Highlights:
-
•
Appraisal of self-relevance is disturbed in borderline personality disorder (BPD).
-
•
We introduce the first neuroimaging study about self-relevance processing in BPD.
-
•
Besides the CMS, the MNS and the SMA are involved in self-relevance processing.
-
•
Functional connectivity of CMS is altered in BPD during self-relevance evaluations.
-
•
In BPD, valence affects the functional connectivity during self-relevance ratings.
Keywords: Borderline personality disorder, Self-relevance, Cortical midline structures, Mirror neuron system, Functional connectivity
Abstract
Self-relevant functional abnormalities and identity disorders constitute the core psychopathological components in borderline personality disorder (BPD). Evidence suggests that appraising the relevance of environmental information to the self may be altered in BPD. However, only a few studies have examined self-relevance (SR) in BPD, and the neural correlates of SR processing has not yet been investigated in this patient group. The current study sought to evaluate brain activation differences between female patients with BPD and healthy controls during SR processing. A task-based fMRI paradigm was applied to evaluate SR processing in 23 female patients with BPD and 23 matched healthy controls. Participants were presented with a set of short sentences and were instructed to rate the stimuli. The differences in fMRI signals between SR rating (task of interest) and valence rating (control task) were examined. During SR rating, participants showed elevated activations of the cortical midline structures (CMS), known to be involved in the processing of self-related stimuli. Furthermore, we observed an elevated activation of the supplementary motor area (SMA) and the regions belonging to the mirror neuron system (MNS). Using whole-brain, seed-based connectivity analysis on the task-based fMRI data, we studied connectivity of networks anchored to the main CMS regions. We found a discrepancy in the connectivity pattern between patients and controls regarding connectivity of the CMS regions with the basal ganglia-thalamus complex. These observations have two main implications: First, they confirm the involvement of the CMS in SR evaluations of our stimuli and add evidence about the involvement of an extended network including the MNS and the SMA in this task. Second, the functional connectivity profile observed in BPD provides evidence for an altered functional interplay between the CMS and the brain regions involved in salience detection and reward evaluation, including the basal ganglia and the thalamus.
1. Introduction
Borderline personality disorder (BPD) is a severe mental disorder with a prevalence of 2–5% (Lenzenweger et al., 2007, Torgersen et al., 2001), contributing significantly to the global burden of disease (Soeteman et al., 2008). Patients with BPD commonly suffer from instabilities in affect regulation, impulse control, self-image, and interpersonal relationships (American Psychiatric Association, 2013, Lieb et al., 2004). The fifth version of the “Diagnostic and Statistical Manual of Mental Disorders” (DSM-5®) acknowledges identity disorders and self-related functioning impairments to be a central difficulty in BPD (Bender and Skodol, 2007, Jørgensen, 2006). Therefore, alterations in self-related processes are considered to be important targets in designing and improving therapeutic approaches.
Evaluating the relevance of environmental and mental events to the self is an important part of self-related processing. A seminal study by Rogers and colleagues (1977) found a memory advantage for information encoded with reference to the self. Also, it is quite well established that information processing differs significantly across self-relevant and self-irrelevant contexts (Ertac, 2011), indicating a “self-reference effect” in the cognitive, affective and executive domains of brain functioning. Evaluations of self-relevance (SR) affect the perception of social cues (Lombardo et al., 2010) as well as emotion control and reappraisal (Neacsiu et al., 2015), which are likely to be impaired in BPD. In fact, an increased tendency to attribute events to themselves has been observed in patients with BPD (Moritz et al., 2011). This may be associated with pathological manifestations such as arousal (Bayer et al., 2017), impulsivity, affective instability and, most importantly, identity disorder.
Unfortunately, there is a paucity of studies that investigate how patients with BPD perceive, evaluate, and respond to self-related information. In a previous study, we demonstrated a higher SR for negative sentences in female patients with BPD compared to healthy controls (HC). We also uncovered a positive correlation between the SR for negative sentences and the severity of BPD symptoms (Sarkheil et al., 2019). Apart from this, most investigations of self-related processing in BPD have focused largely on the evaluation of self-esteem, revealing low (Bungert et al., 2015, Lynum et al., 2008) or unstable (Hochschild Tolpin et al., 2004) self-esteem in this group of patients. According to the available empirical evidence, patients with BPD are highly self-critical (Kopala-Sibley et al., 2012), harboring a pejorative view of themselves (Lieb et al., 2004, Rüsch et al., 2007) characterized by negative rather than positive traits (Beeney et al., 2016, Vater et al., 2015). The construct of self-esteem is very complex (Winter et al., 2017) and is likely to be reactive to self-relevant cues (Winter et al., 2015b) as well as negative emotions with respect to the self (Greenier et al., 1999). In patients with BPD, a negative evaluation bias toward self-referential information is thought to enhance and sustain the disapproving view of themselves (Winter et al., 2015a). The processing of SR on the whole seems to be a key factor in the formation of self-related disorders.
The brain networks responsible for appraising self-relevant information from the environment have already been studied using neuroimaging techniques in the healthy population. The cortical midline structures (CMS) have been shown to be involved in the processing of self-related stimuli (Kedia et al., 2019, Murray et al., 2015, Northoff et al., 2006, Philippi et al., 2012, Schmitz and Johnson, 2007, van der Meer et al., 2010, Wagner et al., 2012). The CMS involve the frontal and parietal parts of the default mode network (DMN) and have strong and reciprocal links to structures in the midbrain, the brain stem, and the limbic system, including the hippocampus, the amygdala, and the insula (Northoff and Bermpohl, 2004). It has been suggested that self-relevant stimuli are represented in the orbital part of the medial prefrontal cortex (MPFC), monitored in the anterior cingulate cortex (ACC), evaluated in the dorsal part of the MPFC, and integrated in the posterior cingulate cortex (PCC) (Northoff and Bermpohl, 2004). Through this loop, the CMS allow for bottom-up and top-down modulation between sensory, self-relevant and higher-order processing (Northoff et al., 2006). Additionally, a second neural system, the so-called “Mirror Neuron System” (MNS), has been shown to be associated with self-related processing (Rizzolatti and Craighero, 2004, Uddin et al., 2007). The MNS comprises the fronto-parietal regions, including the inferior frontal gyrus (IFG), the precuneus, the supramarginal gyrus (SMG), the inferior parietal lobule (IPL), the anterior insula and the anterior mesial frontal cortex (Rizzolatti and Craighero, 2004). The CMS and MNS are linked in the frontal and parietal regions, with the CMS being involved in social and psychological aspects of the self (Qin and Northoff, 2011) while the MNS, strongly associated with imitative behavior (Iacoboni, 2005), performs a physical self-other discrimination (Uddin et al., 2007).
