Abstract
Background
Osteoarthritis (OA) is a chronic disease and a significant cause of joint pain, tenderness, and limitation of motion. At present, no specific treatment is available, and mesenchymal stem cells (MSCs) have shown promising potentials in this regard. Herein, we aimed to evaluate the repairing potentials of stem cells derived from the synovium and fat pad in the treatment of OA.
Methods
Twenty-eight male rats (220 ± 20 g, aged 10-12 weeks), were randomly divided into four groups (n = 7): C1: nontreated group, C2: Hyalgan-treated group, E1: adipose tissue-derived stem cell-treated group, and E2: synovial membrane-based stem cell-treated group. Collagenase type II was injected into the left knee; after eight weeks, OA was developed. Then, stem cells were injected, and rats were followed for three months. Afterward, specimens and radiological images were investigated. p value ≤ 0.05 was set as statistically significant.
Results
Compared to the C1 group, the E1 and E2 groups showed significantly better results in all six pathological criteria as well as joint space width and osteophytes of medial tibial, medial femoral, and medial fabellar condyles (p ≤ 0.001). Similarly, compared to the C2 group, the E1 and E2 groups had better scores regarding surface, matrix, cell distribution, and cell population viability (p < 0.05). E2 showed considerably higher scores compared to C2 regarding subchondral bone and cartilage mineralization (p < 0.05). The joint space width was similar between the C2 and E groups.
Conclusion
Treatment of OA with MSCs, particularly synovial membrane-derived stem cells, not only prevented but also healed OA of the knee to some extent in comparison to the Hyalgan and nontreatment groups.
1. Introduction
Osteoarthritis (OA) is a common joint disorder and one of the leading causes of pain and disability, particularly in the elderly [1]. The epidemiology of this disorder is complex and multifactorial [2]. It is estimated that about 60% of the seniors up to the age of 65 years old present symptoms of OA [3, 4]. The precise pathologic mechanisms leading to the destruction of articular cartilage are unclear. Inadequate mobility may limit sufficient nourishment of the joint through synovial fluid and may lead to cell death [5, 6]. Mediators, such as catabolic cytokines and nitric oxide (NO), interleukin 1 (IL1), and tumor necrosis factor-alpha (TNF-a), modulate the production of destructive enzymes as well as the synthesis of collagen bundles and proteoglycans; these play important roles in the destruction of cartilage structures [5, 6]. Treatment methods of OA may include pharmacological therapy (i.e., intra-articular therapy, corticosteroids, capsaicin, duloxetine, topical NSAIDs, and herbal medicines), physiotherapy, surgical procedures, and tissue engineering methods [7].
Mesenchymal stem cells (MSCs) have shown noticeable potential as therapeutic agents facilitating the regeneration of the cartilaginous tissue in OA considering their multilineage potential, limited immunogenicity, and relative simplicity of growth in culture [8]. Using MSCs as an autologous source of stem cells eliminates the concerns regarding the chances of rejection and disease transmission via MSC transplant, and they are less tumorigenic compared to embryonic stem cells [9]. MSCs, in order to be able to reconstruct, refine themselves and differentiate along a mesodermal lineage, and rely on osteoblasts and chondrocytes because of their intrinsic potential in tissue repair and regeneration [10–13].
In this study, we aimed to determine and compare the efficacy of treatment of OA using MSCs derived from two different origins, synovial layer, and adipose tissue isolated from the inguinal fat pad, in rats based on pathological and radiological criteria.
