Abstract
Glucose 6-phosphate dehydrogenase (G6PD) deficiency facilitates human coronavirus infection due to glutathione depletion. G6PD deficiency may especially predispose to hemolysis upon coronavirus disease-2019 (COVID-19) infection when employing pro-oxidant therapy. However, glutathione depletion is reversible by N-acetylcysteine (NAC) administration. We describe a severe case of COVID-19 infection in a G6PD-deficient patient treated with hydroxychloroquine who benefited from intravenous (IV) NAC beyond reversal of hemolysis. NAC blocked hemolysis and elevation of liver enzymes, C-reactive protein (CRP), and ferritin and allowed removal from respirator and veno-venous extracorporeal membrane oxygenator and full recovery of the G6PD-deficient patient. NAC was also administered to 9 additional respirator-dependent COVID-19-infected patients without G6PD deficiency. NAC elicited clinical improvement and markedly reduced CRP in all patients and ferritin in 9/10 patients. NAC mechanism of action may involve the blockade of viral infection and the ensuing cytokine storm that warrant follow-up confirmatory studies in the setting of controlled clinical trials.
Keywords: Coronavirus 19, COVID-19, N-acetylcysteine, Glutathione, Glucose 6-phosphate dehydrogenase, Mechanistic target of rapamycin, C-reactive protein, Ferritin, Respirator, Extracorporeal membrane oxygenation
1. Introduction
New York is currently the epicenter of the current COVID-19 pandemic caused by SARS-CoV-2. Up until this report, cases of hydroxychloroquine-induced hemolysis in G6PD-deficiency patients affected by COVID-19 have been lacking despite the widespread use of hydroxychloroquine as a treatment option for COVID-19. Hydroxychloroquine oxidative properties decreases glutathione (GHS) levels. Normally, cellular GSH can be regenerated from its oxidized form at the expense of reduced nicotinamide adenine dinucleotide phosphate (NADPH), which is an essential product of the pentose phosphate pathway (PPP). G6PD is a rate-limiting enzyme of NADPH synthesis by the PPP [1]. Thus, diminished production of NADPH leads to a profound depletion of GSH and the subsequent risk of hemolysis in G6PD-deficient patients. NAC can reverse the depletion of GSH imposed by diminished production of NADPH through the PPP [2]. Here, we report a case of hydroxychloroquine-induced severe hemolysis in a G6PD-deficient patient with COVID-19 infection and the successful treatment with IV NAC.
2. NAC treatment of G6PD-deficient patient and nine additional respirator-dependent subjects
A 44-year-old man presented to NYU Langone emergency department on March 20th 2020 with a 5-day history of fever, cough, and shortness of breath. He was earlier diagnosed with G6PD deficiency after hemolytic reaction to sulfa drugs. On admission, physical exam was notable for a body mass index of 37, blood pressure of 138/81 mmHg, pulse of 102 beats/min, respiratory rate of 25 per minute, and temperature of 39.5C. His oxygen (O2) saturation was 85% on room air and 94% on 4 l of O2 via nasal cannula. Patient tested positive for SARS-CoV-2 by PCR. Upon admission, his inflammatory markers, such as C-reactive protein (CRP), ferritin, and D-dimer, neutrophil to lymphocyte ratio (NLR) were elevated. His liver function tests, hemoglobin (Hb), and white blood cell count were normal (Table 1 ). Patient was started on hydroxychloroquine on March 21st and received only one dose of 400 mg. His respiratory status continued to worsen and required intubation on March 24th. Despite intubation and maximum ventilation settings the patient respiratory status continued to deteriorate requiring veno-venous extracorporeal membrane oxygenator (VV ECMO) which was started on March 26th.
Table 1.
Laboratory test values of G6PD-deficient patient upon admission for COVID-19 infection before administration of hydroxychloroquine.