Neural correlates of altered SR involving the CMS and the MNS have been reported in psychiatric diseases such as schizophrenia, autism spectrum disorder and unipolar depression (Zhao et al., 2013). Although evidence suggests that individuals with BPD exhibit a deviant appraisal of SR (Sarkheil et al., 2019, Sauer et al., 2014, Winter et al., 2015a), neural correlates of SR have yet to be investigated in this group of patients. Given the importance of self-related disturbances in the BPD psychopathology, neuroimaging studies that focus on the processing of SR are particularly well-suited for facilitating symptom stratification measures and treatment development.
In the current study, we aimed to evaluate the differences in SR judgment-related neural activation between patients with BPD and HC. We used short affective sentences and an SR evaluation task to induce various degrees of SR processing, while measuring the event-related fMRI activation in participants. Based on the available knowledge about the role of CMS in appraising the self-relevant content of the environment, we investigated the following hypotheses: 1) BPD and HC differ in terms of CMS activation, 2) the affective content of the stimuli affects SR-related activation in the CMS, and 3) BPD and HC exhibit differential connectivity patterns during SR.
2. Materials and methods
2.1. Participants
Twenty-three female patients with BPD (mean age: 24.7 years ± 6.6 [range = 18–42]), who met the diagnostic criteria for BPD according to the DSM-5 (American Psychiatric Association, 2013), as assessed by a psychiatrist using the German version of the structured clinical interview for DSM-5, Personality Disorder Version (SCID-5-PD) (First et al., 2016a), and twenty-three matched HC (mean age: 24.7 years ± 2.9 [range = 20–33]) participated in the present study. To account for gender-specific clinical presentation of BPD (Sansone and Sansone, 2011), only female subjects were included. Patients were recruited from the inpatient psychiatric unit at the University Hospital RWTH Aachen. Healthy volunteers were recruited through public advertisements and matched with respect to age. The exclusion criteria for all participants included current pregnancy, MRI contraindications, acute psychotic symptoms, severe head trauma, current substance use disorder, or a history of alcohol and substance abuse within the past 6 weeks. Patients with benzodiazepine medication were also excluded. None of the HC subjects met the criteria for a psychiatric or neurological disease, neither currently nor historically. All participants were native German-speakers. Of the 23 patients, 18 presented with a comorbid psychiatric diagnosis, assessed with the SCID-5, Clinical Version (SCID-5-CV) (First et al., 2016b), and 16 were under psychotropic medication (for detailed diagnosis and medication list see Table 1). The study was conducted at the University Hospital RWTH Aachen, Germany. The research protocol was approved by the local ethics committee (The Independent Ethics Committee, medical faculty, RWTH Aachen University (EK048-16)). Human research in this study was conducted according to the principles expressed in the Declaration of Helsinki. All participants gave written informed consent to participate in the study and received financial compensation.
Table 1.
Patients characteristic: Clinical data.
| BPD (N = 23) | |
|---|---|
| Medication (n), number of patients (%) Medication type |
total: 16 (69.5%) 4 (17.4%) SSNRI 3 (13.0%) SSNRI + AAP 3 (13.0%) NDRI 1 (4.3%) SSRI 1 (4.3%) AAP 1 (4.3%) SSRI + AAP 1 (4.3%) AAP + ATC 1 (4.3%) AAP + PPI 1 (4.3%) SSRI + AAP + NDRI |
| Comorbidities (n), number of patients (%) Comorbidity type |
total: 18 (78.3%) 9 (39,1%) MDD 6 (26.1%) eating disorder 2 (8.7%) MDD + eating disorder 1 (4.3%) MDD + eating disorder + PTSD |
Abbreviations: AAP, atypical antipsychotics (quetiapine/aripiprazole/pipamperone); ATC, anticonvulsant (topiramate); NDRI, noradrenalin-dopamine-reuptake-inhibitor (bupropion); PPI, proton-pump inhibitor (pantoprazole); SSNRI, selective serotonin-noradrenalin-reuptake-inhibitor (venlafaxine/duloxetine); SSRI, selective serotonin reuptake inhibitor (fluoxetine/sertraline); MDD, major depressive disorder; PTSD, posttraumatic stress disorder; BPD, borderline personality disorder.
2.2. Experimental assessments
2.2.1. Questionnaires
One day prior to the MRI measurement, all patients completed the BSL-95 (Borderline Symptom List) (Bohus et al., 2001) which comprises 95 items rated on a 5-point Likert scale (0 = not at all, 4 = very strong), to help assess borderline symptom severity. The items can be divided into the following subscales: Self-perception, affect regulation, auto-aggression, dysthymia, social isolation, intrusions and hostility. Additionally, all participants completed the German version (Kühner et al., 2007) of the BDI-II (Beck Depression Inventory-II) (Beck et al., 1996) to help measure depressive symptoms.
2.2.2. Task
To assess the perceived SR of information, a set of 56 sentences with third-person pronouns was created as stimuli. Twenty-eight negative and 28 positive terms from the German version (Schmidtke et al., 2014) of “Affective Norms for English Words (ANEW)” (Bradley and Lang, 1999) were selected. The selection was based on affective valence categories (positive and negative) and were balanced across other dimensions such as arousal and dominance. The selected set of words was used to build 56 short sentences (28 for each condition) containing a third-person female pronoun (she, her). The sentences had a simple structure and consisted of 4–7 words. A sample of the sentences both in the German original and in English translation is presented in the Supplementary Material (Table S 1).