2. Materials and Methods
2.1. Isolation of Mesenchymal Stem Cells
The synovial membrane-derived stem cells were harvested from the cartilage tissue of the rats' knees. Isolation of adipose tissue-derived MSCs from the inguinal fat pad was done based on a previously described method by Bunnel et al. with some alterations [14]. Characterization of the stem cells was based on flow cytometry methods [14, 15]. To prepare the stem cells, after repeated washing (3 times here) of the acquired specimen with a solution containing phosphate-buffered saline and supplemented with 1% penicillin-streptomycin (PBS-PS; Sigma, USA) until all connective tissues and blood vessels were liberated, the sample was minced into smaller parts. The specimen was then digested using 0.1% collagenase type I 37 ± 1°C and shaking for 3 hours. Then, an equal volume of Dulbecco's modified Eagle's medium (DMEM; Biovet, Bulgaria) containing 10% fetal bovine serum (FBS; Biovet, Bulgaria) was added to the mixture. The suspension was then filtered through a 100 μm filter (Falcon, USA) to remove the redundant solid aggregates and was centrifuged at 1500 rpm for 5 minutes at a temperature of 24 ± 2°C. Using 1 mL of lysis buffer (Promega, Germany), the pellet was resuspended, incubated (15 minutes), washed using PBS-PS, and centrifuged at 1500 rpm for 5 minutes, in order to clear the suspension from red blood cells. The supernatant was then removed, and the pellet was resuspended in a complete medium containing DMEM, 20% FBS, and 1 penicillin-streptomycin in 75 cm2 culture flask and held in an incubator with a humidified atmosphere of 37°C and 5% CO2.
2.2. Induction of Osteoarthritis and Grouping
The study protocol was approved by the medical ethics committee of Shiraz University of Medical Science, Shiraz, Iran (Reg. no. 97-01-67-19128). OA was induced by using the intra-articular injection of collagenase type II. At first, the rats were anesthetized by using a mixture of Ketamine (10% Alfasan, Netherlands, 100 mg/kg for rat) and Xylazine (2% Rompun Bayer, Germany, 10 mg/kg for rat); then, in aseptic surgery condition, collagenase type II was injected into the left knees of the rats, and after 8 weeks, OA was developed. Treatments were started after OA was developed (8 weeks after injection of collagenase type II) [16].
Twenty-eight Sprague Dawley male adult rats, weighing 220 ± 20 g and aged between 10 and 12 weeks, were randomly divided into 4 groups (n = 7) as follows: C1—nontreated control group, rats did not receive any treatment; C2—Hyalgan-treated control group, rats received 0.1 cc intra-articular Hyalgan (Fidia, Italy) once every two weeks during the treatment as described by Jo et al. [17]; E1—stem cell-treated group 1, rats were injected intra-articular adipose tissue-derived stem cells with a dose of 2.5 × 106 in a volume of 50 μL once during the treatment; E2—stem cell-treated group 2, rats were injected with synovial membrane-based stem cells with a dose of 2.5 × 106 cells in a volume of 50 μL once during the treatment.
2.3. Radiological and Pathological Evaluations
After the treatments, the rats were followed up for three months; then, radiological imaging was done in anterior-posterior (AP) and lateral positions. All images were taken by the same operator and equipment (Axiom Multix M radiographic unit, Siemens™, Germany) and using a standard method. Grading was done by a blind observer by using the International Cartilage Repair Society (ICRS) scores (Table 1).
Table 1.
Radiological grading for knee osteoarthritis (OA) in rat models.
| Radiographic OA feature of medial compartment | Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|---|
| Joint space width | Normal | Reduced | Absent | N/A | |
| Osteophyte | Medial tibial condyle | Absent | Small | Moderate | Sever |
| Medial femoral condyle | Absent | Small | Moderate | Sever | |
| Medial fabella | Absent | Present | N/A | N/A | |
| Total osteophyte | 0-7 | ||||
| Global OA score | 0-9 | ||||
Rats were euthanized with CO2 70% at the end of the third month. Specimens from the knee joint were obtained and fixed in 10% buffered formaldehyde and then were transferred into paraffin. Serial sagittal sections were provided and stained with hematoxylin and eosin (H&E, for cellular architecture), Toluidine blue, and Safranin O (for proteoglycan contents). All pathological specimens were assessed by a pathologist who was blinded from the study data. The degree of cartilage repair of each rat was evaluated based on ICRS scores which consisted of 6 indices including surface, matrix, cell distribution, cell population viability, subchondral bone, and cartilage mineralization (Table 2).
