Abstract
Low plasma triiodothyronine (T3) concentration indicates nonthyroidal illness syndrome (NTIS), which might be associated with a poor outcome in patients in the intensive care unit (ICU). This study evaluated the relationship between NTIS and prognostic indicators in patients admitted to the ICU and examined the fT3 cut-off points that could be associated with 28-day mortality. This prospective observational study included patients admitted to the ICU of The Third Hospital of Hebei Medical University from February to November 2018. The baseline variables and the occurrence of low free T3 (FT3) were collected. The patients were divided into the NTIS (FT3 < 3.28) and non-NTIS groups. Among 305 patients, 118 (38.7%) were in the NTIS group. FT3 (P < 0.001) and FT4 (P = 0.001) were lower, while the 28-day mortality rate (P < 0.001) and hospitalization expenses in ICU (P = 0.001) were higher in the NTIS group. The univariable analyses identified NTIS, FT3, free thyroxine/FT3, APACHEII, sequential organ failure score, duration of mechanical ventilation, creatinine, oxygenation index, white blood cells, albumin, age, and brain natriuretic peptide as being associated with 28-day mortality (all P < 0.05). The cut-off value of FT3 for 28-day mortality was 2.88 pmol/L. The 28-day mortality rate and hospitalization expenses in the ICU were higher in patients with NTIS. NTIS was independently associated with 28-day mortality.
1. Background
Patients with normal basic thyroid function often present with abnormal thyroid hormone levels because of trauma, infection, surgery, inflammation, and other factors. This commonly results in decreased plasma triiodothyronine (T3), decreased or normal thyroxine (T4) and thyroid-stimulating hormone (TSH), and increased plasma reverse T3 (rT3) [1]. These alterations in thyroid hormones are known as the nonthyroidal illness syndrome (NTIS), euthyroid sick syndrome, or low T3 syndrome [2]. The magnitude of the changes in serum T3 and rT3 reflects the severity of the illness; patients with mild to moderate NTIS usually have normal plasma T4 and TSH concentrations, whereas patients with a more severe or prolonged illness will often have low serum T4 and TSH [3].
Some authors consider NTIS not to be a disease by itself but rather a manifestation of a self-protection mechanism in critically ill patients [4]. This syndrome is possibly associated with decreased 5′-deiodinase activity and increased 5-deiodination that results in more inactivation of thyroid hormone and the production of 3T3 caused by various factors activated by a systemic inflammatory reaction, decreasing the metabolism of T4 into active T3 [5, 6], but the mechanism remains unclear [3]. Studies have shown that NTIS is associated with an adverse prognosis in acute myocardial infarction [7], heart failure [8], sepsis [9], severe trauma [10], and acute respiratory distress syndrome [11]. T3 or free T3 (FT3) can be considered a prognostic indicator in myocardial infarction and heart failure [7, 8]. It was also found that there was an association between the decrease in T3 or FT3 levels and mortality in intensive care unit (ICU) patients [9, 11–13].
A study of 480 ICU patients from China published in 2012 suggested that FT3 can be used as a predictor of all-cause mortality in ICU patients [14]. Moreover, the predictive ability was improved when combined with the APACHE II score [14]. Nevertheless, over half of the subjects had cardiovascular disease and cardiopulmonary failure, with a mean age of 72 years and overall mortality rate as high as 19.2%. Therefore, the results of that study could not be representative of most ICU patients in China and around the world. Subgroup analysis suggested that FT3 might only have a prognostic value in cardiac diseases and not sepsis or other major ICU diseases [14]. Other studies in smaller numbers of critically ill patients suggest that the FT3 levels might help predict mortality in a more general population of ICU patients [15, 16]. A systematic review of nine studies concluded that whether current thyroid hormone level could be used as a prognostic indicator of sepsis mortality needed further study [9].
Therefore, further research is needed to clarify the association between NTIS and the prognosis of patients in the ICU. In particular, the value of FT3 as a prognostic marker for mortality in the general ICU population needs further investigation. The aim of this study was to undertake a prospective investigation of patients admitted to the ICU to investigate the incidence of NTIS and the value of a decreased FT3 level as a prognostic marker of mortality. The study also examined the fT3 cut-off point that could be associated with 28-day mortality.
2. Material and Methods
2.1. Patients
This prospective observational study recruited patients admitted to the ICU of The Third Hospital of Hebei Medical University between February 2018 and November 2018. The inclusion criteria were (1) 16-80 years old, (2) patients with organ failure who required supportive treatments or intensive monitoring, and with an APPACHE II score > 10, (3) no history of abnormal thyroid function according to the patient's medical chart, and (4) no history of thyroid diseases such as hyperthyroidism, hypothyroidism, and thyroid tumors. The exclusion criteria were (1) patients with less than 24 h stay in the ICU, (2) patients who donate organs, (3) pregnant women or breastfeeding women, (4) patients with endocrine tumors, or (5) patients administered with antithyroid drugs or other iodine-containing drugs, e.g., amiodarone. The enrolled patients were divided into the NTIS group (FT3 < 3.28 pmol/L) and the non-NTIS group based on their FT3 levels measured soon after hospital admission.