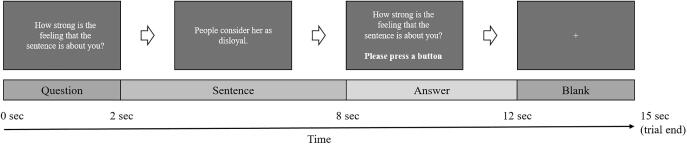
During the fMRI measurements (4 sessions, 28 trials each), the participants viewed the sentences and were asked to rate them based on either SR (“How strong is the feeling that the sentence is about you?”) or affective valence (“How do you evaluate the valence of the sentence?”). Each of the 56 sentences was presented twice for both SR rating and affective valence rating. Every trial started with a 2-second presentation of a question, which served as a cue for the subsequent rating and was followed by the display of a sentence for 6 seconds. Afterwards, the question was presented again for 4 seconds with the participants being asked to choose their response on a 4-point Likert scale (SR rating: 1 = low SR, 4 = high SR; valence rating: 1 = highly negative, 4 = highly positive) by pressing a button. At the end of each trial, a blank screen appeared for 3 seconds before the start of the next trial. A schematic diagram of the trial structure is shown in Fig. 1. Sentences (positive and negative) as well as question types (SR rating and affective valence rating) were presented in a randomized order. In 50% of the participants the 4-point Likert scale was mirrored (SR rating: 1 = high SR, 4 = low SR; valence rating: 1 = highly positive, 4 = highly negative) to cancel out the handedness laterality effect. All participants were instructed to react spontaneously and respond as quickly as possible. Six baseline blocks (each 15 seconds) appeared in randomized intervals in each session. During baseline blocks participants viewed a gray screen. They were instructed to rest and not perform any task. The fMRI analyses were based on the time window of the sentence presentation that did not involve motor activation.
Fig. 1.
Trial design. Twenty-eight trials were presented in each session. A trial started with a question displayed for 2 seconds, followed by a sentence presented for 6 seconds. Subsequently, the question was presented again for 4 seconds with the participants being asked to choose their response by pressing a button. Finally, a blank screen was presented for 3 seconds.
2.2.3. MRI imaging
Brain imaging was performed on a 3.0 Tesla Siemens Prisma fit MRI scanner (Magnetom, Siemens Medical Systems, Erlangen, Germany) with a 32-channel head coil. Functional T2*-weighted images of the BOLD contrast were acquired with an echo planar imaging (EPI) sequence (repetition time (TR) = 2000 ms, echo time (TE) = 28 ms, flip angle = 77°, voxel size 3 × 3 mm, matrix size = 64 × 64 × 64, 34 transverse slices (interleaved acquisition) with whole-brain coverage, slice thickness = 3 mm, 0.75 mm gap). Each experimental run comprised 258 volumes. T1-weighted anatomical images were acquired with a Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence (TR = 2000 ms, TE = 30.3 ms, inversion time TI = 900 ms, flip angle = 9°, voxel size 1 × 1 mm, 176 sagittal slices, 1 mm slice thickness, FOV = 256 × 256 mm2, GRAPPA factor 2).
2.3. Data analysis
2.3.1. Preprocessing
Data preprocessing for the whole-brain and region of interest (ROI) analyses was conducted with the SPM12 software (Wellcome Center for Human Neuroimaging, London, UK) implemented in MATLAB R2017b (The MathWorks, Inc., Natick, MA, USA). The preprocessing included realignment, normalization to Montreal Neurological Institute (MNI) template space and spatial smoothing with an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. The first five images were excluded for T1 equilibration. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
The preprocessing for connectivity analysis was conducted using the MATLAB-based functional connectivity toolbox CONN v.18.a (Whitfield-Gabrieli and Nieto-Castanon, 2012) and included realignment, unwarp, slice-time correction, outlier detection (ART-based identification of outlier scans for scrubbing), segmentation to gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF), normalization to MNI template space and spatial smoothing with an 8 mm FWHM Gaussian kernel. The de-noising step in the CONN toolbox uses linear regression to identify and remove confounding effects including motion and physiological artifacts. Individual time courses from WM and CSF (5 dimensions each) as well as global signal intensity fluctuations and 12 motion parameters (rigid body transformations and their first-order derivatives) were extracted and used as nuisance regressors in the de-noising step. Additionally, the general task effect has been regressed out based on confounds specified for each condition (SR rating of positive sentences, SR rating of negative sentences, valence rating of positive sentences and valence rating of negative sentences). After regression, band-pass filtering was applied between 0.008 and 0.09 Hz.
2.3.2. Whole-brain first- and second-level analysis
The task-related blood oxygen level-dependent (BOLD) signal changes at the subject level were estimated using whole-brain first-level analysis based on General Linear Models (GLM) in SPM12. For each subject, the following conditions of interest were defined: Positive sentences with SR rating task, positive sentences with valence rating task, negative sentences with SR rating task and negative sentences with valence rating task. To reduce motion artifacts, head movement parameters were included in the model. The first-level contrasts used for group analysis were: [positive sentences with SR rating task > baseline], [positive sentences with valence rating task > baseline], [negative sentences with SR rating task > baseline] and [negative sentences with valence rating task > baseline]. For the second-level analysis, beta values representing baseline‐corrected responses were entered into a 3-factor 2-level mixed-model ANOVA, with the between-subjects factor group (BPD, HC) and within-subjects factors task (SR rating, valence rating) and valence (positive, negative), generating contrasts for all main effects and interactions. A voxel-level family-wise error correction (pFWE < .05) was applied for the group analysis.