Table 2.
Histopathological grading using international cartilage repair society (ICRS) histological score.
| Variables | Scores |
|---|---|
| Surface | |
| Smooth/continuous | 3 |
| Discontinuous/irregular | 0 |
| Matrix | |
| Hyaline | 3 |
| Mixture: hyaline+fibrocartilage | 2 |
| Fibrocartilage | 1 |
| Fibrous tissue | 0 |
| Cell distribution | |
| Columnar | 3 |
| Mixture/columnar cluster | 2 |
| Cluster | 1 |
| Individual cell/disorganized | 0 |
| Cell population variability | |
| Predominantly viable | 3 |
| Partially viable | 1 |
| <10% viable | 0 |
| Subchondral bone | |
| Normal | 3 |
| Increase remodeling | 2 |
| Bone necrosis/granulation tissue | 1 |
| Detached/fracture/cell at base | 0 |
| Cartilage mineralization | |
| Normal | 3 |
| Abnormal/inappropriate location | 0 |
2.4. Statistical Analysis
All nonparametric data were analyzed by using Kruskal-Wallis and Mann–Whitney U tests through SPSS software 21.0 (IBM, USA). Data are presented as the median ± interquartile range (IQR), and the significance level of the p value was less than 0.05.
3. Results
Pathologic evaluations showed that both stem cell-treated groups, E1 and E2, had significantly higher scores concerning cartilaginous surface, matrix, cell distribution, viability, subchondral bone, and cartilage mineralization, in comparison with the C1 group (p < 0.001, Figure 1). The C2 group showed significantly higher scores concerning the matrix (p < 0.001), cell viability (p = 0.03), subchondral bone (p = 0.001), and cartilage mineralization (p = 0.004), compared to the C1 group. Stem cell-treated groups had better results regarding surface (p < 0.001), matrix (p < 0.001 and p = 0.01, respectively), cell distribution (p = 0.009 and p = 0.011, respectively), and cell population viability (p = 0.016 and p = 0.021, respectively) in contrast with the Hyalgan-treated group—C2; however, considering subchondral bone and cartilage mineralization, only E2 showed considerably higher scores compared to C2 (p = 0.021 and p = 0.013, respectively) (Figures 1 and 2).
Figure 1.
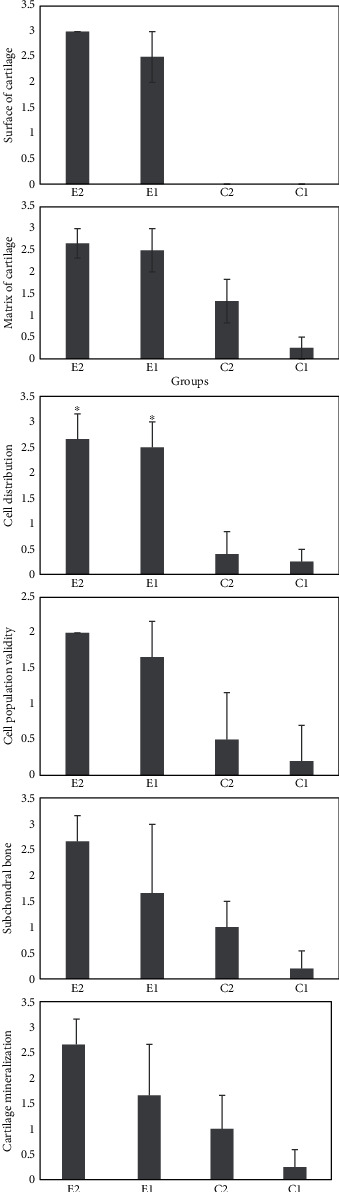
Histological scores of OA in rat models including E2 group: synovial membrane-based stem cell, E1 group: adipose tissue-derived stem cells, C2 group: Hyalgan-treated, and C1 group: nontreated group, based on International Cartilage Repair Society (ICRS). Values are shown as the median and interquartile range. ∗p value < 0.05 vs. C1.
