Abstract
Objective
Patients with chronic stroke have been shown to have failure to release interhemispheric inhibition (IHI) from the intact to the damaged hemisphere before movement execution (premovement IHI). This inhibitory imbalance was found to correlate with poor motor performance in the chronic stage after stroke and has since become a target for therapeutic interventions. The logic of this approach, however, implies that abnormal premovement IHI is causal to poor behavioral outcome and should therefore be present early after stroke when motor impairment is at its worst. To test this idea, in a longitudinal study, we investigated interhemispheric interactions by tracking patients’ premovement IHI for one year following stroke.
Methods
We assessed premovement IHI and motor behavior five times over a 1-year period after ischemic stroke in 22 patients and 11 healthy participants.
Results
We found that premovement IHI was normal during the acute/subacute period and only became abnormal at the chronic stage; specifically, release of IHI in movement preparation worsened as motor behavior improved. In addition, premovement IHI did not correlate with behavioral measures cross-sectionally, whereas the longitudinal emergence of abnormal premovement IHI from the acute to the chronic stage was inversely correlated with recovery of finger individuation.
Interpretation
These results suggest that interhemispheric imbalance is not a cause of poor motor recovery, but instead might be the consequence of underlying recovery processes. These findings call into question the rehabilitation strategy of attempting to rebalance interhemispheric interactions in order to improve motor recovery after stroke.
It has been proposed that one contributor to chronic hemiparesis is an imbalanced inhibitory interaction between the lesioned and intact hemispheres via transcallosal connections. This interhemispheric-competition model proposes that the two hemispheres, which normally exert mutual inhibition in healthy individuals, become imbalanced after stroke, and that unopposed inhibition from the healthy to the damaged side impedes recovery.1 This framework is largely based on a seminal study that showed persistent premovement interhemispheric inhibition (IHI) from the contra- to ipsilesional motor cortex before movement execution in patients with chronic stroke.2 This failure to release IHI before movement onset (abnormal premovement IHI) correlated with weakness and impaired finger tapping performance.2 Influenced by this stroke-recovery model, numerous studies in the neurorehabilitation field have used different approaches (e.g. brain stimulation, peripheral stimulation, and transient deafferentation) in an attempt to downregulate excitability in the unaffected hemisphere and thus rebalance putative abnormal IHI (see recent studies3,4 and reviews5–7).
The problem with the interhemispheric-competition model is that abnormal premovement IHI has only been described in patients with chronic stroke and relatively mild impairment. Stinear and colleagues,8 using an indirect measure of IHI, recently found no evidence for interhemispheric imbalance in the first 3 months after stroke. To date, it remains unclear whether imbalanced interhemispheric interactions are present in the context of movement early after stroke, whether they evolve over time, and whether they have any predictive value for motor recovery. If interhemispheric interactions are normal early after stroke, then designing rehabilitation strategies based on the interhemispheric-competition model is questionable. Here, in a longitudinal observational study of patients with mild-to-moderate hemiparesis, we investigated the evolution of premovement IHI over the first year after stroke and related it to motor recovery of the hand. To this end, we followed the same inclusion-exclusion criteria and procedures as the seminal study of Murase and colleagues.2
Participants and Methods
Participants
Twenty-two patients with hemiparesis from first-time ischemic stroke (7 female; mean age, 57.5 ± 16 years; 15 righthanded according to the Edinburgh Handedness Inventory9) were recruited from three centers (The Johns Hopkins Hospital and Affiliates [JHM], Columbia University Medical Center [CU], and The University Hospital of Zurich & Cereneo Center for Neurology and Rehabilitation [UZ]) for a prospective cohort study over the course of four years. All patients met the following inclusion criteria: (1) first-ever ischemic stroke confirmed by magnetic resonance imaging within the previous two weeks; (2) one-sided upper extremity weakness (Medical Research Council <5). We excluded patients with the following criteria: contraindications to magnetic stimulation, age <21 years, hemorrhagic stroke, space-occupying hemorrhagic transformation, bilateral hemiparesis, traumatic brain injury, encephalopathy, global inattention, visual-field cut larger than a quadrantanopia, receptive aphasia, inability to give informed consent or understand the tasks, major neurological or psychiatric illness that could confound performance/recovery, or a physical or other neurological condition that would interfere with arm, wrist, or hand function recovery. See Table 1 for details of patient characteristics.
TABLE 1.
Patient Characteristicsa
| Patient | Sex | Age at Stroke | Handedness | Paretic Side | Initial FMA | Initial MoCA |
|---|---|---|---|---|---|---|
| 1 | M | 57 | R | R | 48 | 27 |
| 2 | M | 66 | R | L | 65 | 25 |
| 3 | M | 65 | R | L | 30 | 25 |
| 4 | F | 66 | R | L | 60 | 19 |
| 5 | F | 63 | L | L | 57 | 26 |
| 6 | M | 56 | R | L | 64 | 24 |
| 7 | F | 64 | L | R | 20 | 16 |
| 8 | F | 60 | L | R | 55 | 21 |
| 9 | M | 64 | L | L | 63 | 25 |
| 10 | M | 24 | R | L | 35 | 23 |
| 11 | F | 67 | R | R | 16 | 23 |
| 12 | M | 42 | L | R | 54 | 25 |
| 13 | M | 35 | R | L | 4 | 29 |
| 14 | M | 48 | L | L | 16 | 25 |
| 15 | M | 74 | R | R | 5 | 25 |
| 16 | F | 80 | R | R | 9 | 24 |
| 17 | F | 64 | R | L | 58 | 19 |
| 18 | M | 22 | R | R | 63 | 27 |
| 19 | M | 84 | R | R | 30 | 26 |
| 20 | M | 53 | L | R | 30 | 29 |
| 21 | M | 54 | R | L | 59 | 21 |
| 22 | M | 58 | R | L | 61 | 23 |
Sex, age (yr), handedness, paretic side, initial FMA (Fugl-Meyer upper limb score, maximum = 66), and initial MoCA (Montreal Cognitive Assessment, maximum = 30).
We also recruited 11 age-matched healthy control participants (4 female; mean age, 64 ± 9 years; all right-handed) at the three centers. All participants gave written consent and the respective institutional research board at each study center approved all procedures. All procedures were in compliance with the Declaration of Helsinki. All patients were tested at five time points over a 1-year period (Table 2).
TABLE 2.