2.3.3. ROI analysis
Based on the a priori hypotheses, a ROI analysis was performed including the following SR-related brain areas: The ACC, the PCC and the MPFC. All masks were generated based on the automated anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) included in the WFU PickAtlas toolbox v3.0 (Maldjian et al., 2003) in SPM12. The MPFC mask was created by combining the bilateral medial orbital frontal regions, the rectus regions and the superior medial frontal regions (Yoon et al., 2018). Masks for the cingulate regions were generated by combining the left and right hemisphere masks of the ACC and the PCC, respectively. The ROI analysis was performed in SPM12 using the small volume correction. Second-level whole-brain results of the previous analysis were used while restricting to the predefined ROIs only. A voxel-level family-wise error correction (pFWE < .05) was applied for the group analysis.
2.3.4. Connectivity analysis
Seed-based functional connectivity analysis was performed using the functional connectivity toolbox CONN v.18.a (Whitfield-Gabrieli and Nieto-Castanon, 2012) with the masks used for the ROI analysis serving as the seed regions. The connectivity was calculated based on the time series of each seed region and correlations with the time series of all other voxels in the brain. The resulting correlations were Fisher’s z-normalized. On the group level, a 2x2 ANOVA investigated the interaction effects in seed-based connectivity between group (BPD, HC) and task (SR rating, valence rating). The results of the 2 × 2 mixed ANOVA interactions [BPD > HC; SR rating > valence rating] were corrected for multiple testing using a cluster‐level false discovery rate correction pFDR < .05 combined with a voxel‐level threshold of p < .01. In the patient group, we additionally investigated the effect of affective valence in SR evaluations using a paired t-test corrected for multiple comparisons applying a cluster‐level false discovery rate correction pFDR < .05 combined with a voxel‐level threshold of p < .01. To investigate the relationship between symptom severity and functional connectivity, correlations of BSL-95 scores and connectivity estimates were performed in the patient group.
3. Results
3.1. Demographics
Patients with BPD and HC did not differ significantly in regard to age (t(44) = -0.028, p = .978). The BDI-II scores in the BPD group (mean ± SD: 27.17 ± 14.45) were significantly higher compared to the HC group (3.74 ± 3.26; t(44) = 7.59, p < .001) with the patients having a mean BSL-95 score of 1.82 ± 0.77. Sixteen out of 23 patients were medicated and 18 had at least one comorbid disorder (for detailed information see Table 1).
3.2. Whole-brain analysis
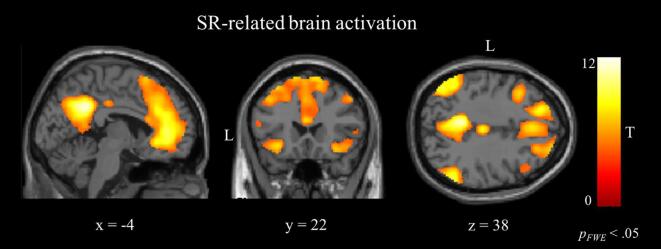
The contrast [SR rating task > valence rating task] in the GLM revealed increased activation in a widespread network including the ACC, the PCC, the MPFC, the middle frontal gyrus (MFG), the supplementary motor area (SMA), the precuneus, the angular gyrus, the middle temporal gyrus (MTG), the middle cingulate gyrus (MCG), the posterior orbital gyrus, the left orbitofrontal cortex (OFC) and the left cerebellum, (Fig. 2, Table 2). The contrast [valence rating task > SR rating task] showed an increased activation in the bilateral central operculum, the bilateral parietal operculum, the left postcentral gyrus, the left supramarginal gyrus, and the bilateral superior parietal lobule (SPL) (Table 2). The GLM analysis did not show any effect of group, nor any interaction between group and task or valence on the whole-brain level.
Fig. 2.
Results of the whole-brain analysis: GLM random-effect group maps for [SR rating > valence rating] in all participants. The network includes the ACC, the PCC, the MPFC, the bilateral precuneus, the bilateral anterior insula, the bilateral SMA and the bilateral angular gyrus. ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; SMA, supplementary motor area.
Table 2.
Clusters showing significant activation for the contrasts [SR rating > valence rating] and [valence rating > SR rating] in the GLM in both groups on whole-brain level.
| Regions | Size (kE) | T value | MNI coordinates (x, y, z) |
|---|---|---|---|
| [SR rating > valence rating] | |||
| PCC, precuneus L, R | 2366 | 12.77 | −08 –48 +34 |
| angular G. L | 1996 | 10.52 | −56 –58 +28 |
| ACC, MPFC/MFG, SMA L, R | 11272 | 10.26 | −02 +40 +06 |
| angular G. R | 897 | 9.96 | +58 –56 +36 |
| ant. insula L, post. orb. G. L, MTG L | 2107 | 8.95 | −30 +16 –12 |
| MCG | 176 | 8.88 | +00 –24 +38 |
| ant. insula R, post. orb. G. R | 397 | 8.67 | +32 +18 –12 |
| MFG R | 380 | 6.54 | +48 +18 +46 |
| cerebellum L | 125 | 6.18 | −40 –60 −30 |
| OFC L | 78 | 5.78 | −50 +40 –14 |
| MTG R | 178 | 5.59 | +60 –24 −08 |
| [valence rating > SR rating] | |||
| central operculum L | 196 | 6.59 | −40 +02 +14 |
| postcentral G. L, supramarginal G. L, parietal operculum L | 1910 | 6.27 | −48 –26 +40 |
| inferior temporal G. L | 170 | 6.22 | −42 –52 −06 |
| SPL R | 165 | 5.86 | +24 –46 +74 |
| parietal operculum R | 55 | 5.49 | +48 –28 +26 |
| SPL L | 136 | 5.41 | −26 –64 +32 |
| central operculum R | 101 | 5.33 | +42 +02 +14 |
| SPL R | 127 | 5.27 | +28 –62 +38 |
Abbreviations: ACC, anterior cingulate cortex; MCG, middle cingulate gyrus; MFG, middle frontal gyrus; MPFC, medial prefrontal cortex; MTG, middle temporal gyrus; OFC, orbitofrontal Cortex; PCC, posterior cingulate cortex; SMA, supplementary motor area; SPL, superior parietal lobule. Voxel-level pFWE < .05.
