tools are more generalizable (surveys) and others are mode descriptive (in-depth interviews, focus groups or patients’ panels). In the end, through patient experience evaluation the healthcare organizations aim to identify unmet needs of the patients and to improve respiratory care. Measuring patient experience is important not only to guide service improvement, but also because an individual’s experiences may be linked to clinical outcomes and costs [41].
Based on the discussions around which patients will benefit from MPV the group concluded that MPV is not specific to a single patient diagnosis but may be applicable to many conditions as long as the prerequisites for MPV are met. To be successful these include the following:
-
•
Able to reliably retain mouthpiece. This can be assessed by asking the patient to keep their mouth closed and blow out their cheeks.
-
•
Able to perform a maximum insufflation capacity which is greater than their vital capacity.
-
•
Capable of understanding tracheostomy MPV trade-off.
7. Areas for further work
The workshop group identified that ventilator companies need to involve clinicians and patients when developing new technology for the provision of MPV. More commercially available options for arm supports and mouth interfaces are needed for those who find customisation difficult. New innovations need to be bench tested to ensure devices reach the desired level of function and address outstanding needs.
Further work is needed to evaluate the effects of MPV on speech and swallowing and quality of life. As MPV has the potential to stabilise patients for years, a registry for patients on MPV is essential. This will provide further insight around timing for MPV for the initiation of MPV and to evaluate the amount of exacerbations a patient may have.
Further education is required so that appropriate patients may be offered MPV in the ICU, either to prevent intubation or to help with successful extubation and transition to NIV. Individualised MPV pathways and information sheet may aid in successful provision of therapy inside the critical care environment. Finally, social media and social networks could help patients support each other with MPV.
8. Conclusion
Clinical experience of local teams appears to be the greatest barrier to individuals being offered MPV. Evidence base resources are a major driver for the provision of therapy in any condition. Where there is a lack of an evidence base or publications of best practice, therapies are not offered to patients who may benefit. This is because clinicians often are uncertain about the potential benefit in any given patient. As a result, MPV is not offered or is quickly discontinued due to a lack of clinical confidence and availability of equipment. Patient limiting factors include; the inability to close their mouth to seal around the interface, not wanting to trial MPV and a lack of available interfaces / equipment. The 252nd ENMC international workshop report provides documents to support best practice for the provision and management of MPV in NMD's.
List of participants
Michel Toussaint, Belgium
Michelle Chatwin, UK
Jésus Gonzalez-Bermejo, France
Miguel Gonçalves, Portugal
Joshua O. Benditt, USA
Doug McKim, Canada
Brit Hov, Norway
Valeria Sansone, Italy
Hélène Prigent, France
Annalisa Carlucci, Italy
Peter Wijkstra, The Netherlands
Tiina Andersen, Norway
Barbara Garabelli, Italy
Joan Escarrabill, Spain
Tiago Pinto, Portugal
Nicolas Audag, Belgium
Laura Verweij-van den Oudenrijn, The Netherlands
Adam Onga, Switzerland
Wendy Hughes, UK
Christian Devaux, France
Johann Chaulet, France
Jesus Sancho, Spain
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nmd.2020.07.008.
Contributor Information
ENMC Respiratory Therapy Consortium:
Michel Toussaint, Michelle Chatwin, Jésus Gonzalez-Bermejo, Miguel Gonçalves, Joshua O. Benditt, Doug McKim, Brit Hov, Valeria Sansone, Hélène Prigent, Annalisa Carlucci, Peter Wijkstra, Tiina Andersen, Barbara Garabelli, Joan Escarrabill, Tiago Pinto, Nicolas Audag, Laura Verweij-van den Oudenrijn, Adam Onga, Wendy Hughes, Christian Devaux, Johann Chaulet, and Jesus Sancho
Appendix. Supplementary materials
References
- 1.Shneerson J.M., Simonds A.K. Noninvasive ventilation for chest wall and neuromuscular disorders. Eur Respir J. 2002;20:480–487. doi: 10.1183/09031936.02.00404002. [DOI] [PubMed] [Google Scholar]
- 2.Hull J., Aniapravan R., Chan E. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax. 2012;67(Suppl 1):i1–40. doi: 10.1136/thoraxjnl-2012-201964. [DOI] [PubMed] [Google Scholar]
- 3.Round Table Conference on Poliomyelitis Equipment, Roosevelt Hotel, White Plaines, New York City National Foundation for Infantile Paralysis-March of Dimes, IncMay 1953.