The study was approved by the ethics committee of The Third Hospital of Hebei Medical University (No. 2018-019-1), and written informed consent was obtained.
2.2. Clinical Data Collection and Examination Methods
During the study period, 400 patients were admitted to the ICU, but 95 were not included because of the eligibility criteria. No patient withdrew from the study. A total of 305 patients were enrolled in the study. The cause of ICU admission included sepsis or sepsis shock, body injury, brain injury, severe disease, after operation, tumor, and pulmonary embolism. Body injury was defined as trauma to limbs and internal organs. Brain injury was defined as brain trauma and nerve damage. Severe disease included severe liver and kidney damage.
Levels of FT3, FT4, and TSH were measured in the enrolled patients within 24 h of admission. A radioimmunoassay was used for their detection in the Department of Nuclear Medicine of our hospital (Beckman DXI800, USA), with the normal reference values of 3.28-6.47 pmol/L for FT3, 7.64-16.3 pmol/L for FT4, and 0.49-4.91 mIU/L for TSH, respectively.
The vital signs and related laboratory indicators of patients were recorded, including albumin (ALB), brain natriuretic peptide (BNP), procalcitonin (PCT), white blood cells (WBC), hemoglobin (HGB), platelet (PLT), creatinine (CREA), total bilirubin (TBil), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen 9FIB), blood gas analysis, and lactic acid. Blood gas analysis and lactic acid levels were detected in the ICU, with arterial blood extracted in patients and tested using a multifunction blood gas analyzer (Rayto, USA). The remaining laboratory indexes were measured by the Laboratory Department of our hospital.
The enrolled patients were subjected to the acute physiology and chronic health evaluation II score (APACHE II), Glasgow coma scale (GCS), and sequential organ failure score (SOFA). All tests and scores were completed within 24 h of admission to the ICU. The duration of mechanical ventilation, length of stay in ICU, and costs of hospitalization were recorded.
2.3. Definitions and Follow-Up
The diagnostic criteria of NTIS was an FT3 level lower than the lowest normal value in our hospital, i.e., <3.28 pmol/L, with FT4 lower than or equal to the normal range of detection, and TSH in the normal range [1]. The patients were followed up for 28 days (in the hospital) to evaluate their survival outcomes and by phone when discharged.
2.4. Statistical Analysis
All data were analyzed using SPSS 19.0 (IBM Corp., USA). Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Continuous data were expressed as means ± standard deviation (SD) if they met the normal distribution, and the independent-sample t-test was used for intergroup comparison. Nonnormally distributed continuous data were expressed as medians (interquartile range (IQR)). The Mann–Whitney U-test was used for comparison. >Categorical data were expressed as percentages, and the chi-squared test was used for intergroup comparison. Univariable and multivariable logistic analyses were conducted. Receiver operating characteristic (ROC) curves were used to detect the cut-off value of FT3 for 28-day mortality. Variables with P < 0.05 were included in the multivariable logistic analysis (stepwise), and multicollinearity was considered. Bilateral α = 0.05 was used as the threshold to determine significance.
3. Results
3.1. Baseline of Patient Characteristics
There were 209 males and 96 females, with a mean age of 55.3 ± 15.7 years. There were 118 patients in the NTIS group, accounting for 38.7%, and 187 cases in the non-NTIS group. Details of the two groups are shown in Table 1.
Table 1.
Baseline of characteristics of the study subjects.