3.3. ROI analysis
Based on our hypothesis, a small volume correction for three a priori ROIs (the ACC, the PCC and the MPFC) was applied on whole-brain results. The positive effect of SR task [SR rating > valence rating] reported in whole-brain results was also found in the ROIs (Table 3). The contrast [valence rating task > SR rating task] did not show significant clusters of activation. The ROI analysis did not reveal any effect of group or valence nor any interaction between group and task or valence within the predefined ROIs.
Table 3.
Increased brain activities after small volume correction analysis in self-relevance rating task as compared to affective valence rating.
| Brain region | Size (kE) | T value | MNI coordinates (x, y, z) |
|---|---|---|---|
| [SR rating > valence rating] | |||
| ACC | 2214 | 10.26 | −02 +40 +06 |
| MPFC | 3190 | 9.73 | +00 +42 +22 |
| PCC | 413 | 12.70 | −08 –50 +32 |
Abbreviations: ACC, anterior cingulate cortex; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; Voxel-level pFWE < .05.
3.4. Connectivity analysis
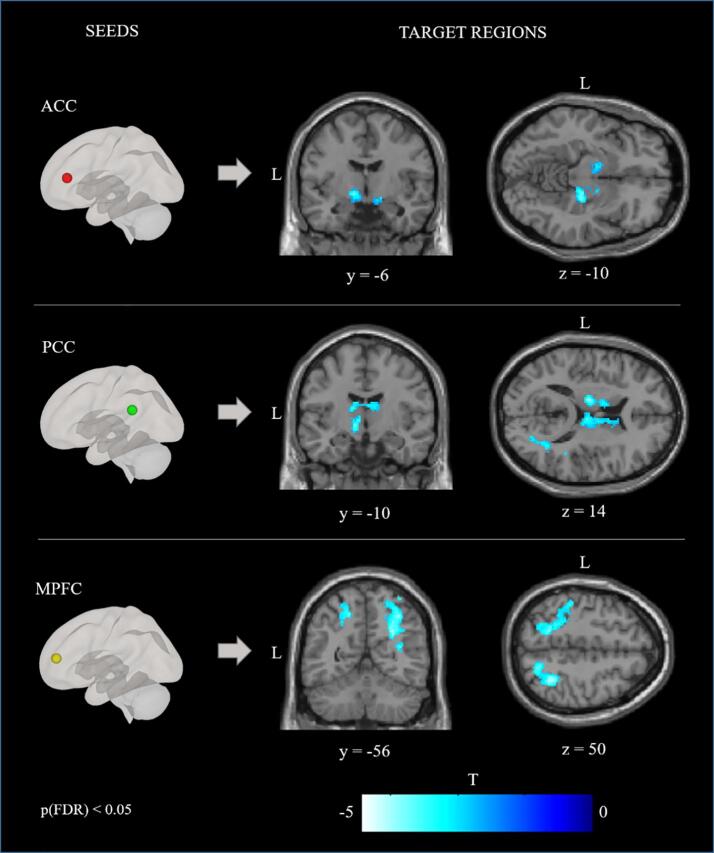
The functional coupling of the ACC, the PCC and the MPFC was analyzed by means of a seed-based connectivity analysis. The analysis revealed patterns of significantly reduced and elevated connectivity across conditions. An increased connectivity is represented by higher correlations between the time series of the seed region and the time series of other voxels in the brain. A decreased connectivity means lower correlations between the time series of the seed region and the time series of other voxels in the brain. Compared to the HC, the patients with BPD demonstrated reduced functional connectivity between the ACC seed and the bilateral basal ganglia, the right amygdala, the right hippocampus, and the left SPL. For the same contrast, the BPD group, relative to the HC, also exhibited reduced functional connectivity between the PCC seed and the bilateral basal ganglia, the bilateral thalamus, and the right lateral occipital gyrus (LOG). The seed-based connectivity analysis for the MPFC seed revealed reduced functional connectivity, for patients with BPD relative to HC, with the bilateral LOG, the bilateral SPL, the right thalamus, the right angular gyrus and the left postcentral gyrus, and enhanced functional connectivity with the left MFG, the superior frontal gyrus (SFG) and the frontal pole. Fig. 3 shows patterns of reduced connectivity for the contrast [BPD > HC; SR rating > valence rating]. The comparison of SR rating of negative and positive sentences in the patient group [BPD; SR rating of negative sentences > SR rating of positive sentences] revealed decreased functional connectivity between the PCC and the bilateral LOG in SR ratings of negative content. There were no significant differences in SR-related connectivity of the ACC and the MPFC for negative vs. positive sentences in the patient group. Table 4 shows the coordinates of cluster regions demonstrating reduced and enhanced functional connectivity for the ACC, the PCC and the MPFC seeds for the contrasts [BPD > HC; SR rating > valence rating] and [BPD; SR rating of negative sentences > SR rating of positive sentences]. The correlation analysis of connectivity estimates with symptom severity (BSL-95) did not reveal a relationship between symptom severity and the functional connectivity strength of any of the brain regions in which between-group differences were found in the patient group.
Fig. 3.
Results of the seed-based connectivity analysis. Images show functional connectivity for the seeds ACC, PCC and MPFC for the contrast [BPD > HC; SR rating > valence rating]. Blue-white color indicates clusters demonstrating reduced connectivity. Cluster‐level pFDR < .05; voxel‐level p < .01. Seeds: ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex. BPD, borderline personality disorder, HC, healthy controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Seed-based connectivity analysis. Brain regions showing significant changes in functional connectivity with ROI seeds for the contrasts [BPD > HC; SR rating > valence rating] and [BPD; SR rating of negative sentences > SR rating of positive sentences].