- 4.Bach J.R., Alba A.S., Saporito L.R. Intermittent positive pressure ventilation via the mouth as an alternative to tracheostomy for 257 ventilator users. Chest. 1993;103:174–182. doi: 10.1378/chest.103.1.174. [DOI] [PubMed] [Google Scholar]
- 5.Annane D., Orlikowski D., Chevret S. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database Syst Rev. 2014;2014 doi: 10.1002/14651858.CD001941.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toussaint M., Chatwin M., Gonzales J., Berlowitz D.J. 228th ENMC International Workshop: airway clearance techniques in neuromuscular disorders Naarden, The Netherlands, 3-5 March 2017. Neuromuscular Disord: NMD. 2018;28:289–298. doi: 10.1016/j.nmd.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Chatwin M., Toussaint M., Goncalves M.R. Airway clearance techniques in neuromuscular disorders: a state of the art review. Respir Med. 2018;136:98–110. doi: 10.1016/j.rmed.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Yang G.F., Alba A., Lee M., Khan A. Pneumobelt for sleep in the ventilator user: clinical experience. Arch Phys Med Rehabil. 1989;70:707–711. [PubMed] [Google Scholar]
- 9.Khirani S., Ramirez A., Delord V. Evaluation of ventilators for mouthpiece ventilation in neuromuscular disease. Respir Care. 2014;59:1329–1337. doi: 10.4187/respcare.03031. [DOI] [PubMed] [Google Scholar]
- 10.Pinto T., Chatwin M., Banfi P., Winck J.C., Nicolini A. Mouthpiece ventilation and complementary techniques in patients with neuromuscular disease: a brief clinical review and update. Chron Respir Dis. 2017;14:187–193. doi: 10.1177/1479972316674411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boitano L.J., Benditt J.O. An evaluation of home volume ventilators that support open-circuit mouthpiece ventilation. Respir Care. 2005;50:1457–1461. [PubMed] [Google Scholar]
- 12.Hess D.R. Noninvasive Ventilation for Neuromuscular Disease. Clin Chest Med. 2018;39:437–447. doi: 10.1016/j.ccm.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Ogna A., Prigent H., Falaize L. Accuracy of tidal volume delivered by home mechanical ventilation during mouthpiece ventilation: a bench evaluation. Chron Respir Dis. 2016;13:353–360. doi: 10.1177/1479972316647177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorentino G., Esquinas A.M. Home ventilator performances with mouthpiece ventilation: does resistance change effectiveness? Clin Respir J. 2018;12:1765–1766. doi: 10.1111/crj.12676. [DOI] [PubMed] [Google Scholar]
- 15.Nardi J., Leroux K., Orlikowski D., Prigent H., Lofaso F. Home monitoring of daytime mouthpiece ventilation effectiveness in patients with neuromuscular disease. Chron Respir Dis. 2016;13:67–74. doi: 10.1177/1479972315619575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toussaint M., Steens M., Wasteels G., Soudon P. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J. 2006;28:549–555. doi: 10.1183/09031936.06.00004906. [DOI] [PubMed] [Google Scholar]
- 17.Fauroux B., Leroux K., Pepin J.L., Lofaso F., Louis B. Are home ventilators able to guarantee a minimal tidal volume? Intensive Care Med. 2010;36:1008–1014. doi: 10.1007/s00134-010-1785-9. [DOI] [PubMed] [Google Scholar]
- 18.Khirani S., Louis B., Leroux K., Delord V., Fauroux B., Lofaso F. Harms of unintentional leaks during volume targeted pressure support ventilation. Respir Med. 2013;107:1021–1029. doi: 10.1016/j.rmed.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Sogo A., Montanya J., Monso E., Blanch L., Pomares X., Lujan M. Effect of dynamic random leaks on the monitoring accuracy of home mechanical ventilators: a bench study. BMC Pulm Med. 2013;13:75. doi: 10.1186/1471-2466-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlucci A., Schreiber A., Mattei A. The configuration of bi-level ventilator circuits may affect compensation for non-intentional leaks during volume-targeted ventilation. Intensive Care Med. 2013;39:59–65. doi: 10.1007/s00134-012-2696-8. [DOI] [PubMed] [Google Scholar]
- 21.Finder J.D., Birnkrant D., Carl J. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004;170:456–465. doi: 10.1164/rccm.200307-885ST. [DOI] [PubMed] [Google Scholar]
- 22.Bedard M.E., McKim D.A. Daytime mouthpiece for continuous noninvasive ventilation in individuals with amyotrophic lateral sclerosis. Respir Care. 2016;61:1341–1348. doi: 10.4187/respcare.04309. [DOI] [PubMed] [Google Scholar]