| Variable | Admission value | Reference value |
|---|---|---|
| G6PD U/g Hemoglobin | 0.5 | > 9 |
| White blood cells x103 /μL | 5.3 | 4.2–9.1 |
| Hemoglobin mg/dL | 12.6 | 13.7–17.5 |
| Platelets x 103 / μL | 205 | 150–400 |
| Neutrophil % | 73 | 34–68 |
| Lymphocyte % | 18 | 22–53 |
| C-reactive protein mg/L | 45 | 0–5 |
| Ferritin ng/ml | 491 | 22–248 |
| D-dimer ng/ml | 520 | < 230 |
| Bilirubin, total mg/dL | 1.0 | 0.2–1.2 |
| Bilirubin, direct mg/dL | 0.5 | 0–0.5 |
| Interlukin-6 pg/ml | 20 | < 5 |
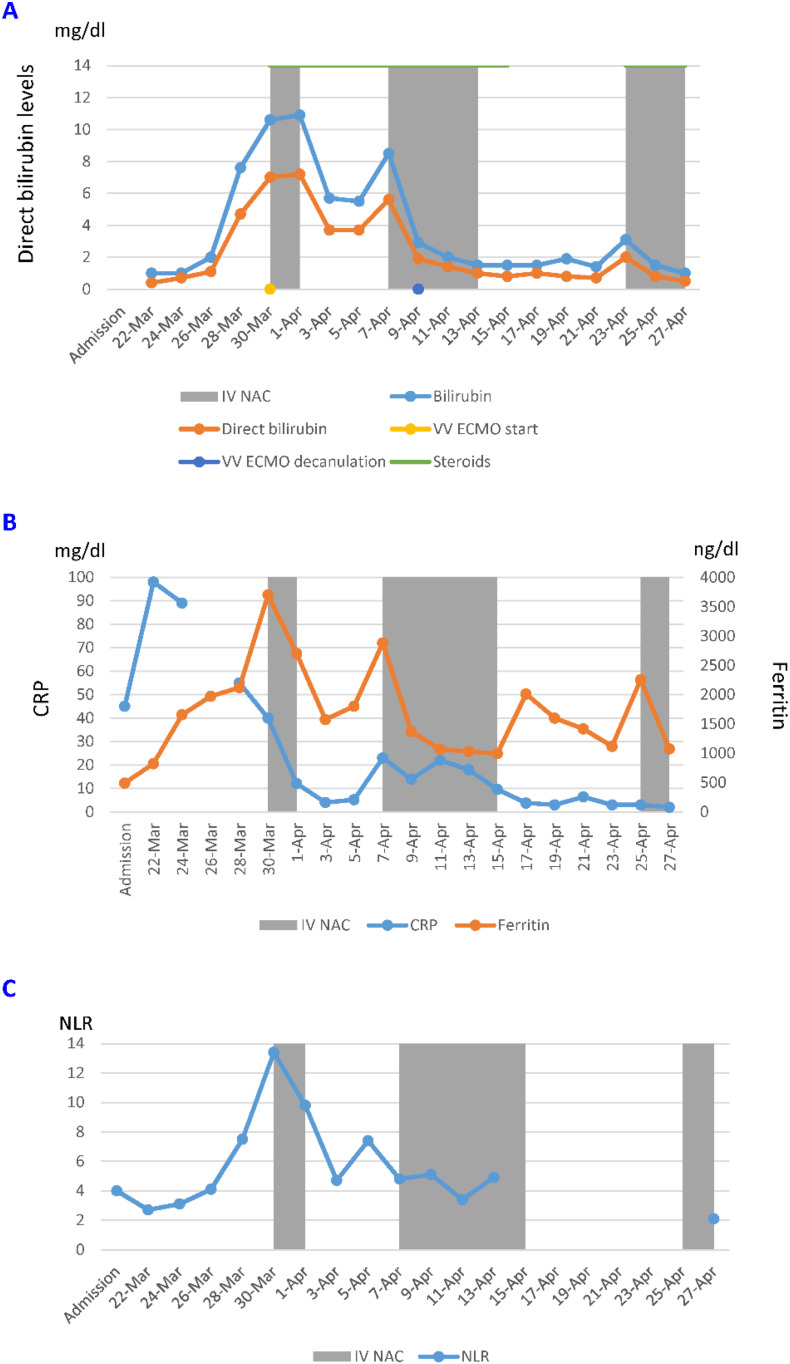
On March 29th, his Hb level dropped (7.9 g/dL) and his direct (6.0 mg/dL) and total bilirubin have become dramatically elevated (9.0 mg/dL). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) peaked at 263 U/L and 338 U/L, respectively. Further testing revealed low G6PD level (0.5 U/g Hb) and haptoglobin (2 mg/dL). Blood smear showed bell cells suggesting G6PD-deficiency hemolysis. The patient was started on IV NAC on March 30th (30,000 mg divided into three doses over 24 h). This was followed by immediate improvement in hemolysis indices (Fig. 1A). ALT and AST improved to 100 U/L and 62 U/L, respectively. On April 7th, one week after IV NAC discontinuation, total and direct bilirubin started rising again and IV NAC was re-started at 600 mg every 12 h for one week. Again, IV NAC administration was associated with resolution of hemolysis as evident by sustained reduction in bilirubin (total and direct) (Fig. 1A) and an increase in haptoglobin. Patient oxygenation continued to improve and his VV ECMO was discontinued on April 9th. Ten days after discontinuation of the second round of IV NAC, a slight increase in both total and direct bilirubin was noted, IV NAC was started again on April 25th at 600 mg every 12 h, this again was associated with reduction in total and direct bilirubin (Fig. 1A). Patient continued to improve clinically and was discharged to rehab on April 27th and was then discharged home on April 30th. Of note, the patient was treated with steroids starting March 30th. Interestingly, we observed a reduction in inflammatory markers (CRP and ferritin) that coincided with IV NAC administration (Fig. 1B). Additionally, IV NAC was associated with a decrease in NLR that was sustained after the first dose (Fig. 1C).