Figure 2.
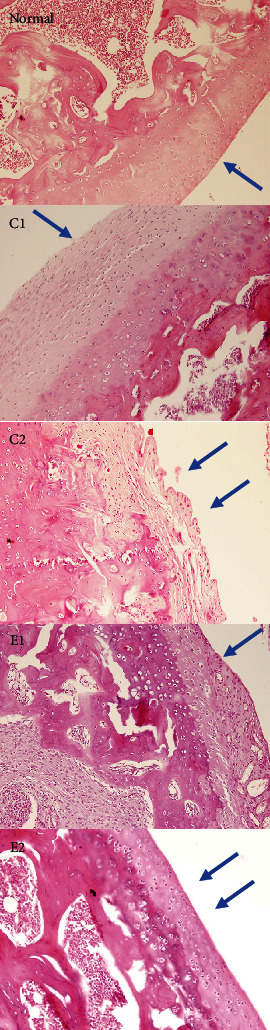
A typical histological section from all groups' surface of articular cartilage (H&E, ×200). E2 group: synovial membrane-based stem cells, arranged chondrocyte in columnar cluster along with continuous and smooth surface of articular hyaline cartilage with no foci of abnormal calcification; E1 group: adipose tissue-derived stem cells, the surface of articular cartilage was mainly continues composed of fibrocartilage tissue arranged in clusters, and the subchondral bone was detached with increased remodeling; C2 group: treated with Hyalgan, the surface of articular cartilage was continuous mainly composed of fibrocartilage with foci of hyaline cartilage cluster; and C1 nontreated group, the surface of articular cartilage was completely destructive and composed of disorganized fibrocartilage and fibrous tissues, and the subchondral bone was detached.
Radiographic images depicted that the E1 and E2 groups had significantly lower scores indicative of better healing effects regarding medial tibial condyle osteophytes (p < 0.001), medial femoral condyle osteophytes (p < 0.001), medial fabellar condyle osteophytes (p < 0.001), and joint space width (p = 0.03 and p = 0.02, respectively) compared to the C1 group. The C2 group showed lower scores compared to C1 concerning medial tibial condyle score (p = 0.04); moreover, the E1 and E2 groups showed significantly lower scores compared to C2 regarding medial tibial condyle (p < 0.001), medial femoral condyle (p < 0.001), and medial fabellar condyle (p < 0.001). The joint space width score was significantly lower merely in the E2 group (p = 0.01, respectively) (Figure 3).
Figure 3.
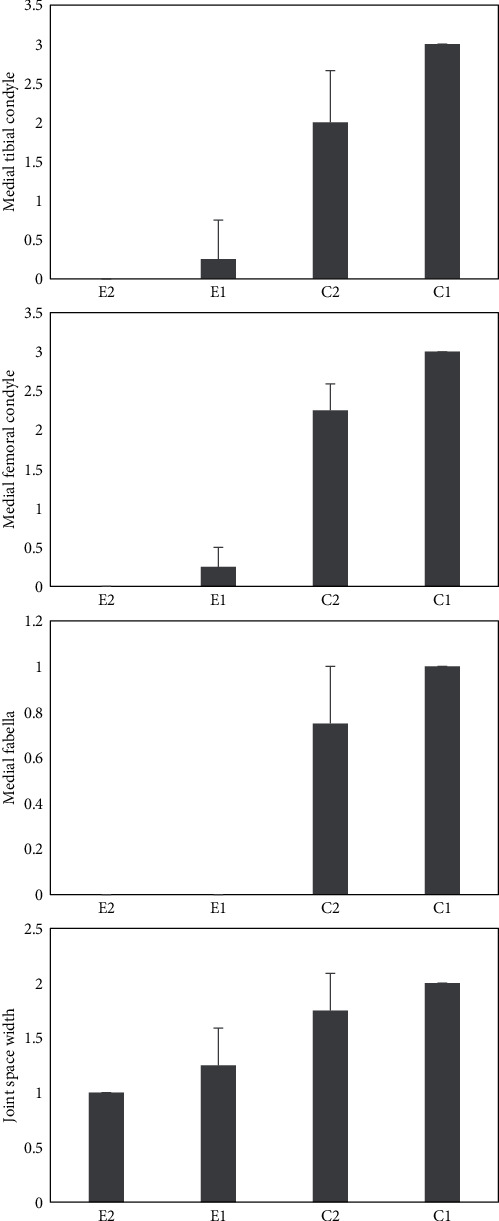
Radiological scores regarding OA in rat models including E2 group: synovial membrane-based stem cells, E1 group: adipose tissue-derived stem cells, C2 group: treated with Hyalgan, and C1 group: nontreated, based on International Cartilage Repair Society (ICRS). Values are shown as the median and interquartile range. ∗p value < 0.05 vs. C1.