| Seed | Connectivity | Cluster Regions | Size (kE) | T value | MNI coordinates (x, y, z) |
|---|---|---|---|---|---|
| [BPD > HC; SR rating > valence rating] | |||||
| ACC | lower | Basal ganglia L | 447 | −5.53 | −12 –02 −04 |
| Hippocampus R, amygdala R, basal ganglia R | 305 | −4.67 | +24 –22 −10 | ||
| SPL L | 301 | −3.63 | −18 –54 +50 | ||
| PCC | lower | LOG R | 594 | −4.95 | +28 –56 +28 |
| Thalamus L, R, basal ganglia L, R | 963 | −4.62 | −14 –14 +14 | ||
| MPFC | higher | MFG L, SFG L, frontal pole L | 665 | 4.43 | −36 +26 +46 |
| lower | LOG R, SPL R, angular G. R | 1205 | −4.53 | +26 –54 +30 | |
| Thalamus R | 296 | −4.30 | +06 –14 +20 | ||
| SPL L, postcentral G. L, LOG L | 792 | −3.73 | −16 –56 +50 | ||
| [BPD; SR rating of negative sentences > SR rating of positive sentences] | |||||
| ACC | lower | LOG L | 530 | −4.92 | –22 –86 +04 |
| LOG R | 726 | −3.76 | +34 –86 +00 | ||
Abbreviations: Seeds: ACC, anterior cingulate cortex; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex. Correlated regions: LOG, lateral occipital gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; SPL, superior parietal lobule. BPD, borderline personality disorder; HC, healthy controls. Cluster‐level pFDR < .05; voxel‐level p < .01.
4. Discussion
We investigated the fMRI activation in our subjects in search of the neural response that might underlie the altered sense of SR in BPD. To that end, we implemented an experimental paradigm, relying on the judgements of SR as opposed to emotional valence elicited by a set of statements. In line with our hypothesis, the regions belonging to the CMS were found to be particularly involved in the SR judgement task, confirming the applicability of our paradigm. Between-group comparisons of the ROI-focused data revealed an altered pattern of connectivity between the CMS regions and the basal ganglia-thalamus complex. In the patient group, the connectivity between the PCC and the bilateral LOG was reduced during the SR evaluations of negative sentences as compared to positive sentences.
The results of the task-based whole-brain fMRI analyses concerning SR indicated the involvement of the midline structures of the DMN (ACC, PCC and MPFC), known as the CMS (Northoff and Bermpohl, 2004, Qin et al., 2013), proving the involvement of an extended cortical network (the OFC, the middle temporal gyrus, the angular gyrus and the precuneus) associated with the MNS. The overlap of the midline regions with the DMN (Gusnard et al., 2001) is thought to reflect a link between self-related processing and intrinsic brain activity (Qin et al., 2013, Qin and Northoff, 2011). The role of the CMS in the evaluation of SR (Araujo et al., 2013, Northoff et al., 2006, Philippi et al., 2012, Schmitz and Johnson, 2007, van der Meer et al., 2010, Wagner et al., 2012) and its interaction with the MNS have been outlined in previous investigations (Molnar-Szakacs and Uddin, 2013, Qin and Northoff, 2011), as has been the involvement of MNS in self-other differentiations (Rizzolatti and Craighero, 2004). Our data, which are based on the evaluation of short sentences, contribute to the body of research with respect to the supramodal representation of SR in the brain (Schmitz and Johnson, 2007).
Remarkably, our findings indicate the involvement of the SMA in SR processing. Previous studies showing SMA activation during self-initiated movements (Jenkins et al., 2000) and self-agency (Seghezzi et al., 2019) suggest a functional role of this region in self-related processing. Our data corroborate those reports regarding SMA involvement in self-referential processing without overt motor activity (Northoff et al., 2006). The SMA activation in association with SR is compatible with the widely acknowledged cognitive role of the SMA in the planning and control of voluntary actions as a part of the motor system (Svoboda and Li, 2018).
The ability to properly judge the SR of the stimuli underlies rapid and vigilant reactions and is essential for adaptive behavior (Sui and Humphreys, 2015). Delineating the neural correlates of SR evaluation in the brain can provide key insights into the physiological basis of behavior in general and the SR-related pathologies in particular. The clinical reports about aberrant judgements of SR in BPD (Bender and Skodol, 2007, Jørgensen, 2006, Sarkheil et al., 2019) and the importance of SR biases in treatment strategies (Lin et al., 2018) serve as a motivation for further explorations of disorder-specific neural alterations. To this end, we adopted an ROI approach as the next exploratory step. The pre-defined ROIs corresponding to the ACC, the PCC and the MPFC as key regions of the CMS showed higher activation during the SR task. That the CMS contain a representation of SR was anticipated and confirmed by the independent whole-brain results. No significant between-group differences were found in the activation levels of these regions. Our results suggest that the activation of a network of regions concerning SR task, which is confirmed by literature, might be comparable between patients with BPD and HC. However, including a larger sample of patients and choosing sentences more individually adopted might reveal more information about the deviant activation patterns. Furthermore, the CMS regions are components of large-scale neural systems underlying self-related processing, which may not be delineated by the simple activation maps. Thus, functional connectivity analyses can potentially reveal additional information about how the components of these neural systems differ in their functional coupling during SR judgements.