Fig. 1.
Effect of IV NAC on clinical and laboratory outcomes in a G6PD-deficient patient infected by COVID-19. Gray shaded areas represent intervals of IV NAC administration. Initiation and termination of CC-ECMO are indicated along the horizontal axis with yellow and blue dots, respectively. A) Display of total and direct bilirubin levels. B) Tracking of CRP and ferritin levels. C) Monitoring of neutrophil/lymphocyte ratio (NLR). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Due to this successful outcome, IV NAC was given to 9 consecutive COVID-19 patients without G6PD deficiency (Table 2 ). Eight of the nine patients required VV ECMO. We have observed a significant overall reduction in inflammatory markers (CRP and ferritin) during IV NAC administration. A rebound of inflammation was noted in six patients following discontinuation of NAC (Supplementary Figs. S1-S6). In the other three patients IV NAC was associated with decrease in CRP and ferritin without rebound increase after discontinuation. The median CRP level during IV NAC administration 55 mg/dL [interquartile range (29–109)] was significantly lower than during the periods without IV NAC (either before administration 143 mg/dL (46–235), or after IV NAC discontinuation 69 mg/dL (27–114) (Fig. S7). Table 2 displays peak CRP levels before NAC as well as CRP level at the completion of NAC administration for each individual patient.
Table 2.
Effect of IV NAC on inflammation assessed by serum levels of CRP (mg/ml) and ferritin (ng/ml) and clinical outcome of COVID-19 infection in 9 patients without G6PD deficiency. *, p = .0022; **, p = .0301, using two-tailed paired t-test.
| Patient (Age/Gender) |
CRP before NAC |
CRP after NAC |
Ferritin before NAC | Ferritin after NAC | NAC duration | NAC dose (mg) | ECMO | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1(44/M) | 89 | 14 | 3700 | 1500 | 2 days | 30,000 | Yes | Discharged home |
| 2 (44/M) | 90 | 13 | 9000 | 2000 | 2 days | 20,000 | Yes | Discharged Home |
| 3 (48/M) | 243 | 72 | 5900 | 2700 | 7 days | 600 every 12 h | Yes | Discharged Home |
| 4 (38/M) | 280 | 26 | 4900 | 900 | 9 days | 600 every 12 h | Yes | Hospitalized |
| 5 (38/M) | 46 | 5 | 1100 | 800 | 4 days | 600 every 12 h | Yes | Discharged home |
| 6 (42/M) | 235 | 31 | 4000 | 2500 | 5 days | 600 every 12 h | Yes | Hospitalized |
| 7 (48/F) | 99 | 45 | 300 | 330 | 4 days | 600 every 12 h | Yes | Discharged Home |
| 8 (48/M) | 307 | 23 | 2700 | 1100 | 6 days | 600 every 12 h | Yes | Discharged Home |
| 9 (71/M) | 145 | 71 | 2200 | 1800 | 5 days | 600 every 12 h | No | Discharged home |
| 10 (65/M) | 63 | 11 | 2800 | 1800 | 4 days | 600 every 12 h | Yes | Discharged home |
| Mean ± SD | 160 ± 97 | 31 ± 24* | 3630 ± 2526 | 1543 ± 762** |
3. Discussion
We describe a remarkable benefit of IV NAC in severe COVID-19 infection. As expected, IV NAC initially mitigated the hemolysis in a G6PD-deficient, COVID-19-infected patient treated with hydroxychloroquine for a single day. We also observed an obvious drop in inflammatory markers following initial NAC administration. This was followed by rebound rises of CRP and ferritin upon discontinuation of NAC. Re-started IV NAC for two additional intervals resulted in repeated drops in CRP and ferritin levels (Fig. 1B). NAC administration allowed the discontinuation of ECMO and eventual discharge of the patient to his home.