| Variable | Total (n = 305) | NTIS (n = 118) | Non-NTIS (n = 187) | P |
|---|---|---|---|---|
| Males (%) | 31.5 | 30.5 | 32.1 | 0.96 |
| Age (years) | 55.3 ± 15.7 | 57.5 ± 16.6 | 54.0 ± 15.0 | 0.06 |
| Principal diagnosis leading to ICU admission (%) | <0.001 | |||
| Sepsis or sepsis shock | 31 | 25 | 6 | |
| Bodily injury | 137 | 44 | 99 | |
| Brain injury | 71 | 18 | 53 | |
| Severe disease of medicine | 23 | 9 | 14 | |
| After operation | 32 | 18 | 14 | |
| Tumor | 9 | 7 | 2 | |
| Pulmonary embolism | 2 | 1 | 1 | |
| APACHE II score | 12.69 ± 8.06 | 15.44 ± 9.09 | 10.95 ± 6.80 | <0.001 |
| GCS score | 12.2 ± 4.6 | 12.2 ± 4.5 | 12.2 ± 4.6 | 0.97 |
| SOFA score | 5.1 ± 3.9 | 6.3 ± 4.3 | 4.4 ± 3.4 | <0.001 |
| FT3 (pmol/L) | 3.48 ± 0.82 | 2.72 ± 0.40 | 3.97 ± 0.63 | <0.001 |
| FT4 (pmol/L) | 12.89 ± 4.64 | 11.79 ± 2.83 | 13.59 ± 5.37 | 0.001 |
| TSH (mIU/L) | 1.31 ± 3.35 | 1.67 ± 5.14 | 1.09 ± 1.27 | 0.148 |
| Vital signs when enrolled | ||||
| T (°C) | 35.93 ± 7.01 | 36.38 ± 5.97 | 35.66 ± 7.60 | 0.387 |
| HR | 90.9 ± 34.6 | 94.3 ± 37.0 | 88.8 ± 33.0 | 0.172 |
| SBP (mmHg) | 119.8 ± 40.2 | 115.6 ± 42.2 | 122.4 ± 38.7 | 0.149 |
| DBP (mmHg) | 65.6 ± 22.9 | 63.2 ± 24.4 | 67.2 ± 21.8 | 0.134 |
| RR | 21.4 ± 12.0 | 20.7 ± 8.8 | 21.8 ± 13.6 | 0.448 |
| ALB (g/L) | 28.29 ± 7.93 | 26.40 ± 6.93 | 29.48 ± 8.30 | 0.001 |
| PCT (μg/L) | 2.6 ± 8.5 | 4.0 ± 11.2 | 1.7 ± 5.9 | 0.02 |
| BNP (pg/mL) | 103.8 ± 23.6 | 353.9 ± 978.9 | 115.2 ± 374.1 | 0.003 |
| WBC (×109/L) | 12.0 ± 6.4 | 12.5 ± 7.56 | 11.6 ± 5.5 | 0.222 |
| HGB (g/L) | ||||
| PLT (×109/L) | 146.5 ± 101.4 | 128.9 ± 105.3 | 157.5 ± 97.6 | 0.016 |
| PT (s) | 13.8 ± 8.2 | 14.8 ± 11.8 | 13.2 ± 4.7 | 0.101 |
| APTT (s) | 31.2 ± 14.2 | 33.4 ± 16.6 | 29.8 ± 12.3 | 0.032 |
| FIB (g/L) | 3.32 ± 2.00 | 3.35 ± 2.61 | 3.30 ± 1.50 | 0.848 |
| Lactic acid (mmol/L) | 2.78 ± 6.91 | 3.58 ± 10.46 | 2.26 ± 2.91 | 0.113 |
| PCO2 (mmHg) | 27.8 ± 17.7 | 26.7 ± 20.5 | 28.5 ± 15.7 | 0.379 |
| Oxygenation index (PaO2/FIO2) | 277.0 ± 140.4 | 270.6 ± 147.2 | 281.1 ± 136.2 | 0.535 |
| CREA (μmol/L) | 65.7 ± 124.4 | 120.2 ± 165.8 | 80.2 ± 85.8 | 0.006 |
| TBil (mmol/L) | 29.0 ± 38.7 | 33.8 ± 51.0 | 25.9 ± 28.0 | 0.084 |
| Na (mmol/L) | 136.6 ± 22.3 | 136.0 ± 27.0 | 137.0 ± 18.9 | 0.744 |
| K (mmol/L) | 3.77 ± 0.92 | 3.74 ± 1.07 | 3.78 ± 0.81 | 0.664 |
NTIS: nonthyroidal illness syndrome; APACHE II score, acute physiology and chronic health evaluation II score; GCS: Glasgow coma scale; SOFA: sequential organ failure score; FT3: free triiodothyronine; FT4; free thyroxine; TSH: thyroid-stimulating hormone; T: temperature; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; RR: respiratory rate; ALB: albumin; PCT: procalcitonin; BNP; brain natriuretic peptide; WBC: white blood cell; HGB: hemoglobin; PLT: blood platelet; PT: prothrombin time; APTT: activated partial thromboplastin time; FIB: fibrinogen; PCO2: pressure of carbon dioxide; PaO2: pressure of oxygen; FIO2: fraction of inspired oxygen; CREA: creatinine; TBil: total bilirubin.
There were differences between the NTIS and non-NTIS group in terms of age, diagnosis leading to ICU admission, APACHE II, SOFA, FT3, FT4, ALB, PCT, CREA, BNP, and PLT (all P < 0.05). The levels of FT3 and FT4 were lower in the NTIS group than those in the non-NTIS group (2.72 ± 0.40 pmol/L vs. 3.97 ± 0.63, P < 0.001; 11.79 ± 2.83 pmol/L vs. 13.59 ± 5.37, P = 0.001).
Compared with the survivors, the nonsurvivors were older, had a lower proportion of trauma, had higher APACHE II scores, had lower GCS scores, had higher SOFA scores, and had lower fT3 levels (all P < 0.05) (Table 2).
Table 2.