4. Discussion
Currently, the treatment of OA is mainly focused on symptom therapies, pain reduction, and improving the quality of life [18, 19]. There is no specific treatment either for stopping the progress of OA or vanishing the underlying causes [20]. In this study, we aimed to evaluate the efficacy of the treatment of OA using MSCs derived from two various origins, adipose tissue, and synovial membrane. Another aim of this study was to compare these treatments with intra-articular Hyaluronic acid (Hyalgan) as a potential agent that was shown to alleviate the degenerative process in the joint [21]. Our results showed that both adipose tissue-derived and synovial membrane-derived stem cells possess promising effects in reducing the degeneration of the joint cartilage based on radiologic and pathologic parameters compared to the untreated control group. Moreover, compared to Hyalgan, stem cell treatment results were significantly better regarding all the evaluated parameters, although Hyalgan has also shown significant improvements in the joint regarding cartilage matrix, cell viability, subchondral bone, and cartilage mineralization compared to the untreated control group. Comparing the results of synovial and adipose-derived MSCs with the control group, regarding some parameters including subchondral bone and cartilage mineralization, synovium-derived MSCs showed even better effects on the joint affected with OA.
According to the outcome of the present study, a single intra-articular injection of 2.5 × 106 adipose tissue-derived MSCs exert a protective impact on the OA in rats compared to the nontreated group. Radiologic evaluation of the joint, as well as the pathologic results comparing this treatment with Hyalgan, as a currently used treatment for OA, also depicted the superiority of this method of treatment. Adipose tissue is an easy access rich source of MSCs, and stem cells obtained from adipose tissue have remarkable proliferation and differentiation potentials into chondrocytes that can be due to secretion of various signaling molecules and growth factors by these cells [22, 23]. In a study by Toghraie et al. MSCs derived from infrapatellar fat pad were used to treat OA induced by anterior cruciate ligament (ACL) dissection in rabbits [24]. They showed that a single intra-articular injection of 106 MSCs led to decreased cartilage degeneration, subchondral sclerosis, and osteophyte formation compared to the nontreated group. Kuroda et al. also observed decreased joint erosions in both lateral and medial femoral condyles, suppressed cartilage degeneration, and increased cell viability, treating OA-induced rabbits with adipose tissue-derived MSCs [25]. ter Huurne et al. reported positive outcome using the inguinal fat pad in mice model of OA induced by collagenase [26]. In their study, the MSC-treated group showed reduced synovial thickening, enthesophyte formation, and cartilage destruction compared to nontreated mice. Additionally, in human case-control studies in 2012 and 2013 by Koh et al., promising results were presented after treating patients suffering from knee OA with MSCs obtained from infrapatellar fat pad [27, 28]. After 25 intra-articular injections of MSCs combined with arthroscopic debridement in the patients, compared to the control group that had undergone arthroscopic debridement and platelet-rich plasma injections, the patients showed significant improvement in activity as well as radiologic and arthroscopic evaluation of the joint in both short- and long-term follow-ups [27, 28]. Overall, several published papers as well as the results of the present study show that adipose tissue can play the role of a noticeable source for MSCs useful in the treatment of OA. However, clinical evidence in this regard seems to be still unsatisfactory [29–33].