- 23.McKim D.A., Griller N., LeBlanc C., Woolnough A., King J. Twenty-four hour noninvasive ventilation in Duchenne muscular dystrophy: a safe alternative to tracheostomy. Can Respir J. 2013;20:e5–e9. doi: 10.1155/2013/406163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bach J.R. Noninvasive Respiratory Management of Patients With Neuromuscular Disease. Ann Rehabil Med. 2017;41:519–538. doi: 10.5535/arm.2017.41.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garuti G., Nicolini A., Grecchi B., Lusuardi M., Winck J.C., Bach J.R. Open circuit mouthpiece ventilation: concise clinical review. Rev Port Pneumol. 2014;20:211–218. doi: 10.1016/j.rppneu.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Fiorentino G., Esquinas A.M. Tidal volume during mouthpiece non-invasive home ventilation: when the choice is the right answer. Chron Respir Dis. 2016;13:383–384. doi: 10.1177/1479972316661927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogna A., Lofaso F. Mouthpiece ventilation: individualized patient care is the key to success. Chron Respir Dis. 2016;13:385–386. doi: 10.1177/1479972316661928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalziel J. On sleep and an apparatus for promoting artificial respiration. Br Assoc Advancement Sci. 1838;2 [Google Scholar]
- 29.Toussaint M., Chatwin M., Verhulst S., Reychler G. Preference of neuromuscular patients regarding equipment for daytime mouthpiece ventilation: a randomized crossover study. Clin Respir J. 2020;14:214–221. doi: 10.1111/crj.13118. [DOI] [PubMed] [Google Scholar]
- 30.Bach J.R., O'Brien J., Krotenberg R., Alba A.S. Management of end stage respiratory failure in Duchenne muscular dystrophy. Muscle Nerve. 1987;10:177–182. doi: 10.1002/mus.880100212. [DOI] [PubMed] [Google Scholar]
- 31.Ward K., Ford V., Ashcroft H., Parker R. Intermittent daytime mouthpiece ventilation successfully augments nocturnal non-invasive ventilation, controlling ventilatory failure and maintaining patient independence. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-209716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garuti G., Nicolini A., D R. Mouthpiece ventilation in patients with neuromuscular disease: a brief clinical review. Phys Med Rehab - Int. 2014;1:1–4. [Google Scholar]
- 33.Gross R.D., Atwood C.W., Jr., Grayhack J.P., Shaiman S. Lung volume effects on pharyngeal swallowing physiology. J Appl Physiol (1985) 2003;95:2211–2217. doi: 10.1152/japplphysiol.00316.2003. [DOI] [PubMed] [Google Scholar]
- 34.Terzi N., Orlikowski D., Aegerter P. Breathing-swallowing interaction in neuromuscular patients: a physiological evaluation. Am J Respir Crit Care Med. 2007;175:269–276. doi: 10.1164/rccm.200608-1067OC. [DOI] [PubMed] [Google Scholar]
- 35.Garguilo M., Lejaille M., Vaugier I. Noninvasive mechanical ventilation improves breathing-swallowing interaction of ventilator dependent neuromuscular patients: a prospective crossover study. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0148673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Britton D., Hoit J.D., Benditt J.O. Swallowing with noninvasive positive-pressure ventilation (NPPV) in individuals with muscular dystrophy: a qualitative analysis. Dysphagia. 2019 doi: 10.1007/s00455-019-09997-6. [DOI] [PubMed] [Google Scholar]
- 37.Britton D., Hoit J.D., Pullen E., Benditt J.O., Baylor C.R., Yorkston K.M. Experiences of speaking with noninvasive positive pressure ventilation: a qualitative investigation. Am J Speech Lang Pathol. 2019;28:784–792. doi: 10.1044/2019_AJSLP-MSC18-18-0101. [DOI] [PubMed] [Google Scholar]
- 38.Glerant J.C., Rose D., Oltean V., Dayen C., Mayeux I., Jounieaux V. Noninvasive ventilation using a mouthpiece in patients with chronic obstructive pulmonary disease and acute respiratory failure. Respiration. 2007;74:632–639. doi: 10.1159/000105163. [DOI] [PubMed] [Google Scholar]
- 39.Bach J.R., Gonçalves M.R., Hamdani I., Winck J.C. Extubation of patients with neuromuscular weakness: a new management paradigm. ChestChest. 2010;137:1033–1039. doi: 10.1378/chest.09-2144. [DOI] [PubMed] [Google Scholar]
- 40.Koopman M., Vanfleteren L., Steijns S., Wouters E.F.M., Sprooten R. Increased exercise tolerance using daytime mouthpiece ventilation for patients with diaphragm paralysis. Breathe (Sheff) 2017;13:225–229. doi: 10.1183/20734735.005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manary M.P., Boulding W., Staelin R., Glickman S.W. The patient experience and health outcomes. N Engl J Med. 2013;368:201–203. doi: 10.1056/NEJMp1211775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.