Baseline characteristics of the subjects according to 28-day mortality.
| Characteristics | Survivors (n = 270) | Nonsurvivors (n = 35) | P |
|---|---|---|---|
| Males (%) | 68.9 | 65.7 | 0.7 |
| Ages (years) | 54.3 ± 15.5 | 63.3 ± 15.0 | 0.001 |
| Principal diagnosis leading to ICU admission (%) | <0.001 | ||
| Sepsis or sepsis shock | 8.6 | 25.7 | |
| Severe trauma | 50 | 5.7 | |
| Brain injury or diseases | 22.2 | 31.5 | |
| Severe disease | 6.3 | 17.1 | |
| After operation | 10.7 | 8.6 | |
| Tumor | 1.9 | 11.4 | |
| Pulmonary embolism | 0.7 | 0 | |
| APACHE II score | 11.19 ± 6.49 | 24.23 ± 9.60 | <0.001 |
| GCS score | 12.81 ± 4.09 | 7.43 ± 5.21 | <0.001 |
| SOFA score | 4.46 ± 3.14 | 10.00 ± 5.21 | <0.001 |
| FT3 (pmol/L) | 2.77 ± 0.37 | 2.53 ± 0.47 | 0.009 |
| FT4 (pmol/L) | 11.95 ± 2.71 | 11.14 ± 3.25 | 0.22 |
| TSH (mIU/L) | 1.81 ± 5.70 | 1.06 ± 1.21 | 0.537 |
| Vital signs when enrolled | |||
| T (°C) | 37.27 ± 0.84 | 37.36 ± 1.10 | 0.565 |
| HR | 93.1 ± 29.7 | 103.8 ± 34.7 | 0.052 |
| SBP (mmHg) | 127.56 ± 31.72 | 99.06 ± 35.41 | <0.001 |
| DBP (mmHg) | 69.61 ± 18.37 | 56.38 ± 22.84 | 0.002 |
| R | 22.1 ± 9.9 | 23.4 ± 19.8 | 0.524 |
| ALB (g/L) | 29.04 ± 7.11 | 26.57 ± 6.66 | 0.049 |
| PCT (μg/L) | 3.21 ± 8.51 | 9.77 ± 17.03 | 0.002 |
| BNP(pg/mL) | 439.1 ± 937.3 | 1193.0 ± 1301.4.4 | 0.005 |
| WBC (×109/L) | 11.3 ± 5.1 | 17.0 ± 11.1 | <0.001 |
| HGB (g/L) | 104.7 ± 21.7 | 99.9 ± 30.7 | 0.242 |
| PLT (×109/L) | 151.4 ± 102.5 | 113.0 ± 84.7 | 0.035 |
| PT (s) | 13.7 ± 8.35 | 15.2 ± 6.94 | 0.329 |
| APTT (s) | 31.1 ± 13.2 | 32.9 ± 20.3 | 0.488 |
| FIB (g/L) | 3.35 ± 1.52 | 3.21 ± 4.15 | 0.697 |
| Lac (mmol/L) | 2.48 ± 6.91 | 5.08 ± 6.53 | 0.041 |
| pH | 7.32 ± 0.94 | 7.32 ± 0.17 | 0.937 |
| BE (mmol/L) | −1.45 ± 4.62 | −6.46 ± 8.45 | <0.001 |
| PCO2 (mmHg) | 35.05 ± 10.50 | 36.56 ± 18.14 | 0.676 |
| Oxygenation index (PaO2/FIO2) | 287.3 ± 141.2 | 205.5 ± 104.8 | 0.001 |
| CREA (μmol/L) | 81.9 ± 99.8 | 204.5 + 214.4 | <0.001 |
| TBil (mmol/L) | 27.9 ± 35.2 | 40.3 ± 60.4 | 0.085 |
| Na (mmol/L) | 137.7 ± 16.1 | 139.7 ± 27.4 | 0.532 |
| K (mmol/L) | 3.81 ± 0.77 | 3.80 ± 1.28 | 0.931 |
APACHE II score: acute physiology and chronic health evaluation II score; GCS: Glasgow coma scale; SOFA; sequential organ failure score; fT3: free triiodothyronine; FT4: free thyroxine; TSH: thyroid-stimulating hormone; SBP: systolic blood pressure; DBP: diastolic blood pressure; PCT: procalcitonin; BNP: brain natriuretic peptide; HGB: hemoglobin; PLT: blood platelet; CREA: creatinine; TBil: total bilirubin; PT: prothrombin time; APTT: activated partial thromboplastin time; FIB; fibrinogen.
3.2. Comparison of Outcomes in the Two Groups
The 28-day mortality in the NTIS group was 19.5%, which was higher than that in the non-NTIS group (6.4%, P < 0.001). There was no obvious difference in the use of mechanical ventilation between the two groups. The length of stay in the ICU in the NTIS group was slightly longer than that in the non-NTIS group, but there was no significant difference between the groups (P = 0.094). The costs of hospitalization in ICU were higher in the NTIS group than in the non-NTIS group (P < 0.001) (Table 3).
Table 3.