Indeed, seed-based functional connectivity analysis revealed differences in functional coupling during SR judgements between the BPD and control groups. The connectivity of the CMS regions with the basal ganglia and the thalamus was found to be consistently reduced in the BPD group. Thus, the results of our task-based connectivity analysis highlight the association of the basal ganglia-thalamus complex with the self-system. Altered functioning of the basal ganglia in salience detection (Peters et al., 2016), reward evaluation (Haber, 2011), forecasting the outcome of actions (Seger, 2008) and action selection (Friend and Kravitz, 2014) may affect the evaluation of SR. Disturbed thalamus functioning may result in abnormal sensory gating and misinterpretation of the sensory inputs contributing to alterations of cognitive processing (McCormick and Bal, 1994). Based on our results the connectivity between the MPFC and the PCC of the DMN and the thalamus (salience network) was disturbed, which may reflect a dysregulation within the cortico-thalamic loops. Our findings on reduced interaction between the core regions of the default-mode and the salience networks could indicate limited flexibility in switching between networks. Disturbed inter-network connectivity between the default-mode and the salience network was also reported in a resting-state fMRI study in patients with BPD (Doll et al., 2013). Reduced functional connectivity between the DMN and occipital areas may be associated with an inflexible integration of sensory stimuli into self-referential processing in patients with BPD (Wolf et al., 2011). A disturbed SR for negative statements in BPD has been indicated in previous literature (Sarkheil et al., 2019). Our finding about reduced functional connectivity between the PCC and the occipital cortex for SR ratings of negative sentences provide additional evidence about SR is affected by negative contents in patients with BPD. Linehan’s model for BPD (Lieb et al., 2004) postulates that a vulnerability to negative affects underlies evolution of BPD symptoms like a disturbed self-image. The widespread connections of the basal ganglia and the thalamus within the cortico-basal ganglia-thalamo-cortical loop (Parent and Hazrati, 1995) and its role in psychiatric diseases (Neuner et al., 2014, Peters et al., 2016, Zhu et al., 2018) are widely acknowledged. Our findings support the notion that the appraisal of SR is not purely ‘top-down’ (Northoff et al., 2006), but also integrates bottom-up processes. The cortico-basal ganglia-thalamo-cortical loops may represent the integration of sensory input toward shaping SR as well as mediating the effect of SR on perception, decision making and action planning. At the same time, an increased connectivity between the MPFC and the executive control regions in the dorsal PFC likely indicates a shift in resource allocation from brain networks that support salience and reward to the executive control networks.
Choosing appropriate control tasks is of critical importance in task-based fMRI protocols. In the current task-based fMRI paradigm, we used valence ratings as a control task for SR ratings. This design enables a highly sensitive isolation of SR-related activity by controlling for possible confounding effects on the processing of the sentences. In light of previous findings regarding the role of PCC activation in the detection of personal significance (Maddock et al., 2001) and its link to the processing of emotional stimuli (Maddock et al., 2003), the decreased PCC connectivity we observed may reflect a deviant neural response during SR or valence rating, or a mixture of the two in BPD. Further investigations are needed to disentangle these effects.
This study has some limitations within which our findings need to be interpreted carefully. A possible confounding factor might be the medication of the patients. It cannot be excluded that psychiatric medications (present in 16 out of 23 patients) might have their effects through network reorganization in the brain (Flanagan et al., 2019). Second, the presence of comorbid disorders limits the specificity of our results, although most patients with BPD have additional conditions of Axis I disorders (Leichsenring et al., 2011). Third, although analyses of seed-based connectivity are appropriate to address hypothesis-driven questions, the results are mainly limited to the seeds selected a priori. Thus, differences between patients with BPD and HC in neural circuits not associated with any of our seeds may have gone unobserved in the current study.
5. Conclusion
Our study has identified a number of regions that appear to be involved in the evaluation of SR, adding to the existing evidence of a central brain system being attuned to decisions with respect to SR. The connectivity of these cortical brain areas with the subcortical reward-, salience- and emotion-related (e.g., the basal ganglia, the hippocampus and the amygdala) structures and the thalamus is compromised in patients with BPD. The altered functional connectivity likely indicates a neural correlate for the disturbed sense of self, which is marked by an unstable sense of self-esteem, culminating in self-destructive behavior and self-harm. The characterization of neural deviations related to SR processing may help identify the neural endophenotypes for self-related symptoms in BPD, facilitating the development of relevant diagnostic and treatment strategies.
CRediT authorship contribution statement
Linda Orth: Data curation, Formal analysis, Data interpretation, Visualization, Writing - Original Draft. Jana Zweerings: Formal analysis, Writing - review & editing. Camellia N. Ibrahim: Investigation, Writing - review & editing. Irene Neuner: Data interpretation, Writing - review & editing. Pegah Sarkheil: Funding acquisition, Conceptualization, Supervision, Data interpretation, Writing - review & editing.
Acknowledgements
This research project is supported by the START-Program of the Medical Faculty, RWTH Aachen University and the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF; Project No. APIC 01EE1405A-C). The authors would like to thank Niko Goik for his help in producing the stimuli for this study and the Brain Imaging Facility of the Interdisciplinary Center for Clinical Research at the RWTH Aachen University for technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102324.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub.
- Araujo H.F., Kaplan J., Damasio A. Cortical midline structures and autobiographical-self processes: an activation-likelihood estimation meta-analysis. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M. Bayer K. Ruthmann A. Schacht The impact of personal relevance on emotion processing: Evidence from event-related potentials and pupillary responses 2017 Cogn. Affect. Neurosci Soc 10.1093/scan/nsx075. [DOI] [PMC free article] [PubMed]
- Beck, A.T., Steer, R.A., Brown, G.K., 1996. Beck Depression Inventory, 2nd edn. M. ed. The Psychological Corporation, San Antonio.