G6PD deficiency was first described in the setting of hemolysis induced by the anti-malarial medication primaquine [3]. In red blood cells, G6PD is essential for the PPP to produce NADPH, which is used by glutathione reductase to regenerate GSH from oxidized glutathione (GSSG) [1]. The depletion of GSH in G6PD deficient cells can be reversed by NAC that helps replenish cellular GSH [4]. An in-vitro study showed that G6PD deficient cells were more susceptible to infection by the human coronavirus (HCoV 229E) [5]. The association between G6PD deficiency and COVID 19 has not yet been reported despite the widespread use of hydroxychloroquine. This could be due to the minimal oxidative stress that hydroxychloroquine exerts compared to chloroquine and primaquine [6]. Additionally, G6PD deficiency can vary in severity based on specific genetic polymorphisms. Nonetheless, our case shows that caution should be exerted when considering hydroxychloroquine as a treatment option for these patients. Reports from other epicenters where G6PD deficiency is far more prevalent -such as Italy- may shed more light on a potentially overlooked association.
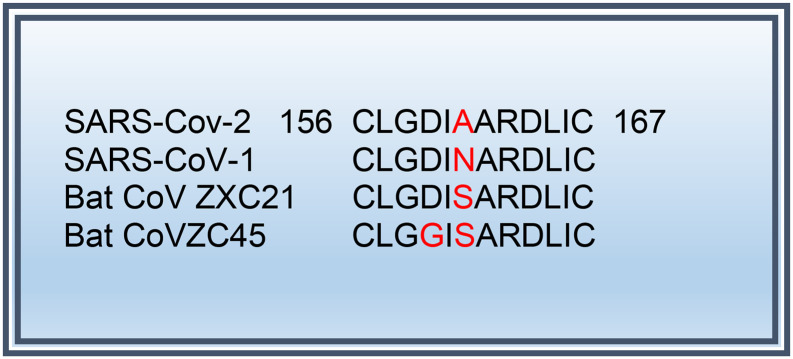
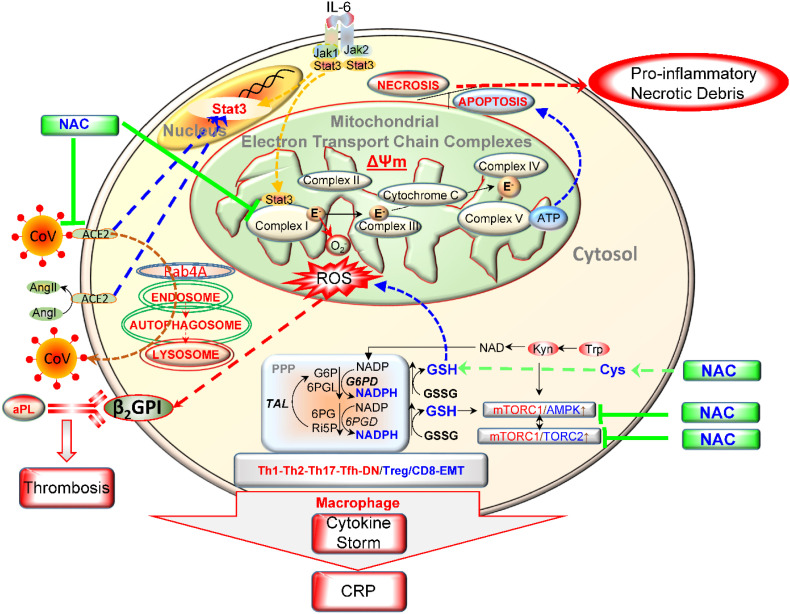
The CRP and ferritin responses to IV NAC were favorable in our patient, and we observed similar benefit in nine additional patients without G6PD deficiency. Anti-viral [7,8] and anti-inflammatory properties of NAC have been well documented [[9], [10], [11]]. The morbidity and mortality of human coronaviruses causing lower respiratory tract infections appears to stem from the exuberant immune response of the host. High serum levels of pro-inflammatory cytokines have been reported. Interleukin-6 (IL-6) has been proposed to play an essential role in COVID-19 associated cytokine storm [12]. NAC has been found to reduce IL-6-dependent CRP elevation during H1N1 influenza pneumonia [13]. NAC is a cell-permeable precursor of reduced GSH. Preclinical studies have shown that GSH-capped nanoclusters inhibit coronavirus replication through blockage of viral RNA synthesis and budding [7]. Furthermore, an in vitro study showed that NAC was able to reduce H5N1 viral replication [8]. Apparently, host cell infection by COVID-19 depends on the interaction between the receptor binding domain (RBD) of the viral spike glycoprotein S2 subdomain and the peptidase domain of the angiotensin converting enzyme 2 (ACE2) receptor [14]. The S2 subdomain of SARS-CoV-1, which lies 6 amino acids away from the fusion peptide, is flanked by two cysteine residues that are essential for membrane fusion [15], is conserved across all coronaviruses (Fig. 2 ). The post- translational disulfide bond between the two cysteine residues (C156 and C167) is apparently essential for fusion complex exposure and the subsequent membrane fusion [15], which may be disrupted by NAC. Moreover, NAC blocks mTOR [9] which is a central regulator of inflammation within the immune system (Fig. 3 ) [[16], [17], [18]] and required for binding of its substrates LARP1 and FKBP7 to viral N and ORF8 proteins [19].