Outcomes of the two groups.
| Outcomes | NTIS (n = 118) | Non-NTIS (n = 187) | P |
|---|---|---|---|
| 28-day mortality rate, n (%) | 23 (19.5) | 12 (6.4) | <0.001 |
| MV rate, n (%) | 92 (77.8) | 140 (74.9) | 0.78 |
| Duration of MV (h), median (IQR) | 56.5 (11.5, 117.6) | 34 (7, 114) | 0.097 |
| Length of ICU stay (h), median (IQR) | 109 (69.7, 219.5) | 90 (58, 161) | 0.094 |
| Hospitalization expenses in ICU (×10,000 yuan), median (IQR) | 4.57 (2.97, 9.00) | 3.35 (2.27, 6.03) | 0.001 |
MV: mechanical ventilation; ICU: intensive care unit; NTIS: nonthyroidal illness syndrome; IQR: interquartile range.
3.3. Factors Associated with 28-Day Mortality
The univariable analyses suggested that the 28-day mortality of patients admitted to the ICU was associated with age, NTIS, FT3, FT4/FT3, APACHE II, SOFA, duration of mechanical ventilation, CREA, oxygenation index, WBC, ALB, and BNP (P < 0.05), as shown in Table 4.
Table 4.
Univariable and multivariable logistic regression analyses for 28d mortality.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| NTIS | 3.531 | (1.68, 7.41) | 0.001 | 2.966 | (1.272, 6.915) | 0.012 |
| FT3 | 0.433 | (0.26, 0.722) | 0.001 | |||
| FT4 | 1.01 | (0.941, 1.083) | 0.789 | |||
| FT4/FT3 | 1.426 | (1.121, 1.814) | 0.004 | |||
| TSH | 0.837 | (0.585,1.197) | 0.329 | |||
| APACHE II | 1.231 | (1.160, 1.308) | <0.001 | |||
| SOFA | 1.378 | (1.248, 1.520) | <0.001 | |||
| The duration of MV (h) | 1.002 | (1.000, 1.003) | 0.046 | |||
| Lactic acid (mmol/L) | 1.03 | (0.991, 1.072) | 0.137 | |||
| CREA (μmol/L) | 1.005 | (1.002, 1.007) | <0.001 | |||
| Oxygenation index | 0.995 | (0.992, 0.998) | 0.003 | 0.995 | (0.991, 0.999) | 0.007 |
| WBC | 1.121 | (1.062, 1.183) | <0.001 | 1.118 | (1.052, 1.187) | <0.001 |
| ALB | 0.962 | (0.925, 1.000) | 0.049 | 1.004 | (1.002, 1.007) | 0.001 |
| Age (years) | 1.045 | (1.016, 1.075) | 0.002 | |||
| BNP | 1.001 | (1.000, 1.001) | 0.003 | |||
Variables included in the multivariable logistic analysis were NTIS, the duration of MV, CREA, oxygenation index, WBC, ALB, and age. NTIS: nonthyroidal illness syndrome; FT3: free triiodothyronine; FT4: free thyroxine; TSH: thyroid-stimulating hormone; APACHE II score: acute physiology and chronic health evaluation II score; SOFA: sequential organ failure score; MV: mechanical ventilation; CREA: creatinine; WBC: white blood cell; ALB: albumin; BNP: brain natriuretic peptide.
Using multivariable logistical regression analysis of the 28-day mortality rate, after adjusting for duration of mechanical ventilation, CREA, oxygenation index, WBC, ALB, age, and BNP, NTIS was an independent factor for 28-day mortality (OR = 2.966, 95% CI: 1.68-7.41, P = 0.012) (Table 4).
3.4. ROC Curve Analysis of FT3 and 28-Day Mortality
The ROC curve analysis that included all patients suggested that a cut-off value of 2.88 pmol/L FT3 was related to 28-day mortality with an AUC of 0.671, sensitivity of 0.514, and specificity of 0.815. This suggests that patients who have a level of FT3 lower than 2.88 pmol/L are at high risk of death in the ICU (Figure 1 and Table 5). When the patients were reanalyzed after excluding those who underwent operations, a cut-off value of 2.875 pmol/L provided an AUC of 0.701 with sensitivity 0.504 and specificity 0.813. Further excluding patients with brain injury along with those who underwent operation provided an AUC of 0.76 with sensitivity 0.81 and specificity 0.669.
Figure 1.