Distribution of Assessment Time and Data Obtained
| Total N = 21 | W1 | W4 | W12 | W24 | W52 |
|---|---|---|---|---|---|
| No. of weeks post stroke | 1 to 2 | 4 to 6 | 12 to 14 | 24 to 26 | 52 to 54 |
| No. of days post stroke | 12 ± 3 | 34 ± 5 | 93 ± 8 | 184 ± 12 | 369 ± 10 |
| No. of patients | |||||
| IHI obtained | 10 (48%) | 13 (62%) | 14 (67%) | 18 (86%) | 16 (76%) |
| ΔIHI obtained | 8 (38%) | 11 (52%) | 12 (57%) | 16 (76%) | 15 (71%) |
| MEP count <9 (early TMS epochs) | 0 (0%) | 1 (5%) | 1 (5%) | 0 (0%) | 0 (0%) |
| MEP count <9 (late TMS epochs) | 2 (10%) | 1 (5%) | 1 (5%) | 2 (10%) | 1 (5%) |
| No MEP | 4 (19%) | 2 (10%) | 0 (0%) | 0 (0%) | 0 (0%) |
| TS >90% MSO | 3 (14%) | 4 (19%) | 2 (10%) | 0 (0%) | 0 (0%) |
| No reliable movement | 6 (29%) | 3 (14%) | 2 (10%) | 0 (0%) | 0 (0%) |
| Other missing | 2 (10%) | 0 (0%) | 3 (14%) | 3 (14%) | 5 (24%) |
The first two rows are the number of weeks and days poststroke at each time point of assessment. The following two rows are patient counts with obtained data. The following six categories are counts of missing data for various reasons: MEP count <9 at early TMS epochs (20 and 50% RT) are likely attributed to lack of big enough MEP size (>50 μV), whereas those at late TMS epochs (80 and 95% RT) are likely attributed to TMS pulses occurring during the movement; “No MEP” or “TS >90% MSO” can sometimes be overlapping with “No reliable movement (index finger abduction)” count in cases of complete plegia; “Other missing” cases include data missing for random reasons: missed the time window, patient dropped out of the study, patients refused to continue the session, or technical issues during the session. Percentages out of the total N = 21 are presented in parentheses.
IHI = interhemispheric inhibition; MEP = motor-evoked potential; MSO = maximal stimulator output; RT = reaction time; TMS = transcranial magnetic stimulation; TS = test stimulus.
Assessment of Interhemispheric Inhibition with Transcranial Magnetic Stimulation
Transcranial Magnetic Stimulation Procedures And IHI Assessments
Participants were comfortably seated in an armchair, arms resting on a pillow, and faced a computer monitor. IHI was assessed by a double-pulse paradigm2,10 (Fig 1A), with two figure-of-eight coils (diameters of wings 70 and 50 mm), each connected to a Magstim-200 magnetic stimulator (Magstim Co Ltd, Whitland, UK). The larger coil was placed tangentially over the lesioned Ml (for testing stimulus [TS]), with the handle oriented toward the back of the head and laterally at a 45-degree angle from the midsagittal line. The smaller coil was oriented perpendicular to midsagittal line over the unaffected Ml (for conditioning stimulus [CS]). For healthy age-matched controls, the CS was always applied to the right M1 and the TS to the left M1, contralateral to the moving right hand. The positions of the coils on the skull were adjusted to produce a maximal response in the contralateral first dorsal interosseus (FDI) muscles (the hotspots). A frameless stereotactic neuronavigation device (Brainsight; Rogue Research Inc, Montreal, QC, Canada) was used to track coil positions within and across sessions.
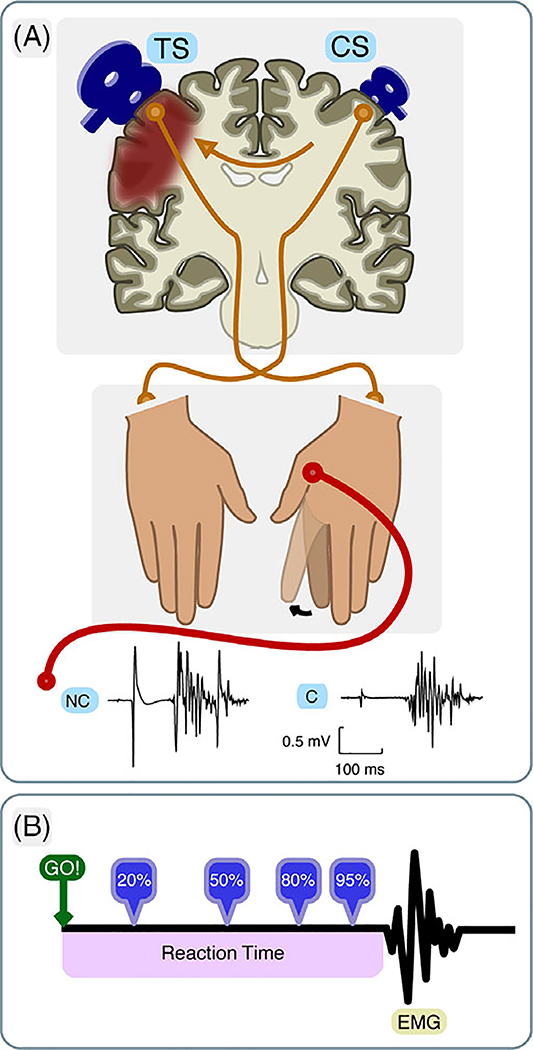
FIGURE 1:
Schematic illustration of the premovement Interhemispheric Inhibition (IHI) paradigm. (A) A test stimulus (TS) was delivered over the lesioned hemisphere, and a conditioning stimulus (CS) was applied over the intact hemisphere before index finger abduction of the paretic hand (or right hand in healthy age-matched controls). In nonconditioned (NC) trials, only the TS was delivered, whereas in conditioned (C) trials, the CS preceded TS by 10 ms. EMG signals were recorded from the first dorsal interosseous muscle (FDI) of the moving hand. (B) TMS pulses were delivered at four timing epochs relative to the individual’s mean reaction time, estimated from a simple-reaction task. EMG = electromyography.
Two stimulation conditions were used to calculate IHI: nonconditioned (NC) trials (NC: TS-only), where only a TS pulse was delivered, and conditioned (C) trials (C: CS + TS), where a CS pulse was delivered before a TS pulse with an interstimulus interval (ISI) of 10 ms. Conditioned and unconditioned trials were intermixed and randomized throughout the testing session.