Fig. 2.
Highly Conserved motif in the S2 subdomain of some coronaviruses (including SARS-CoV1 and SARS-CoV2). This motif, that lies six residues away from the fusion peptide, is flanked by two highly conserved cysteine residues between which a disulfide bond is essential for membrane fusion 1. More CoVs sequences available in [15].
Fig. 3.
Schematic diagram of metabolic pathways that control oxidative stress and mTOR-dependent generation of cytokine storm. The depicted surface receptors and transducers exemplify those that operate in T cells and underlie pro-inflammatory lineage development as well as hepatocytes that secrete apolipoprotein H, also known as β2-glycoprotein I (β2GPI). Oxidized β2GPI in the primary antigen that elicits the formation of antiphospholipid antibodies (aPL) in patients with antiphospholipid syndrome [22]. Thus, oxidation of β2GPI induces not only aPL but also promotes cardiovascular disease [24] in the setting of COVID-19 infection [[25], [26], [27]]. IL-6, the primary cytokine that drives inflammation in COVID-19 infected patients, elicits mitochondrial oxidative stress at complex I of the mitochondrial electron transport chain (ETC). In turn, this leads to redox-dependent activation of mTORC1. Further downstream, uncontrolled activation of mTORC1 promotes inflammation [28]. NAC inhibits oxidative stress by serving as a cell-permeable amino acid precursor of the main intracellular antioxidant, GSH. Acting outside the cell, NAC may break disulfide bonds within ACE2 that serves as the cellular receptor for COVID-19 [15]. NAC may also block COVID-19 binding by disrupting disulfide bind within its receptor-binding domain [29]. In addition to epithelial, endothelial, and myocardial cells [30,31], ACE2 is expressed on T lymphocytes [32], macrophages [33], and hepatocytes [[34], [35], [36], [37]]. ACE2 controls the expression of pro-inflammatory transcription factor Stat3 [[38], [39], [40], [41], [42], [43]], which also modulates the production of reactive oxygen intermediates by complex I of the mitochondrial electron transport chain (ETC) [44]. ACE2 also attenuates signaling through mTORC1 [[45], [46], [47], [48]].
We propose that NAC restrains the pro-inflammatory metabolic pathways that control oxidative stress and mTOR-dependent generation of cytokine storm emanating from the immune system [20]. mTOR blockade also abrogates the production of oxidized apolipoprotein H, also known as β2-glycoprotein I (β2GPI) by hepatocytes [21]. Oxidized β2GPI is the primary antigen that elicits the formation of antiphospholipid antibodies (aPL) in patients with antiphospholipid syndrome [22]. Direct blockade of mTOR with sirolimus also attenuates aPL production in patients with lupus [23].Thus, oxidation of β2GPI induces not only aPL but also promotes cardiovascular disease [24] in the setting of COVID-19 infection [[25], [26], [27]]. IL-6, the primary cytokine that drives inflammation in COVID-19 infected patients, elicits mitochondrial oxidative stress at complex I of the mitochondrial electron transport chain (ETC). In turn, this leads to redox-dependent activation of mTORC1. Further downstream, uncontrolled activation of mTORC1 promotes inflammation [28]. NAC inhibits oxidative stress by serving as a cell-permeable amino acid precursor of the main intracellular antioxidant, GSH. Acting outside the cell, NAC may break disulfide bonds within ACE2 that serves as the cellular receptor for COVID-19 [15]. NAC may also block COVID-19 binding by disrupting disulfide bind within its receptor-binding domain [29]. In addition to epithelial, endothelial, and myocardial cells [30,31], ACE2 is expressed on T lymphocytes [32], macrophages [33], and hepatocytes [[34], [35], [36], [37]]. ACE2 controls the expression of pro-inflammatory transcription factor Stat3 [[38], [39], [40], [41], [42], [43]], which also modulates the production of reactive oxygen intermediates by complex I of the mitochondrial electron transport chain (ETC) [44]. ACE2 also attenuates signaling through mTORC1 [[45], [46], [47], [48]] (Fig. 3). In turn, mTOR-dependent promoter hypomethylation causes increased expression of ACE2 which may underlie severe infection and poor outcomes in patients with preexisting comorbidities, such as lupus and cancer [[49], [50], [51]]. Our findings support the notion that mTOR blockade with sirolimus is expected to improve the clinical outcome of COVID-19 infection [52]. Several anti-inflammatory medications have been shown to mitigate the cytokine storm in COVID-19 infection, such as corticosteroids [53], colchicine [54], imatinib [55], and complement C3 inhibitor AMY-101 [56]. However, the safety of mTOR blockade stands out based on its propensity to extend overall lifespan [57].