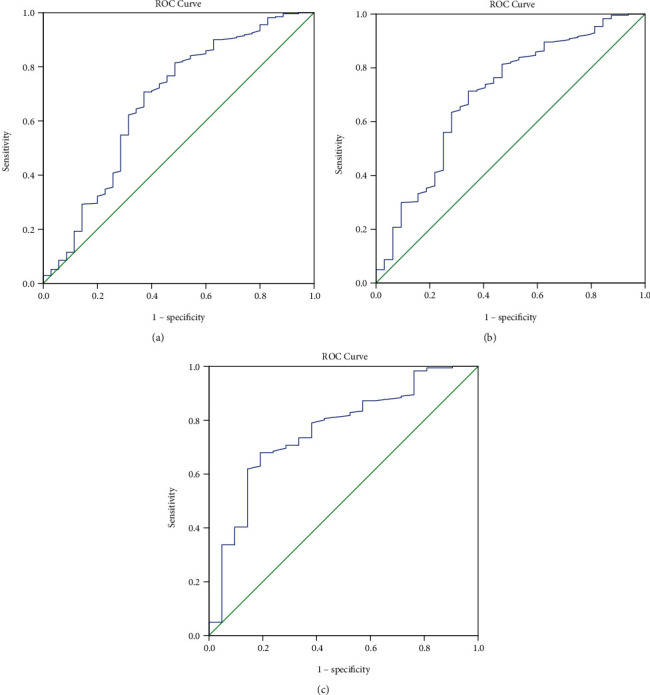
Receiver operating characteristic (ROC) curve for free triiodothyronine (FT3) level and 28-day mortality. The cut-off value of FT3 for 28-day mortality was 2.88 pmol/L, determined based on the Youden index. (a) ROC for all the patients, when cut-off of FT3 was 2.88 pmol/L, area under the curve (AUC) was 0.671 (95% CI: 0.563, 0.779), sensitivity was 0.514, and specificity was 0.815. (b) ROC for patients excluding those after an operation, when cut-off of FT3 was 2.875 pmol/L, AUC was 0.701 (95% CI: 0.598, 0.804), sensitivity was 0.504, and specificity was 0.813. (c) ROC for patients excluding those after an operation and those with brain injury, when cut-off of FT3 was 3.165 pmol/L, AUC was 0.76 (95% CI: 0.652, 0.869), sensitivity was 0.81, and specificity was 0.669.
Table 5.
Receiver operating characteristic curve (ROC) of FT3 for 28-day mortality.
| AUC (95% CI) | Cut-off | Sensitivity | Specificity | P value | |
|---|---|---|---|---|---|
| All patients | 0.671 (0.563, 0.779) | 2.88 | 0.815 | 0.514 | <0.001 |
| Patients excluding those after an operation | 0.701 (0.598, 0.804) | 2.875 | 0.813 | 0.504 | <0.001 |
| Patients excluding those after an operation and those with brain injury | 0.76 (0.652, 0.869) | 3.165 | 0.669 | 0.81 | <0.001 |
4. Discussion
This study investigated the relationship between NTIS and prognosis in patients admitted to the ICU. The results showed that 38.7% of the patients had NTIS in the ICU. There were differences between the NTIS and non-NTIS groups in terms of age, diagnosis leading to ICU admission, APACHE II, SOFA, FT3, FT4, ALB, PCT, CREA, BNP, and PLT. The 28-day mortality in the NTIS group was 19.5%, which was significantly higher than 6.4% in the non-NTIS group (P < 0.001). The costs of hospitalization in ICU were higher in the NTIS group than in the non-NTIS group (P < 0.001). The univariable analyses suggested that the 28-day mortality of patients admitted to the ICU was associated with age, NTIS, FT3, FT4/FT3, APACHE II, SOFA, duration of mechanical ventilation, CREA, oxygenation index, WBC, ALB, and BNP. The ROC analysis suggested that a cut-off value of 2.88 pmol/L FT3 indicated a higher risk of 28-day mortality. Excluding the patients who underwent operations or had brain injury further increased the AUC of the ROC analysis. Both the APACHE II and SOFA scores are complex clinical scoring systems consisting of large numbers of indicators that offer significant advantages over a single indicator, and no single indicator has been reported to date to exceed the APACHE II score in terms of the prognostic value for mortality [17]. Although FT3 has a lower diagnostic value than both scores, it has a specificity of 81.5% as a single indicator, which is still of great prognostic value. High FT3 specificity indicates low false-positive rates. In clinical practice, FT3 is a single laboratory test and might quickly and objectively provide information about the prognosis. FT3 results can be available within 2-4 h after admission. In contrast, APACHE II and SOFA scores take more time to be determined and can be subjectively influenced. They are impractical in ICUs with a heavy workload.
This study suggests that NTIS is an important indicator of 28-day mortality in patients in the ICU. The ROC analysis suggested that patients admitted to the ICU with an FT3 level of <2.88 pmol/L are at a high risk of death. Overall, these results agree with the study by Wang et al. [14]. Nevertheless, there are differences in disease types and patient age between the two studies. The previous study had a high rate of patients over 71 years with cardiovascular diseases and cardiorespiratory function failure [12], while the present study had a younger population with many cases of severe trauma. Thyroid hormones can increase the heart rate and myocardial contractility, improve diastolic cardiac function, and reduce systemic vascular resistance [18], having a significant effect on cardiac and circulatory function. A decreased thyroid level has a great influence on the function of the heart and circulation [19]. Changes in thyroid hormone levels are evident in myocardial infarction, heart failure, and other diseases and can thus be considered a predictor of multiple prognostic indicators [7, 8, 20]. Furthermore, other studies [21, 22] also suggested that the selective application of thyroid hormone in patients with myocardial infarction and extracorporeal circulation can improve the survival rates. The results of the present study suggest that NTIS might also be an important prognostic marker in patients with other diagnoses, not cardiac related, in the ICU. At the same time, it has been suggested that decreased FT3 is related to a low autometabolic rate. When the patients who had undergone operations were excluded from the ROC analysis, the AUC increased, suggesting that FT3 levels might be more useful in some patient populations than in others. The AUC increased further when the patients with brain injury were excluded along those who had undergone operations, further supporting the view that the specific diagnoses should be considered when using FT3.