IHI was assessed in two contexts: at rest (resting) and during movement preparation (premovement). Following a previous study, IHI at rest was obtained in order to determine the stimulation parameters for premovement IHI.2 For resting IHI, intensities of TS and CS were first set at the minimum level of maximal stimulator output (MSO) that produced a contralateral motor-evoked potential (MEP) with amplitude 0.5 to 1.0 mV. CS intensity was then adjusted to produce a ~50% reduction in TS-MEP amplitude. The resting-IHI assessment consisted of a block of 36 trials with 18 each for NC and C stimulation.
During the premovement IHI task, while the participant performed a simple reaction-time (RT) task, a transcranial magnetic stimulation (TMS) pulse was then delivered on each trial at four possible epochs: 20, 50, 80, and 95% of each participant’s RT (see the section below, Fig. 1B). TS intensity was determined in the same way as for resting IHI. To assess CS intensity in the context of movement execution, participants were asked to perform the same RT task when double-TMS pulses were delivered at an estimated 50% of RT on each trial, and CS intensity was adjusted to the level approximating 50% of the TS-MEP. This adjustment was to probe the largest possible dynamic range of CS modulation during premovement IHI testing. As described previously,2 when probed at different times during the RT, a healthy control’s typical IHI curve shows an initial reduction, followed by increases of MEP when stimulation is delivered closer to movement onset, that is, IHI switches to facilitation (release of inhibition).
A total of six blocks, with 24 premovement IHI trials per block, were run in each testing session, with 18 pulses per stimulation/time-epoch condition. Sessions were not run if patients could not abduct their index finger or if the stimulation intensity was too high to obtain both resting and premovement IHI (required >90% MSO to elicit an MEP >0.5 mV). These patients were still included in the study if IHI could be obtained in subsequent visits.
Resting motor threshold (rMT) for both FDIs were determined as the minimal TMS intensity required to evoke MEPs of ~50 μV (peak-to-peak amplitude) in the targeted muscle on five of ten consecutive trials.
Because MEP amplitudes increase in the moving effector immediately before movement onset, leading to large MEP that can mask the true size of release of inhibition (or contralateral facilitation), we compared MEP amplitudes recorded during the premovement IHI procedure with maximal amplitudes obtained in each participant using assessment of active corticospinal tract (aCST). This was done with 18 single pulses delivered at 100% MSO with an ISI of 5 to 7 seconds, while the participant was actively contracting the contralateral FDI at a constant level of 20% of their maximum voluntary contraction force.
EMG Recording
Electromyogram (EMG) activity was monitored from surface electrodes placed over the FDI in both hands. Three EMG systems were used at the three sites: SX230–100 and K800, Biometrics Ltd. (CU); AMT-8; Bortec Biomedical Ltd. (JHM); and Telemyo desk receiver, Noraxon (UZ). The Biometrics EMG signal was sampled at 1,000 Hz, amplified 1,000×, band-pass filtered at 15 to 450 Hz; the AMT-8 EMG signal was sampled at 1,000 Hz, amplified 1000×, band-pass filtered at 10 to 1,000 Hz; and the Noraxon EMG was sampled at 1,500 Hz, amplified 500×, band-pass filtered at 15 to 450 Hz. EMG signals were used to determine RTs and MEP amplitudes (see below the Measures of Premovement IHI section).
Simple Reaction-Time Task for Premovement IHI Assessment
Premovement IHI was assessed while participants performed a simple RT task. The participants were instructed to make a voluntary index-finger abduction in response to a GO-cue (green dot). Patients used their paretic hand, whereas healthy volunteers always performed the task with their right hand. The GO-cue was displayed on the monitor for 2 seconds and disappeared at the end of the trial. The intertrial interval was 5 seconds plus 0 to 2 seconds of jitter to prevent anticipation.
Before the IHI procedure, each participant performed the simple reaction task for 30 trials to determine their average RT. The last 15 trials were used to calculate the RT.
Stroke-Related Behavioral Assessments
All patients’ and controls’ upper-extremity motor impairment was determined with the Fugl-Meyer Assessment (FMA),11 following the same schedule as premovement IHI. Hand function was also tested within ±4.6 days from the TMS experiment, as previously described.12 Briefly, participants were instructed to move each finger in isolation on an ergonomic device that measures the isometric force generated by each digit. A strength index was calculated from the maximum voluntary force (MVF) of individual finger flexion, normalized to the MVFs on the nonparetic side at the 1-year time point. An individuation index was derived from the activation in the noninstructed fingers as a function of force produced by the instructed finger pressing to four levels of target forces.
Measures of Premovement IHI
EMG was used to measure RT and peak-to-peak amplitudes of the MEPs elicited in FDI of both hands. Both RTs and MEPs were identified using custom-made MATLAB scripts (The MathWorks, Inc., Natick, MA) from the EMG recordings. The RT was manually identified with the following criteria: peak-to-peak waveforms of EMG activity >100 μV and lasting longer than 50 ms following the GO-cue.
The following trial types were excluded from further analysis: (1) trials with any background EMG activity >20 μV in the 150-ms window preceding the TMS pulse in either FDI; (2) MEP size <50 μV; (3) MEP occurrence after movement onset; and (4) RT >1,000 ms. An analysis of the background pretrigger EMG across different TMS epochs was also conducted to rule out the potential influence of systematic differences in background EMG on the premovement IHI results.
Resting and premovement IHI was computed as the ratio C/NC. An IHI ratio of 1 indicates no IHI. To prevent averaging epochs with too few MEP observations, a minimum of nine good MEPs (one-half of the total count) was required to compute the ratio. A good TS-MEP was defined as: (1) no background EMG activity in the 150-ms window before the TMS pulse; (2) the MEP occurred before movement onset; (3) peak-to-peak amplitude was >50 μV; and (4) distinct movement is detectable (EMG >100 μV for >50 ms) within 1,000 ms after the GO-cue. TMS timing epochs with less than nine good MEPs were counted as missing values. To evaluate the reproducibility of the IHI ratio as the main dependent variable in this study, we computed its Cronbach’s alpha.13,1 Mathematically, alpha is equivalent to the averaged split-half correlation of all possible splits of the existing data:
To assess the evolution of IHI during movement preparation, we derived three other measures: IHIEARlY-EPOch = mean (IHI20 % RT IHI50 % RT), IHILATE-EPOCH = mean (IHI80 % RT IHI95 % RT), and ΔIHI = IHIlate-epoch – IHIearly-epoch ΔIHI therefore reflects the amount of release of IHI during movement preparation. A value of ΔIHI = 0 indicates no modulation of inhibition,15 whereas a positive value implies a release of inhibition during movement preparation. Hereinafter, we will use ΔIHI as an operational definition of premovement IHI to refer to the level of release of inhibition preceding movement onset.