IV NAC has long been used to safely treat patients with acetaminophen overdose [58,59], or ARDS [60,61]. NAC was also found to reduce CRP levels in several controlled clinical trials [10,11]. CRP elevation is a prominent risk factor for disease progression in patients infected with COVID-19 [62,63]. Whether these anti-inflammatory changes were specific to the use of NAC is difficult to discern from our study due to sporadic use of steroids and other anti-inflammatory drugs. However, it is conceivable that NAC has beneficial effects in reducing inflammation in patients infected with COVID 19. Our observations warrant prospective studies to look at the clinical and laboratory effects of NAC and to evaluate the role of IV NAC –if any- in the treatment of severe COVID 19 patients.
Acknowledgements
This work was supported in part by grants AI 072648, AI 122176, AI141304, AR068052, and AR076092 from the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2020.108544.
Appendix A. Supplementary data
References
- 1.Perl A., Hanczko R., Telarico T., Oaks Z., Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 2011;7:395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banki K., Hutter E., Colombo E., Gonchoroff N.J., Perl A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J. Biol. Chem. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 3.Carson P.E., Flanagan C.L., Ickes C.E., Alving A.S. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124(3220):484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- 4.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014;141(2):150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y.H., Tseng C.P., Cheng M.L., Ho H.Y., Shih S.R., Chiu D.T.Y. Glucose-6-phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. J. Infect. Dis. 2008;197(6):812–816. doi: 10.1086/528377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovanella F., Ferreira G.K., de Pra S.D., Carvalho-Silva M., Gomes L.M., Scaini G. Effects of primaquine and chloroquine on oxidative stress parameters in rats. An. Acad. Bras. Cienc. 2015;85 doi: 10.1590/0001-3765201520140637. (1678–2690 (Electronic)):1487–96. [DOI] [PubMed] [Google Scholar]
- 7.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L. Glutathione-Capped Ag2S Nanoclusters Inhibit Coronavirus Proliferation through Blockage of Viral RNA Synthesis and Budding. ACS Appl. Mater. Interfaces. 2018;10 doi: 10.1021/acsami.7b13811. (1944–8252 (Electronic)):4369–78. [DOI] [PubMed] [Google Scholar]
- 8.Geiler J., Michaelis M., Naczk P., Leutz A., Langer K., Doerr H.W. N-acetyl-l-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza a virus. Biochem. Pharmacol. 2010;79(3):413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Lai Z.-W., Hanczko R., Bonilla E., Caza T.N., Clair B., Bartos A. N-acetylcysteine reduces disease activity by blocking mTOR in T cells of lupus patients. Arthritis Rheum. 2012;64(9):2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porcu M., Urbano M.R., Verri W.A., Barbosa D.S., Baracat M., Vargas H.O. Effects of adjunctive N-acetylcysteine on depressive symptoms: modulation by baseline high-sensitivity C-reactive protein. Psychiatry Res. 2018;263:268–274. doi: 10.1016/j.psychres.2018.02.056. [DOI] [PubMed] [Google Scholar]
- 11.Zuin R., Palamidese A., Negrin R., Catozzo L., Scarda A., Balbinot M. High-dose N-acetylcysteine in patients with exacerbations of chronic obstructive pulmonary disease. Clin. Drug Invest. 2005;25(6):401–408. doi: 10.2165/00044011-200525060-00005. [DOI] [PubMed] [Google Scholar]
- 12.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5) doi: 10.1172/JCI137244. https://www.jci.org/articles/view/137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai K.Y., Ng W.Y., Osburga Chan P.K., Wong K.F., Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann. Intern. Med. 2010;152(10):687–688. doi: 10.7326/0003-4819-152-10-201005180-00017. [DOI] [PubMed] [Google Scholar]
- 14.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madu I.G., Belouzard S., Whittaker G.R. SARS-coronavirus spike S2 domain flanked by cysteine residues C822 and C833 is important for activation of membrane fusion. Virology. 2009;393(2):265–271. doi: 10.1016/j.virol.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012;12(5):325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang N., Perl A. Metabolism as a target for modulation in autoimmune diseases. Trends Immunol. 2018;39:562–576. doi: 10.1016/j.it.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Fajgenbaum D.C., Langan R.A., Japp A.S., Partridge H.L., Pierson S.K., Singh A. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6-blockade-refractory idiopathic multicentric Castleman disease. J. Clin. Invest. 