In the present study, the cut-off point for predicting mortality was 2.88 pmol/L. Yu et al. [23] reported a cut-off of 3.685 pmol/L for mortality in patients with acute myocardial infarction. Su et al. [24] reported a cut-off point of 2.415 pmol/L for the prediction of cardiovascular death in the ICU. Different patient populations might influence the determination of the cut-off point, and large studies are needed to determine the exact values.
Consistent with other findings, the present study found that NTIS, defined as those with FT3 below the laboratory minimum normal value (FT3 < 3.28), indicated a worse prognosis than non-NTIS. The 28-day mortality of patients admitted to the ICU was much higher than in the non-NTIS patients and was associated with higher hospitalization costs. In a previous study with the duration of mechanical ventilation of NTIS patients as the main objective, published in Chest in 2009 [25], the results suggested that the duration of mechanical ventilation in patients with NTIS was significantly prolonged, but this was not found in this study. Furthermore, our study indicated that there was no significant difference in the use of mechanical ventilation between the two groups, which may be related to the incorporation of noninvasive ventilator-assisted ventilation into the scope of mechanical ventilation. Meanwhile, no significant difference was found, although the ICU duration in the NTIS group was longer than that in the non-NTIS group, which might be attributed to the lack of strict assurance concerning the indications for the discharge of the patients from the ICU. Specifically, many patients were discharged or had transfer delays out of the ICU owing to various reasons other than the condition of illness, whereas many patients who needed further treatments were transferred ahead of schedule due to the costs of treatment.
It should be highlighted that there were some limitations to this study. First, as a prospective predictive study, the sample size was still relatively small, without detailed stratified analysis, and with unbalanced types of diseases and a relatively high proportion of trauma patients, thereby affecting the estimation of overall mortality risk of patients admitted to the ICU. Multicenter studies should be designed in the future to ensure the diversity and balance of diseases. Second, a number of critically ill patients in the study chose to abandon or terminate treatment and voluntarily discharged themselves for various reasons such as economic considerations, which objectively increased the 28-day mortality rate, and had a certain degree of interference with the results. In addition, the 28-day mortality in patients admitted to the ICU is intimately associated with the intensity of treatment. Intensified ICU support therapy can make some patients survive 28 days, but the final prognosis is still poor. Therefore, the 90-day mortality rate should be further analyzed. Third, FT3, FT4, and TSH are routinely measured upon admission to the ICU, but thyroid-related antibody, thyrotropin receptor antibody, thyroglobulin, rT3, and thyroid B-ultrasound are not routinely done. Fourth, due to the wide variety of conditions being included and the different possible treatments for each condition, we could not include the treatments in the analyses. Fifth, the dynamic measurement of FT3 levels could represent more the actual condition of the patients and could detect more cases of NTIS, but the FT3 levels were not dynamically detected in this study. Finally, the total F3 levels were not measured.
5. Conclusion
In conclusion, NTIS was independently associated with a higher risk of 28-day mortality in patients admitted to the ICU. Although fT3 levels are not independently associated with ICU prognosis, the results suggest a cut-off value of fT3 levels that might be used for evaluating the prognosis of the patients. Future studies should examine the combination of fT3 levels with other biomarkers to construct a prognostic model for the ICU.
Acknowledgments
Thanks are due to respiratory therapist Xiaolei Chen for her great support in data collection. The study was supported by the Key Project of Medical Science Research in Hebei Province in 2016 (No. 20160586) and key scientific and technological research plan of Hebei Province (20190626).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The study was approved by the ethics committee of The Third Hospital of Hebei Medical University (No. 2018-019-1), and written informed consent was obtained.
Conflicts of Interest
The authors have nothing to disclose for potential conflict of interest, and the research data are reliable.