Statistical Analysis
Data analysis was done with custom-written MATLAB and R (R Core Team, 2017) routines. Given that there were missing sessions (on average, each patient completed 3.4 sessions and each healthy control completed 3.5 sessions, out of a total of 5), we used two analysis approaches: (1) For the primary analysis, we assumed missing ΔIHI values arose at random (MAR) and used linear mixed-effects models implemented in the lme4 package in R16 to test for changes in the neurophysiology and behavioral measures over time, with a random factor of Subject, and fixed factors of Time-Point (five time points from W1 to W52, or acute/subacute versus chronic), Hand-Condition (paretic, nonparetic, and/or control), and/or TMS-Epoch (early versus late TMS timing). (2) Because there are cases where data were missing due to severity of impairment, specifically when there was no reliable finger abduction and/or MEP at a given assessment session (Table 2), there was a concern about the possibility of a systematic relationship between premovement IHI and missingness. We therefore conducted a sensitivity analysis by imputing missing values under different data-generating mechanisms. Specifically, for all missing values belong to the category of severity dependent (Table 2) we implemented the assumptions of either no dependency or strong dependency between premovement IHI and the severity of initial impairment (Fig 3D,E). No dependency mimics the MAR assumption of the mixed model, with imputed samples drawn from N~(μ(t,patient), σ(t,patient)), Where μ(t,patient) and σ(t,patient) are estimated from patient data at each time point; whereas strong dependency represents a scenario in which severely affected patients have ΔIHI values centered at 0, with imputed samples from N~(0, σpatient), where σpatient is estimated from all patients’ data. For each data set containing imputed values, we fit the linear mixed model as specified above to account for other missingness. Analysis of variance (ANOVA) tests for sensitivity analyses were conducted by pooling significance tests of multiply-imputed data sets.17
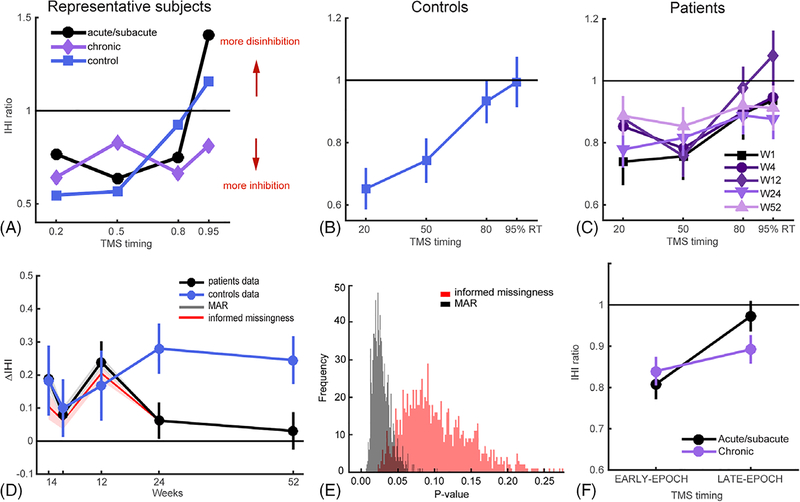
FIGURE 3:
Release of IHI before movement onset. (A) IHI curves for a representative patient and a healthy control. These exemplar IHI profiles illustrate the normal release of IHI in patients at the acute/subacute stage, comparable to control subjects, and the lack of normal release of IHI during the chronic period. (B) Overall mean IHI curves for healthy controls. Because there were no differences over time in premovement IHI in controls (mixed-effects model with Week and TMS-Timing as fixed factors showed no significant effect of Week, χ2 = 0.067, p = 0.80, but significant main effect of TMS-Timing, χ2 = 22.28, p < 0.001), we averaged control data across weeks. (C) IHI curves for each time point over the 1-year period for patients. (D) Evolution of ΔIHI for patients and controls over the 1-year period. Patients showed close to control level of ΔIHI in the acute/subacute periods (W1–12), but their ΔIHIs became abnormal at the chronic stage. Shaded plots in gray and red are sensitivity analysis with two imputation schemes with MAR and informed-missingness cases, respectively, where missing not at random (MNAR) cases are imputed with 1,000 samples from N~(μ(t,patient), σ(t,patient)) or N~(0, σpatient). (μ(t,patient) and σ(t,patient)) are estimated from patients data at each time point and σpatient is estimated from all patients’ data. (E) Distribution of p values from sensitivity analysis with multiple imputation for the MAR and informed-missingness cases. (F) Change of IHI level at different movement preparation epochs in patients from the acute/subacute to chronic stage after stroke. There was a significant interaction of IHIEARLY-EPOCH vs IHILATE-EPOCH or acute/subacute and chronic stages (χ2 = 4.34, p = 0.037), but no differences when comparing across acute/subacute vs chronic stages for IHIEARLY-EPOCH (t(14) = 0.75; p = 0.47) or IHILATE-EPOCH (t(14) = 1.69; p = 0.11). Means and variances in all plots were estimated by mixed models. IHI = interhemispheric inhibition; TMS = transcranial magnetic stimulation.
For behavioral results, we included all available behavioral data, including the sessions in which we could not obtain IHI.
Results
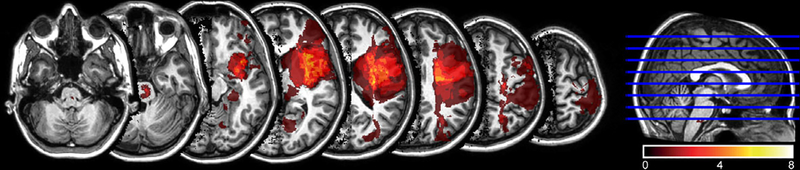
We tested a total of 22 patients from the acute to chronic stages after stroke and 11 healthy controls. Each participant was expected to undergo five testing sessions over the course of a 1-year period. One patient appeared to meet initial inclusion criteria, but was later found to have bilateral strokes and was excluded from further analysis. The final analysis included a total of 110 premovement IHI sessions from 21 patients and 11 controls. Thirteen patients and eight controls completed ≥3 sessions. The distributions of assessment time and missing data are presented in Table 2. Nontested sessions were treated as missing data, and all available data were used in the statistical analyses. The data showed good reliability for the major dependent variable, IHI ratio (α = 0.74 and 0.79 for patients and controls, respectively; Participants and Methods). Figure 2 shows the distribution of lesions defined using diffusion tensor images (details reported in our earlier publication12).
FIGURE 2:
Lesion distribution of patients (N = 21). Averaged lesion distribution mapped to JHU-MNI space,30 with lesion flipped to one hemisphere. Color bar indicates patient count.