2019;130(10):4451–4463. doi: 10.1172/JCI126091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020:581. doi: 10.1038/s41586-020-2286-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clin. Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oaks Z., Winans T., Caza T., Fernandez D., Liu Y., Landas S.K. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheum. 2016;68:2728–2739. doi: 10.1002/art.39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannou Y., Zhang J.Y., Passam F.H., Rahgozar S., Qi J.C., Giannakopoulos B. Naturally occurring free thiols within beta2-glycoprotein I in vivo: nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress-induced cell injury. Blood. 2010;116(11):1961–1970. doi: 10.1182/blood-2009-04-215335. [DOI] [PubMed] [Google Scholar]
- 23.Lai Z., Kelly R., Winans T., Marchena I., Shadakshari A., Yu J. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet. 2018;391:1186–1196. doi: 10.1016/S0140-6736(18)30485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee R., Margaritis M., Channon K.M., Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr. Med. Chem. 2012;19(16):2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020:1533–4406. doi: 10.1056/NEJMc2007575. (Electronic):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. 2020. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B. ST-Segment elevation in patients with Covid-19 - a case series. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perl A. Mechanistic target of rapamycin pathway activation in rheumatic diseases. Nat. Rev. Rheumatol. 2016;12:169–182. doi: 10.1038/nrrheum.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan M., Wu N.C., Zhu X., Lee C.C., So R.T.Y., Lv H. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. eabb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi F., Qian S., Zhang S., Zhang Z. 2020. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. LID - S0006-291X(20)30523–4 [pii] LID (1090–2104 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S. 2018. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration.(1522–1504 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Qixin, Sundar Isaac K., Li Dongmei, Lucas Joseph H., Muthumalage Thivanka, McDonough Samantha R., Rahman Irfan. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir Res. 2020;21(1):154. doi: 10.1186/s12931-020-01396-y. PMID: 32552811 PMCID: PMC7301079 DOI: 10.1186/s12931-020-01396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keidar S., Strizevsky A.F., Raz A.F., Gamliel-Lazarovich A. 2007. ACE2 activity is increased in monocyte-derived macrophages from prehypertensive subjects.(0931–0509 (Print)) [DOI] [PubMed] [Google Scholar]
- 34.Hofmann H., Hattermann K., Marzi A., Gramberg T., Geier M., Krumbiegel M. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 2004;78(12):6134–6142. doi: 10.1128/JVI.78.12.6134-6142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X., Song L.N., Zhang Y.C., Li Q., Shi T.T., Yang F.Y. Angiotensin-converting enzyme 2 inhibits endoplasmic reticulum stress-associated pathway to preserve nonalcoholic fatty liver disease. Diabetes Metab. Res. Rev. 2019;35(4) doi: 10.1002/dmrr.3123. [DOI] [PubMed] [Google Scholar]
- 36.Cao X., Yang F., Shi T., Yuan M., Xin Z., Xie R. 2016. Angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis.(2045–2322 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paizis G., Tikellis C., Cooper M.E., Schembri J.M., Lew R.A., Smith A.I. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54(12):1790. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sodhi C.P., Nguyen J., Yamaguchi Y., Werts A.D., Lu P., Ladd M.R. A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to pseudomonas aeruginosa lung infection in mice. J. Immunol. 2019;203(11):3000–3012. doi: 10.4049/jimmunol.1900579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J., Zhang W., Xu Q., Zhang J., Chen W., Xu Z. Ang-(1-7) protects HUVECs from high glucose-induced injury and inflammation via inhibition of the JAK2/STAT3 pathway. Int. J. Mol. Med. 2018;41(5):2865–2878. doi: 10.3892/ijmm.2018.3507. [DOI] [PubMed] [Google Scholar]