Our results demonstrated that a single injection of 2.5 × 106 synovial MSCs into the knee joint can have a protective effect in collagenase-induced OA in rats based on pathologic and radiologic evaluation of the joint. The efficacy of this treatment was also superior to Hyalgan. The synovial membrane has an intrinsic potential of regeneration leading to its recovery after synovectomy, suggesting that it can play a considerable role as a stem cell resource in cartilage repair [34, 35]. Zhu et al. in collagenase-induced OA mouse models showed that MSC-derived exosomes which contain various modulatory factors for cellular signaling, inflammatory response, and differentiation can enhance the protective effect of synovium-derived MSCs after a single injection into the joint; however, these exosomes have shown a stronger stimulatory effect on human-induced pluripotent stem cells compared to the synovial MSCs in the OA joint [36]. Mak et al. in a surgical-induced knee joint cartilage defect in mice showed that intra-articular injection of 104 synovial MSCs with a specific cell marker (Sca-1) protects the joint against the progression of the cartilage defect [37]. Neybecker et al. using human synovium-derived MSCs in rat models of OA induced by ACL dissection depicted that these cells have a noticeable capacity for chondrogenic gene induction and synthesis of the extracellular matrix, but they observed that intra-articular injection of these xenogenic MSCs obtained from human did not show significant chondroprotection in OA rat models [38]. Ozeki et al. have used synovial MSCs in OA induced via ACL dissection [15]. They, for the first time according to their claim, compared the chondroprotective effects of repeated injections of MSCs with a single injection of 106 MSCs using fluorocytometric methods as well as pathologic evaluation of tibial plateau and femoral condyle. According to their outcome, the weekly injection of MSCs has superior efficacy compared to a single injection in inhibiting OA progression and maintaining the viability of MSCs. They also reported that synovial MSCs can retain their stem cell properties after migration to the synovial tissue without differentiating into another lineage. However, according to their report single injection of 106 MSCs may not be beneficial since migrated MSCs do not survive in the long term and repeated injection is needed to maintain the number of MSCs in the joint. Conclusively, approved by the present study and the previously published papers, synovial MSC seem to be a promising source of stem cell in the treatment of OA; thus, the number of injected stem cells or repeating the injections aiming to maintain the number of viable cells seems to play an important role and should be further investigated.
This study had some limitations to be mentioned. First, though we used a high dose of MSCs for injection (2.5 × 106) we should have considered performing also repeated injection method that could show even better results. However, according to limited facilities, that goal was not obtainable. Secondly, we were not able to estimate inflammatory markers and count the MSCs within the joint using flow cytometry to provide more reliable results in this regard. Lastly, the number of the rats in each group could be higher in order to prove more valid and more reliable outcome.
5. Conclusion
The treatments of OA with MSCs, derived from synovial membrane and adipose tissue, were useful and effective, in comparison to the nontreated group as well as Hyalgan-treated rats, according to the radiological and histopathological analyses. The result of this study was in accordance to the majority of previously published papers on this subject. The differences in the outcomes can be related to the method used to create the AO model, the duration of the study, and the number of injection repeats as well as the number of injected MSCs. Thus, conducting further studies with different designs and greater population as well as human trials is necessary to establish the advantages and disadvantages of stem cell therapy, specifically with the above-mentioned cell sources, in OA.
Acknowledgments
This study is from the doctorate thesis of Reza Zare and was supported by Shiraz University, Shiraz, Iran. The paper was reviewed and edited by Medipress Scientific Writing Group regarding any linguistic errors within the text.