Premovement IHI Changed from Normal to Abnormal as Paresis Improved from the Acute to the Chronic Stage
Our main goal was to determine how IHI before movement onset evolves over the first year after stroke and how this relates to motor recovery. Figure 3A shows a representative patient’s IHI curves at the acute/subacute and chronic stages, as compared to a healthy age-matched control. Figure 3B,C shows the group data for controls and patients. Visual inspection of these curves suggests that, consistent with the previous report by Murase and colleagues,2 patients in the chronic stage had an abnormal IHI pattern, characterized by the absence of release of inhibition at movement onset. Crucially, however, in the acute/subacute period (W1–12) release of IHI at movement onset in patients did not appear to differ from controls. Specifically, the IHI ratio at weeks 1 to 12 poststroke increased over the movement-preparation interval, approaching a ratio of 1 at later stimulation epochs (80–95% RT), indicating a level of release of inhibition before movement onset similar to healthy controls.
Given that in previous reports, and corroborated here, the poststroke abnormality in premovement IHI is most apparent at movement onset, our statistical analyses focused on ΔIHI, as in earlier studies.15,18 ΔIHI is the difference between IHIlate-epoch and IHIearly-epoch, which captures the level of release of IHI immediately preceding movement onset (Participants and Methods). An ANOVA using a mixed-effects model for ΔIHI yielded a significant Week × Group (patients versus controls) interaction (χ2 = 4.59; p = 0.03). The evolution of ΔIHI from the acute/subacute to the chronic stage after stroke clearly showed that at earlier stages (W1–12), patients and controls were similar (t(21) = 0.50; p = 0.62), whereas the two groups started to diverge from W24 onward (t((31)= 3.30, p = 0.0025; Fig. 3D). Our sensitivity tests also indicate that this trend is robust to the differences in the data-generating mechanisms considered (p = 0.028 for MAR and p = 0.10 for informed missingness; Fig. 3D,E, Participants and Methods). To directly compare ΔIHI in the acute versus the chronic stage, we pooled data into two Time-periods: mean (W1–12; acute/subacute for patients) and mean (W24–52; chronic for patients). This data pooling was further supported by our observation that there was no difference in patients’ ΔIHI from W1 to W12 (p = 0.17) or from W24 to 52 (p = 0.70). The mixed-effects model with Time-period and Group (patients versus controls) as fixed factors showed a significant interaction (χ2 = 6.68; p = 0.01). These results show that patients’ premovement IHI progressed from normal in the acute/subacute period to abnormal in the chronic stage in the case of mild-to-moderate paresis (Fig. 3F).
The Development of Abnormal Premovement IHI Was Inversely Correlated with the Extent of Finger Individuation Recovery
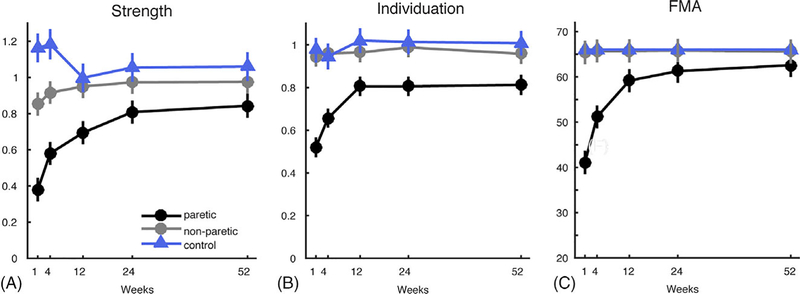
Our cohort of patients was mild to moderately impaired in the acute stage (FMAinitIal Mean = 41 ± 22; Table 1). Motor recovery was quantified using three behavioral measures: FMA, Strength and an Individuation Index for finger (ability to move digits independently; Participants and Methods).12 All three measures showed good early recovery (Strength: χ2 = 28.07; p < 0.001, Individuation: χ2 = 13.64; p < 0.001, and FMA: χ2 = 28.07; p < 0.001), but then plateaued after the subacute stage (Fig 4).
FIGURE 4:
Recovery curves for behavior measures of hand function over 1-year period, from week 1 to 52. (A) Strength indices. (B) Individuation indices. (C) FMA. Means and variances are estimated by mixed model. FMA = Fugl-Meyer Assessment.
We then sought to determine whether there was any correlation between abnormal premovement IHI and motor behavior. To address this question, we first examined the cross-sectional correlation between ΔIHI and all three behavioral measures at both the acute/subacute and chronic stages; none of the correlations were significant with the null value (0) lying within 95% confidence intervals (CIs; Table 3). Thus, there was no clear relationship between abnormal premovement IHI with strength, individuation, or motor impairment at any time point.
TABLE 3.
Cross-Sectional Correlations Between Release of IHI Before Movement Onset (ΔIHI) and Behavioral Measures, FMA, and Strength and Individuation Indices, at Acute/Subacute and Chronic Stages
| FMA | Strength | Individuation | |
|---|---|---|---|
| Acute/subacute (W1–12) | 0.47 [−0.03, 0.78] | 0.46 [−0.05, 0.78] | 0.22 [−0.31, 0.65] |
| Chronic (W24–52) | 0.06 [−0.50, 0.40] | 0.16 [−0.32, 0.57] | 0.43 [−0.03, 0.74] |
Table shows Pearson r values; parentheses represent the 95% confidence interval for each correlation coefficient.
IHI = interhemispheric inhibition; FMA = Fugl-Meyer Assessment.
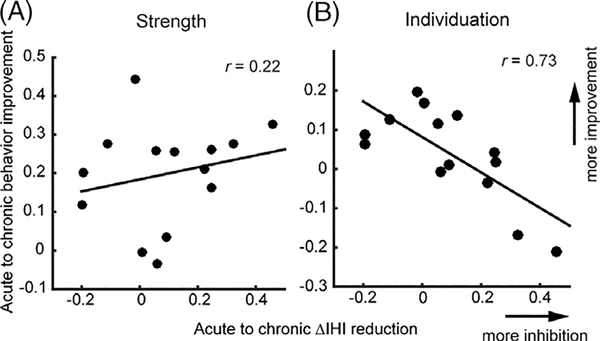
Both the opposite longitudinal time courses for motor recovery and development of abnormal premovement IHI and the lack of significant cross-sectional correlation between the two suggest that the premovement IHI abnormality was not causally related to behavioral impairment. Instead, the emergence of abnormal premovement IHI (failure-to-release inhibition during movement preparation) may be a marker for underlying recovery processes (see Discussion). To address this alternative possibility, we examined the correlation between longitudinal motor-function recovery (change in behavior) and the emergence of the failure-to-release IHI (reduction in ΔIHI) from the acute/subacute to the chronic stages. We found a strong negative correlation between the reduction of ΔIHI and the amount of improvement in the individuation index (r = −0.73; p = 0.003; 95% CI, [−0.91, −0.33]). This suggests that the emergence of failure-to-release IHI during movement preparation and poor finger-individuation recovery share a latent cause. We did not find a significant correlation between changes in ΔIHI and changes in the Strength Index (r = 0.22; p = 0.44; 95% CI, [−0.35, 0.67]; Fig. 5). This observation is consistent with the fact that by week 52 at the group level, patients’ strength was not far from healthy levels (t (26) = 1.43; p = 0.16), but finger individuation was (t (26) = 2.43; p = 0.02).