- 40.Lin C.I., Tsai C.H., Sun Y.L., Hsieh W.Y., Lin Y.C., Chen C.Y. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018;14(3):253–265. doi: 10.7150/ijbs.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu Y., Tao L., Zheng S., Lin R., Fu X., Chen Z. AAV8-mediated angiotensin-converting enzyme 2 gene delivery prevents experimental autoimmune uveitis by regulating MAPK, NF−+¦ B and STAT3 pathways. Sci. Rep. 2016;6 doi: 10.1038/srep31912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song B., Jin H., Yu X., Zhang Z., Yu H., Ye J. Angiotensin-converting enzyme 2 attenuates oxidative stress and VSMC proliferation via the JAK2/STAT3/SOCS3 and profilin-1/MAPK signaling pathways. Regul. Pept. 2013;185:44–51. doi: 10.1016/j.regpep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Zhong J., Basu R., Guo D., Chow F.L., Byrns S., Schuster M. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122(7):717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 44.Wegrzyn J., Potla R., Chwae Y.J., Sepuri N.B.V., Zhang Q., Koeck T. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323(5915):793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X., Zheng J., Yan Y., Ruan Z., Su Y., Wang J. Angiotensin-converting enzyme 2 regulates autophagy in acute lung injury through AMPK/mTOR signaling. Arch. Biochem. Biophys. 2019;672 doi: 10.1016/j.abb.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 46.Yisireyili M., Uchida Y., Yamamoto K., Nakayama T., Cheng X.W., Matsushita T. Angiotensin receptor blocker irbesartan reduces stress-induced intestinal inflammation via AT1a signaling and ACE2-dependent mechanism in mice. Brain Behav. Immun. 2018;69:167–179. doi: 10.1016/j.bbi.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 47.He F., Wu C., Li P., Li N., Zhang D., Zhu Q. Functions and signaling pathways of amino acids in intestinal inflammation. Biomed. Res. Int. 2018;2018 doi: 10.1155/2018/9171905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiao W., Wang C., Chen B., Zhang F., Liu Y., Lu Q. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131(2):97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 49.Sawalha A.H., Zhao M., Coit P., Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pruimboom L. Methylation pathways and SARS-CoV-2 lung infiltration and cell membrane-virus fusion are both subject to epigenetics. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chai P., Yu J., Ge S., Jia R., Fan X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: A pan-cancer analysis. J. Hematol. Oncol. 2020;13(1) doi: 10.1186/s13045-020-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omarjee L., Janin A., FDR Perrot, Laviolle B., Meilhac O., Mahe G. Targeting T-cell senescence and cytokine storm with rapamycin to prevent severe progression in COVID-19. Clin. Immunol. 2020;216:108464. doi: 10.1016/j.clim.2020.108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahase E. Covid-19: demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ Clin. Res. 2020;369:m2512. doi: 10.1136/bmj.m2512. [DOI] [PubMed] [Google Scholar]
- 54.La-Torre E., La-Torre F., Kusanovic M., Scotti R., Ramirez G.A., Dagna L. Treating COVID-19 with colchicine in community healthcare setting. Clin. Immunol. 2020;217:108490. doi: 10.1016/j.clim.2020.108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morales-Ortega A., Bernal-Bello D., Llarena-Barroso C., Frutos-Perez B., Duarte-Millan M.A., Garcia de Viedma-Garcia V. Imatinib for COVID-19: a case report. Clin. Immunol. 2020;218:108518. doi: 10.1016/j.clim.2020.108518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215:108450. doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann. N. Y. Acad. Sci. 2015;1346(1):33–44. doi: 10.1111/nyas.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong A., McNulty R., Taylor D., Sivilotti M., Greene S., Gunja N. The NACSTOP trial: a multicenter, cluster-controlled trial of early cessation of acetylcysteine in acetaminophen overdose. Hepatology. 2019;69(2):774–784. doi: 10.1002/hep.30224. [DOI] [PubMed] [Google Scholar]
- 59.Smilkstein M.J., Bronstein A.C., Linden C., Augenstein W.L., Kulig K.W., Rumack B.H. Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann. Emerg. Med. 1991;20(10):1058–1063. doi: 10.1016/s0196-0644(05)81352-6. [DOI] [PubMed] [Google Scholar]
- 60.Suter P.M., Domenighetti G., Schaller M.D., Laverriere M.C., Ritz R., Perret C. N-acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind. Placebo-Controlled Clinical Study. Chest. 1994;105(1):190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- 61.Ortolani O., Conti A., De Gaudio A.R., Masoni M., Novelli G. Vol. 13. 2000. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome; pp. 14–18. (1) [DOI] [PubMed] [Google Scholar]
- 62.Hou W., Zhang W., Jin R., Liang L., Xu B., Hu Z. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect. Dis. Ther. 2020;52:1–8. doi: 10.1080/23744235.2020.1759817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J., Yu M., Tong S., Liu L.Y., Tang L.V. Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan. China. J. Clin. Virol. 2020;127:104392. doi: 10.1016/j.jcv.2020.104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.