Abbreviations
- OA:
Osteoarthritis
- MSCs:
Mesenchymal stem cells
- ICRS:
International Cartilage Repair Society
- IFP:
Infrapatellar fat pad.
Contributor Information
Nader Tanideh, Email: tanidehn@gmail.com.
Soheil Ashkani-Esfahani, Email: soashkani@gmail.com.
Data Availability
The experimental data of the study is available on demand.
Ethical Approval
The study protocol was approved by Medical Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Reg. No. 97-01-67-19128).
Consent
All authors and subjects have declared consent for the publication of this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest
Authors' Contributions
Nader Tanideh is responsible for the hypothesis and study design, data gathering, and editing the draft. Soheil Ashkani-Esfahani did the data analysis, created the charts and figures, and wrote and edited the draft. Reza Zare, Behrooz Nikahval, Shahrokh Zare, and Omid Koohi Hosseinabadi are involved in the experiments and data gathering. Rohan Bhimani reviewed the data and designed the statisitical analysis method and edited the final draft. Nasrollah Ahmadi and Maryam Sadat Mirtalebi performed the pathological study. All of the authors contributed in writing the final revision of the paper.
References
- 1.Glyn-Jones S., Palmer A., Agricola R., et al. Osteoarthritis. The Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Flaxman A. D., Naghavi M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fransen M., McConnell S., Harmer A. R., Van der Esch M., Simic M., Bennell K. L. Exercise for osteoarthritis of the knee. Cochrane Database of Systematic Reviews. 2015;1, article CD004376 doi: 10.1002/14651858.cd004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palazzo C., Nguyen C., Lefevre-Colau M.-M., Rannou F., Poiraudeau S. Risk factors and burden of osteoarthritis. Annals of Physical and Rehabilitation Medicine. 2016;59(3):134–138. doi: 10.1016/j.rehab.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Lombello C., Reis G., Cohen M. Study on human chondrocyte culture viability for autologous transplantation in clinical application. Einstein. 2003;1:84–88. [Google Scholar]
- 6.Torio C., Andrews R. National inpatient hospital costs: the most expensive conditions by payer, 2011. Statistical brief# 160; 2006. [PubMed] [Google Scholar]
- 7.Frisbie D. D. Future directions in treatment of joint disease in horses. Veterinary Clinics: Equine Practice. 2005;21(3):713–724. doi: 10.1016/j.cveq.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Beitzel K., McCarthy M. B., Cote M. P., et al. Rapid isolation of human stem cells (connective progenitor cells) from the distal femur during arthroscopic knee surgery. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2012;28(1):74–84. doi: 10.1016/j.arthro.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Raghunath J., Salacinski H. J., Sales K. M., Butler P. E., Seifalian A. M. Advancing cartilage tissue engineering: the application of stem cell technology. Current Opinion in Biotechnology. 2005;16(5):503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Khan W. S., Johnson D. S., Hardingham T. E. The potential of stem cells in the treatment of knee cartilage defects. The Knee. 2010;17(6):369–374. doi: 10.1016/j.knee.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Freitag J., Bates D., Wickham J., et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regenerative Medicine. 2019;14(3):213–230. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- 12.Bauer D., Hunter D., Abramson S., et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis and Cartilage. 2006;14(8):723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Saravani S., Tanideh N., Fazeli M., et al. Evaluation of hydroalcoholic extract of Psidium guajava Linn.(Myrtaceae) leave effect with synovium-derived stem cells and platelet rich plasma (PRP) on induced osteoarthritis of the knee in male rat. Iranian Journal of Orthopaedic Surgery. 2017;15(2):28–38. [Google Scholar]