FIGURE 5:
Correlations between the reduction of premovement IHI (ΔIHI) from acute/subacute to chronic stages and the amount of behavioral recovery: (A) Strength; (B) Individuation. x- and y-axes are the mean differences between chronic and acute/subacute behavior measures and ΔIHI, respectively.
Other TMS and Behavioral Measures
In addition to premovement IHI, we also measured the participants’ rMT, aCST, and resting-IHI for the FDI muscle (Participants and Methods). Results from these measures are reported in Table 4. Consistent with the previous literature,2,19 IHiREST in patients and controls did not differ. Patients and controls had comparable TS- and CS-stimulation intensities for both resting and premovement IHI. For rMT, we included sessions when premovement IHI was not obtainable, and consistent with earlier reports,8,20 the results showed higher rMT on the lesioned hemsphere, reflecting lower level of Ml output at acute-subacute stages in severely impaired patients.
TABLE 4.
Other Basic TMS Measures
| Mean (SD) | t Testst- Value (p) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week | 1 | 4 | 12 | 24 | 52 | 1 | 4 | 12 | 24 | 52 |
| IHIrest | IHIR | Patient vs Control | ||||||||
| Patient | 0.69 (0.20) | 0.67 (0.19) | 0.81 (0.16) | 0.79 (0.20) | 0.73 (0.25) | 0.81 (0.44) | 0.60 (0.56) | 0.11 (0.56) | 0.11 (0.91) | 0.35 (0.73) |
| Control | 0.59 (0.26) | 0.73 (0.29) | 0.73 (0.44) | 0.78 (0.32) | 0.70 (0.22) | |||||
| CS Stimulus Intensity (% MSO) | ||||||||||
| Patient | 57 (17) | 57 (15) | 53 (13) | 55 (15) | 55 (16) | 0.15 (0.88) | 1.21 (0.24) | 0.65 (0.53) | 0.20 (0.84) | 0.08 (0.94) |
| Control | 58 (8) | 49 (10) | 49 (4) | 56 (14) | 56 (15) | |||||
| TS Stimulus Intensity (% MSO) | ||||||||||
| Patient | 57 (19) | 59 (20) | 60 (16) | 63 (18) | 57 (17) | 0.19 (0.85) | 0.61 (0.55) | 0.79 (0.44) | 0.01 (0.99) | 0.40 (0.69) |
| Control | 59(9) | 54 (7) | 54 (8) | 63 (11) | 60 (12) | |||||
| IHIpremove | CS Stimulus Intensity (% MSO) | |||||||||
| Patient | 56 (17) | 57 (16) | 55 (18) | 58 (16) | 54 (14) | 0 (1.00) | 1.12 (0.28) | 0.70 (0.49) | 0.23 (0.82) | 0.30 (0.77) |
| Control | 56 (8) | 50 (10) | 49 (4) | 56 (14) | 56 (15) | |||||
| TS Stimulus Intensity (% MSO) | ||||||||||
| Patient | 55 (18) | 52 (16) | 60 (19) | 60 (18) | 56 (14) | 0.35 (0.73) | 0.60 (0.55) | 1.12 (0.28) | 0.32 (0.75) | 0.50 (0.62) |
| Control | 52(7) | 48 (6) | 50(6) | 58(9) | 53 (12) | |||||
| MEP Amplitude in Patient (mV) | aCST vs TS | |||||||||
| aCST | 2.89 (1.36) | 3.71 (1.99) | 3.27 (1.80) | 2.78 (1.53) | 3.27 (2.12) | |||||
| TS at 95% RT | 2.22 (1.46) | 2.51 (1.07) | 2.00 (1.28) | 1.68 (1.32) | 1.81 (0.99) | 1.05 (0.31) | 1.91 (0.07) | 2.13 (0.04*) | 2.28 (0.03*) | 2.48 (0.02*) |
| TS at 80% RT | 1.81 (1.06) | 1.82 (0.91) | 1.64 (1.26) | 1.33 (1.23) | 1.55 (0.81) | 1.97 (0.06) | 3.11 (0.005*) | 2.74 (0.01*) | 3.10 (0.004*) | 3.02 (0.005*) |
| Patient | rMT | Paretic vs Nonparetic | ||||||||
| Paretic | 62 (24) | 54 (21) | 50 (13) | 47 (14) | 46 (13) | 3.34 (0.002*) | 2.74 (0.009*) | 2.72 (0.01*) | 1.70 (0.10) | 1.48 (0.15) |
| Non-paretic | 42 (11) | 40 (9) | 40 (9) | 40 (10) | 40 (10) | |||||
| Control | Paretic vs Control | |||||||||
| Dominant | 47(7) | 46 (8) | 48 (9) | 45 (8) | 45(7) | 1.95 (0.06) | 1.20 (0.24) | 0.51 (0.62) | 0.42 (0.68) | 0.25 (0.81) |
| Non-dominant | 43 (9) | 42(7) | 41 (6) | 42(7) | 40 (6) | 2.43 (0.02*) | 1.87 (0.07) | 2.08 (0.046* | 1.19 (0.24) | 1.42 (0.167) |
Reported here are mean and standard deviations (SD) of IHI at rest (IHIrest), CS and TS stimulation intensities for resting and premovement IHI, MEP amplitude in patients for active corticospinal track integrity (aCST) assessed with 100% MSO, TS at 95 and 80% of RT, and resting motor threshold (rMT) in patients and controls. Independent-samples t tests were done between patients and controls for IHIrest and CS and TS intensities at each time point. MEP amplitudes were compared between aCST and TS at each time point among patients. Comparison of rMT were done between paretic versus nonparetic hands in patients, and dominant versus nondominant hands in healthy controls.