References
- 1.Lee S., Farwell A. P. Euthyroid sick syndrome. Comprehensive Physiology. 2016;6(2):1071–1080. doi: 10.1002/cphy.c150017. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G. Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid : official journal of the American Thyroid Association. 2014;24(10):1456–1465. doi: 10.1089/thy.2014.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langouche L., Jacobs A., Van den Berghe G. Nonthyroidal illness syndrome across the ages. Journal of the Endocrine Society. 2019;3(12):2313–2325. doi: 10.1210/js.2019-00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y.-F., Heng J.-F., Yan J., Dong L. Relationship between disease severity and thyroid function in Chinese patients with euthyroid sick syndrome. Medicine. 2018;97(31):e11756–e11756. doi: 10.1097/MD.0000000000011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeters R. P., Wouters P. J., Kaptein E., Van Toor H., Visser T. J., Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. The Journal of clinical endocrinology and metabolism. 2003;88(7):3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 6.Boelen A., Kwakkel J., Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocrine reviews. 2011;32(5):670–693. doi: 10.1210/er.2011-0007. [DOI] [PubMed] [Google Scholar]
- 7.Kang M. G., Hahm J. R., Kim K.-H., et al. Prognostic value of total triiodothyronine and free thyroxine levels for the heart failure in patients with acute myocardial infarction. The Korean journal of internal medicine. 2018;33(3):512–521. doi: 10.3904/kjim.2016.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okayama D., Minami Y., Kataoka S., Shiga T., Hagiwara N. Thyroid function on admission and outcome in patients hospitalized for acute decompensated heart failure. Journal of Cardiology. 2015;66(3):205–211. doi: 10.1016/j.jjcc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Angelousi A. G., Karageorgopoulos D. E., Kapaskelis A. M., Falagas M. E. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. European journal of endocrinology. 2011;164(2):147–155. doi: 10.1530/EJE-10-0695. [DOI] [PubMed] [Google Scholar]
- 10.Ilias I., Stamoulis K., Armaganidis A., et al. Contribution of endocrine parameters in predicting outcome of multiple trauma patients in an intensive care unit. Hormones. 2007;6(3):218–226. [PubMed] [Google Scholar]
- 11.Türe M., Memiş D., Kurt I., Pamukcu Z. Predictive value of thyroid hormones on the first day in adult respiratory distress syndrome patients admitted to ICU: comparison with SOFA and APACHE II scores. Annals of Saudi medicine. 2005;25(6):466–472. doi: 10.5144/0256-4947.2005.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qari F. A. Thyroid function status and its impact on clinical outcome in patients admitted to critical care. Pakistan journal of medical sciences. 2015;31(4):915–919. doi: 10.12669/pjms.314.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray D. C., Macduff A., Drummond G. B., Wilkinson E., Adams B., Beckett G. J. Endocrine measurements in survivors and non-survivors from critical illness. Intensive care medicine. 2002;28(9):1301–1308. doi: 10.1007/s00134-002-1427-y. [DOI] [PubMed] [Google Scholar]
- 14.Wang F., Pan W., Wang H., Wang S., Pan S., Ge J. Relationship between thyroid function and ICU mortality: a prospective observation study. Critical care. 2012;16(1):p. R11. doi: 10.1186/cc11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutch M., Kumar S., Gupta K. K. Prognostic value of thyroid profile in critical care condition. Indian journal of endocrinology and metabolism. 2018;22(3):387–391. doi: 10.4103/ijem.IJEM_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar K. V. S. H., Kapoor U., Kalia R., NSA C., Singh P., Nangia R. Low triiodothyronine predicts mortality in critically ill patients. Indian journal of endocrinology and metabolism. 2013;17(2):285–288. doi: 10.4103/2230-8210.109715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent J. L., Moreno R. Clinical review: scoring systems in the critically ill. Critical Care. 2010;14(2):p. 207. doi: 10.1186/cc8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Límanová Z., Jiskra J. Thyroid hormones and cardiovascular system. Vnitrni lekarstvi. 2016;62(9 Suppl 3):92–98. [PubMed] [Google Scholar]
- 19.Jabbar A., Pingitore A., Pearce S. H. S., Zaman A., Iervasi G., Razvi S. Thyroid hormones and cardiovascular disease. Nature reviews Cardiology. 2017;14(1):39–55. doi: 10.1038/nrcardio.2016.174. [DOI] [PubMed] [Google Scholar]
- 20.Coceani M., Iervasi G., Pingitore A. Thyroid hormone and coronary artery disease: from clinical correlations to prognostic implications. Clinical cardiology. 2009;32(7):380–385. doi: 10.1002/clc.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heusch G. The regional myocardial flow-function relationship. Circulation research. 2013;112(12):1535–1537. doi: 10.1161/CIRCRESAHA.113.301446. [DOI] [PubMed] [Google Scholar]
- 22.Siribaddana S. Cardiac dysfunction in the CABG patient. Current opinion in pharmacology. 2012;12(2):166–171. doi: 10.1016/j.coph.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Yu T., Tian C., Song J., et al. Value of the fT3/fT4 ratio and its combination with the GRACE risk score in predicting the prognosis in euthyroid patients with acute myocardial infarction undergoing percutaneous coronary intervention: a prospective cohort study. BMC Cardiovascular Disorders. 2018;18(1):p. 181. doi: 10.1186/s12872-018-0916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su W., Zhao X. Q., Wang M., Chen H., Li H. W. Low T3 syndrome improves risk prediction of in-hospital cardiovascular death in patients with acute myocardial infarction. Journal of Cardiology. 2018;72(3):215–219. doi: 10.1016/j.jjcc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Bello G., Pennisi M. A., Montini L., et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest. 2009;135(6):1448–1454. doi: 10.1378/chest.08-1816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