- 14.Bunnell B. A., Flaat M., Gagliardi C., Patel B., Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozeki N., Muneta T., Koga H., et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis and Cartilage. 2016;24(6):1061–1070. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Adães S., Mendonça M., Santos T. N., Castro-Lopes J. M., Ferreira-Gomes J., Neto F. L. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Research & Therapy. 2014;16(1):p. R10. doi: 10.1186/ar4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo C. H., Lee Y. G., Shin W. H., et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 18.Wang A. T., Feng Y., Jia H. H., Zhao M., Yu H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: a concise review. World Journal of Stem Cells. 2019;11(4):222–235. doi: 10.4252/wjsc.v11.i4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravalli S., Szychlinska M. A., Leonardi R. M., Musumeci G. Recently highlighted nutraceuticals for preventive management of osteoarthritis. World Journal of Orthopedic. 2018;9(11):255–261. doi: 10.5312/wjo.v9.i11.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyles C. C., Houdek M. T., Behfar A., Sierra R. J. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells and Cloning: Advances and Applications. 2015;2015:117–124. doi: 10.2147/sccaa.s68073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezende M. U., Hernandez A. J., Oliveira C. R. G. C. M., Bolliger N. R. Experimental osteoarthritis model by means of medial meniscectomy in rats and effects of diacerein administration and hyaluronic acid injection. Sao Paulo Medical Journal. 2015;133(1):4–12. doi: 10.1590/1516-3180.2013.6730001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maumus M., Manferdini C., Toupet K., et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Research. 2013;11(2):834–844. doi: 10.1016/j.scr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Jayaram P., Ikpeama U., Rothenberg J. B., Malanga G. A. Bone marrow–derived and adipose-derived mesenchymal stem cell therapy in primary knee osteoarthritis: a narrative review. PM&R. 2019;11(2):177–191. doi: 10.1016/j.pmrj.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Toghraie F. S., Chenari N., Gholipour M. A., et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. The Knee. 2011;18(2):71–75. doi: 10.1016/j.knee.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda K., Kabata T., Hayashi K., et al. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskeletal Disorders. 2015;16(1, article 236) doi: 10.1186/s12891-015-0701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ter Huurne M., Schelbergen R., Blattes R., et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis & Rheumatism. 2012;64(11):3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 27.Koh Y.-G., Choi Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. The Knee. 2012;19(6):902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Koh Y.-G., Choi Y.-J., Kwon S.-K., Kim Y.-S., Yeo J.-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23(5):1308–1316. doi: 10.1007/s00167-013-2807-2. [DOI] [PubMed] [Google Scholar]
- 29.Yun S., Ku S.-K., Kwon Y.-S. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. Journal of Orthopaedic Surgery and Research. 2016;11(1, article 9) doi: 10.1186/s13018-016-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Y. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. Journal of Cellular and Molecular Medicine. 2017;21(9):2153–2162. doi: 10.1111/jcmm.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurley E. T., Yasui Y., Gianakos A. L., et al. Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2018;26(11):3499–3507. doi: 10.1007/s00167-018-4955-x. [DOI] [PubMed] [Google Scholar]
- 32.Wickham M. Q., Erickson G. R., Gimble J. M., Vail T. P., Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clinical Orthopaedics and Related Research. 2003;412:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 33.English A., Jones E., Corscadden D., et al. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology. 2007;46(11):1676–1683. doi: 10.1093/rheumatology/kem217. [DOI] [PubMed] [Google Scholar]
- 34.Morito T., Muneta T., Hara K., et al. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology. 2008;47(8):1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- 35.Harvanová D., Tóthová T., Sarissky M., Amrichová J., Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biologica (Praha) 2011;57(3):119–124. [PubMed] [Google Scholar]
- 36.Zhu Y., Wang Y., Zhao B., et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Research & Therapy. 2017;8(1):p. 64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mak J., Jablonski C., Leonard C., et al. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Scientific Reports. 2016;6(1, article 23076) doi: 10.1038/srep23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neybecker P., Henrionnet C., Pape E., et al. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Research & Therapy. 2018;9(1):p. 329. doi: 10.1186/s13287-018-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The experimental data of the study is available on demand.