TMS = transcranial magnetic stimulation; IHI = interhemispheric inhibition; CS = conditioning stimulus; TS = testing stimulus; RT = reaction time; MSO = maximal stimulator output; MEP = motor-evoked potential
= statistically significant.
To ensure our premovement IHI results were not attributed to high MEP amplitudes, especially during the later TMS epochs, we compared the MEP sizes obtained from aCST with the single-pulse TS at late TMS epochs (80 and 95% RT; Participants and Methods). If TS-MEPs approach the MEP amplitudes of the aCST, when MEP amplitudes are expected to be near maximal, the amount of IHI modulation during movement preparation could lack sufficient dynamic range or be masked. We found, however, that most late-epoch MEP amplitudes were lower than those obtained during the aCST assessment (see statistics in Table 4).
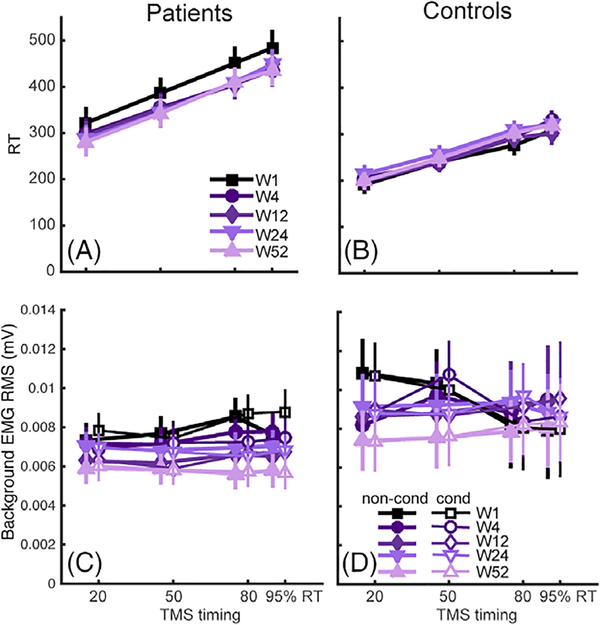
It might be posited that one way that failure-to-release inhibition might influence behavior is to prolong the RT. We therefore examined the relationship between the RT and premovement IHI in the simple RT task. RTs in patients were prolonged compared to controls (Fig 6A,B; χ2 = 9.19; p = 0.002), but this prolongation was not linked to changes in premovement IHI: There was no interaction with ΔIHI and RT (χ2 = 0.31;p = 0.58).
FIGURE 6:
Other behavioral and physiological measures in premovement experiments. Reaction time (RT) for patients (A) and controls (B) at different TMS timing during movement preparation across the 1-year period. RTs for controls were overall faster than patients. Background EMG for patients (C) was overall lower than that in controls (D), but was at a similar level for conditioned vs nonconditioned TMS stimulation. RMS = root mean square; TMS = transcranial magnetic stimulation.
To rule out the possibility of background EMG influencing the observed premovement IHI patterns, we also performed a mixed-effect model analyses on pretrigger EMG (Participants and Methods). Results showed that background EMG was higher in healthy controls (χ2 = 5.46; p = 0.019) and decreased over time in both groups (χ2 = 45.23; p = 1.77 X e−11), possibly attributed to participants becoming more acquainted with the testing procedure (Fig 6C,D). Critically, there was no main effect of conditioned (C) versus nonconditioned (NC) trials, nor any interaction between group and any other factor. Thus, differences in background EMG cannot explain the premovement IHI findings.
Finally, age did not influence the main dependent variable ΔIHI (χ2 = 0.53; p = 0.47), nor did it interact with Week (χ2 = 4.73; p = 0.09). Similarly, age also did not modulate the behavioral outcome variables in our cohort: Strength, Individuation, FMA, and ARAT.
Discussion
In a longitudinal multicenter study, we tracked the evolution of premovement IHI from stroke onset up to 1 year. We used a double-pulse TMS paradigm to test patients and healthy controls at five time points: week 1, 4, 12, 24, and 52. We also tracked patients’ finger strength and individuation, and overall motor impairment (FMA). We found that release of IHI before movement onset was normal in the acute/subacute period and became abnormal in the chronic stage. Conversely, behavioral outcomes were most impaired in the acute/subacute period and improved over time to reach plateau in the chronic stage. In addition to these opposite longitudinal trends for the physiological and behavioral measures, we found no significant cross-sectional correlations between premovement IHI and behavioral measures in the patients (strength and individuation). The only significant correlation was an inverse relationship between the development of abnormal premovement IHI from the acute/subacute to the chronic stage after stroke (i.e. the emergence of the failure-to-release IHI before movement onset) and the amount of recovery in finger individuation across the same period.
In the seminal study by Murase and colleagues,2 impaired premovement IHI was found in nine patients with chronic stroke. This study has become highly influential and, in our view, was prematurely interpreted by the overall neurorehabilitation field as suggesting a possible causal relationship between IHI and recovery of motor impairment. This interpretation is problematic because: (1) premovement IHI is only one kind of interhemispheric measure; it is possible to assess IHI at other ISIs or interhemispheric facilitation.21 (2) Premovement IHI is only obtainable in patients with detectable MEPs and finger movements; it cannot be assessed in patients with more severe motor deficits. (3) The study by Murase and colleagues had a small sample of patients at only one time point in the chronic stage, which makes inference about changes over time, or recovery, impossible. The overinterpretation of the Murase and colleagues results led, in turn, to a large number of studies that attempted, or claimed, to rebalance IHI using noninvasive brain stimulation (NIBS) in the acute and chronic stages after stroke.22–26 What should have been established first, in our view, is the time course of the development of premovement IHI abnormality from the acute/subacute period to the chronic stage.
The critical finding reported here is that in the acute/subacute period, in those patients that could be assessed with this TMS technique, we found normal modulation of premovement IHI despite their motor deficits. Failure-to-release premovement IHI only emerged in the chronic stage, whereas the behavioral measures all improved over the same time period. This diametric contrast makes any claim to a causal relationship between abnormal premovement IHI and the motor deficit implausible. Adding to this, we found no significant cross-sectional correlations between premovement IHI and severity of paresis, assessed by FMA, Strength, or Individuation. Admittedly, given the limited statistical power, we cannot definitively rule out the possibility of an association between premovement IHI and a clinical measure. Interestingly, though, a recent meta-analysis27 of 112 TMS studies concluded that “there is no clear evidence for hyper-excitability of the unaffected hemisphere” in either the acute or chronic phases after stroke. Nevertheless, it is important to note that the interpretation of our results, as well as of previous investigations, should be limited to those patients for whom it is possible to assess premovement IHI and/or obtain MEPs (i.e. those with mild-to-moderate motor deficits). Therefore, it remains unclear what the interhemispheric interaction would be for patients with more severe motor deficits.
It would be puzzling, however, if premovement IHI were to be abnormal in the acute period in severe patients given that our mild-to-moderate patients showed improvement from paresis as IHI became worse. Thus, from parsimony, it would seem that the interhemispheric competition model would not be a satisfactory causal explanation even in patients with severe motor deficits. Unfortunately, methodological limitations prevent us from going beyond this speculation.
The inverse correlation between the emergence of abnormal premovement IHI from the acute/subacute to chronic stages and recovery of individuation suggests that, rather than any direct causal relationship between them, the development of an abnormal pattern of ΔIHI over time might provide an indirect measure of the state of longitudinal recovery. This would mean that the amount of reduction in ΔIHI might reflect a less-optimal form of reorganization, such as a reliance on contralesional corticoreticular projections28,29 or, possibly, the consequence of decreasing use of the paretic hand in dexterity-requiring tasks. Both possibilities are consistent with the finding that finger individuation did not fully recover even at 1 year after stroke (Fig 4B). We cannot disambiguate these two possibilities in this study. However, here we show: (1) There is no cross-sectional correlation between premovement IHI and behavior; (2) behavior gets better as premovement IHI gets worse; and (3) the emergence of abnormal premovement IHI is correlated with poor finger-individuation recovery. These results together suggest that the abnormal interhemispheric interaction in the chronic stage might be the consequence of, and a marker for, the state of recovery of the brain rather than the cause of impairment. Therefore, it is questionable that interhemispheric imbalance should be a therapeutic target.
The results presented here challenge the validity of the interhemispheric-competition recovery model. This is important given that in the past decade, numerous studies have used NIBS in an attempt to downregulate the contralesional hemisphere to promote recovery: From 2005 to 2016, there were 45 published clinical trials using cathodal transcranial direct current stimulation25 and 25 trials up to May 2014 using rTMS.26 The lasting impact of the model is apparent in a recent influential perspective by Di Pino et al,7 in which they introduce a hybrid recovery model that combines vicariation in the ipsilesional hemisphere with interhemispheric-competition. Of note, our results do not negate the fact that on occasions, NIBS over the ipsi-, contra-, or bilateral hemisphere have shown beneficial effects.3,4 What our results do indicate, however, is that any beneficial effect of NIBS is not likely operating by an IHI mechanism, at least for patients with mild-to-moderate hemiparesis.
In conclusion, the results reported here cast doubt on the validity of the interhemispheric-competition model. Future investigations using noninvasive brain stimulation, or other interventions, such as peripheral nerve stimulation, to improve recovery following stroke will require alternative mechanistic justification.
Acknowledgment
This main study was supported by James S. McDonnell Foundation (JSMF) Awards 90043345 and 220020220. Additional support came from R01HD053793 (to P.A.C., J.W.K., and M.B.) and K23NS078052 (to H.S.).
We thank Tziporah Thompson for making the artwork in Figure 1 and thank Isis Martinez-Hernandez, Jessica Berard, and Joachim Cerny for data collection assistance.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol 2004;61:1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 2004;55:400–409. [DOI] [PubMed] [Google Scholar]
- 3.Nair DG, Renga V, Lindenberg R, et al. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci 2011;29:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindenberg R, Renga V, Zhu LL, et al. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 2010; 75:2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 2006; 5:708–712. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer EH, Celnik PA. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci 2011;29:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014;10:597–608. [DOI] [PubMed] [Google Scholar]
- 8.Stinear CM, Petoe MA, Byblow WD. Primary motor cortex excitability during recovery after stroke: implications for neuromodulation. Brain Stimulat 2015;8:1183–1190. [DOI] [PubMed] [Google Scholar]
- 9.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 10.Ferbert A, Priori A, Rothwell JC, et al. Interhemispheric inhibition of the human motor cortex. J Physiol 1992;453:525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fugl-Meyer AR, Jääskö L, Leyman I, Steglind S The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 12.Xu J, Ejaz N, Hertler B, et al. Separable systems for recovery of finger strength and control after stroke. J Neurophysiol 2017;118:1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schambra HM, Ogden RT, Martínez-Hernández IE, et al. The reliability of repeated TMS measures in older adults and in patients with subacute and chronic stroke. Front Cell Neurosc. 2015;9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16:297–334. [Google Scholar]
- 15.Hummel FC, Steven B, Hoppe J, et al. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology 2009;72:1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. ArXiv 2014;arXiv:1406.5823 [stat.CO]. [Google Scholar]
- 17.van Ginkel JR, Kroonenberg PM. Analysis of variance of multiply imputed data. Multivar Behav Res 2014;49:78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liuzzi G, Hörniß V, Lechner P, et al. Development of movement-related intracortical inhibition in acute to chronic subcortical stroke. Neurology 2014;82:198–205. [DOI] [PubMed] [Google Scholar]
- 19.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci 1996;144: 160–170. [DOI] [PubMed] [Google Scholar]
- 20.Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. CerebCortex 2008;18:1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis J, Swayne OB, Vandermeeren Y, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 2008;586:325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsner B, Kwakkel G, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J Neuroeng Rehabil 2017;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev 2013;(5):CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graef P, Dadalt MLR, Rodrigues DAM da S, et al. Transcranial magnetic stimulation combined with upper-limb training for improving function after stroke: a systematic review and meta-analysis. J Neurol Sci 2016;369:149–158. [DOI] [PubMed] [Google Scholar]
- 25.Lefaucheur JP. A comprehensive database of published tDCS clinical trials (2005–2016). Neurophysiol Clin Neurophysiol 2016;46:319–398. [DOI] [PubMed] [Google Scholar]
- 26.Lüdemann-Podubecká J, Bösl K, Nowak DA. Repetitive transcranial magnetic stimulation for motor recovery of the upper limb after stroke. Prog Brain Res 2015;218:281–311. [DOI] [PubMed] [Google Scholar]
- 27.McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimulat 2017;10:721–734. [DOI] [PubMed] [Google Scholar]
- 28.Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 2004;159:284–300. [DOI] [PubMed] [Google Scholar]
- 29.Ellis MD, Drogos J, Carmona C, et al. Neck rotation modulates flexion synergy torques, indicating an ipsilateral reticulospinal source for impairment in stroke. J Neurophysiol 2012;108:3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008; 40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]