- Beeney J.E., Hallquist M.N., Ellison W.D., Levy K.N. Self other disturbance in borderline personality disorder: Neural, self-report, and performance-based evidence. Personal. Disord. Theory, Res. Treat. 2016;7:28. doi: 10.1037/per0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender D.S., Skodol A.E. Borderline Personality as a Self-Other Representational Disturbance. J. Pers. Disord. 2007;21:500–517. doi: 10.1521/pedi.2007.21.5.500. [DOI] [PubMed] [Google Scholar]
- Bohus M., Limberger M.F., Frank U., Sender I., Gratwohl T., Stieglitz R.D. Entwicklung der Borderline-Symptom-Liste. PPmP Psychother. Psychosom. Medizinische Psychol. 2001;51:201–211. doi: 10.1055/s-2001-13281. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. University of Florida; Gainesville, FL: 1999. Affective Norms for English Words (ANEW): Instruction Manual and Affective Ratings. [Google Scholar]
- Bungert M., Liebke L., Thome J., Haeussler K., Bohus M., Lis S. Rejection sensitivity and symptom severity in patients with borderline personality disorder: effects of childhood maltreatment and self-esteem. Borderline Personal. Disord. Emot. Dysregulation. 2015;2:4. doi: 10.1186/s40479-015-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A. Doll C. Sorg A. Manoliu A. Wöller C. Meng H. Förstl C. Zimmer A.M. Wohlschläger V. Riedl Shifted intrinsic connectivity of central executive and salience network in borderline personality disorder 2013 Hum. Neurosci Front 10.3389/fnhum.2013.00727. [DOI] [PMC free article] [PubMed]
- Ertac S. Does self-relevance affect information processing? Experimental evidence on the response to performance and non-performance feedback. J. Econ. Behav. Organ. 2011;80:532–545. doi: 10.1016/j.jebo.2011.05.012. [DOI] [Google Scholar]
- First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. American Psychiatric Association Publishing Arlington, VA; 2016. Structured Clinical Interview for DSM-5 Personality Disorders: SCID-5-PD. [Google Scholar]
- First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. American Psychiatric Association Publishing Arlington; VA: 2016. Structured Clinical Interview for DSM-5 Disorders-Clinician Version SCID-5-CV. [Google Scholar]
- Flanagan R., Lacasa L., Towlson E.K., Lee S.H., Porter M.A. Effect of antipsychotics on community structure in functional brain networks. J. Complex Networks. 2019 doi: 10.1093/comnet/cnz013. [DOI] [Google Scholar]
- Friend D.M., Kravitz A.V. Working together: basal ganglia pathways in action selection. Trends Neurosci. 2014;37:301–303. doi: 10.1016/j.tins.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenier K.D., Kernis M.H., McNamara C.W., Waschull S.B., Berry A.J., Herlocker C.E., Abend T.A. Individual Differences in Reactivity to Daily Events: Examining the Roles of Stability and Level of Self-Esteem. J. Pers. 1999;67:187–208. doi: 10.1111/1467-6494.00052. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haber S.N. Sensation and Reward; 2011. Neuroanatomy of Reward: A View from the Ventral Striatum, Neurobiology of. [PubMed] [Google Scholar]
- Hochschild Tolpin L., Cimbolic Gunthert K., Cohen L.H., O’Neill S.C. Borderline Personality Features and Instability of Daily Negative Affect and Self-Esteem. J. Pers. 2004;72:111–138. doi: 10.1111/j.0022-3506.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Curr. Opin. Neurobiol. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Jenkins I.H., Jahanshahi M., Jueptner M., Passingham R.E., Brooks D.J. Self-initiated versus externally triggered movements: II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Jørgensen C.R. Disturbed Sense of Identity in Borderline Personality Disorder. J. Pers. Disord. 2006;20:618–644. doi: 10.1521/pedi.2006.20.6.618. [DOI] [PubMed] [Google Scholar]
- Kedia G., Mussweiler T., Adam R., Ischebeck A., Ihssen N., Linden D.E.J. So pretty! The neural correlates of self-other vs familiar-other attractiveness comparisons. Soc. Neurosci. 2019;14:41–52. doi: 10.1080/17470919.2017.1397544. [DOI] [PubMed] [Google Scholar]
- Kopala-Sibley D.C., Zuroff D.C., Russell J.J., Moskowitz D.S., Paris J. Understanding heterogeneity in borderline personality disorder: Differences in affective reactivity explained by the traits of dependency and self-criticism. J. Abnorm. Psychol. 2012;121:680–691. doi: 10.1037/a0028513. [DOI] [PubMed] [Google Scholar]
- Kühner C., Bürger C., Keller F., Hautzinger M. Reliabilität und Validität des revidierten Beck-Depressionsinventars (BDI-II) Nervenarzt. 2007;78:651–656. doi: 10.1007/s00115-006-2098-7. [DOI] [PubMed] [Google Scholar]
- Leichsenring F., Leibing E., Kruse J., New A.S., Leweke F. Borderline personality disorder. Lancet. 2011;377:74–84. doi: 10.1016/S0140-6736(10)61422-5. [DOI] [PubMed] [Google Scholar]
- Lenzenweger M.F., Lane M.C., Loranger A.W., Kessler R.C., Lenzenweger M.F., Lane M.C., Loranger A.W., Kessler R.C. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62:553–564. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K., Zanarini M.C., Schmahl C., Linehan M.M., Bohus M. Borderline personality disorder. Lancet. 2004;364:453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- Lin Y., Callahan C.P., Moser J.S. A mind full of self: Self-referential processing as a mechanism underlying the therapeutic effects of mindfulness training on internalizing disorders. Neurosci. Biobehav. Rev. 2018;92:172–186. doi: 10.1016/j.neubiorev.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Wheelwright S.J., Sadek S.A., Suckling J., Baron-Cohen S. Shared Neural Circuits for Mentalizing about the Self and Others. J. Cogn. Neurosci. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Lynum L.I., Wilberg T., Karterud S. Self-esteem in patients with borderline and avoidant personality disorders. Scand. J. Psychol. 2008;49:469–477. doi: 10.1111/j.1467-9450.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/S0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCormick D.A., Bal T. Sensory gating mechanisms of the thalamus. Curr. Opin. Neurobiol. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I., Uddin L.Q. Self-Processing and the Default Mode Network: Interactions with the Mirror Neuron System. Front. Hum. Neurosci. 2013;7:571. doi: 10.3389/fnhum.2013.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S., Schilling L., Wingenfeld K., Köther U., Wittekind C., Terfehr K., Spitzer C. Psychotic-like cognitive biases in borderline personality disorder. J. Behav. Ther. Exp. Psychiatry. 2011;42:349–354. doi: 10.1016/J.JBTEP.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Murray R.J., Debbané M., Fox P.T., Bzdok D., Eickhoff S.B. Functional connectivity mapping of regions associated with self- and other-processing. Hum. Brain Mapp. 2015;36:1304–1324. doi: 10.1002/hbm.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neacsiu A.D., Herr N.R., Fang C.M., Rodriguez M.A., Rosenthal M.Z. Identity Disturbance and Problems With Emotion Regulation Are Related Constructs Across Diagnoses. J. Clin. Psychol. 2015;71:346–361. doi: 10.1002/jclp.22141. [DOI] [PubMed] [Google Scholar]
- Neuner I., Werner C.J., Arrubla J., Stöcker T., Ehlen C., Wegener H.P., Schneider F., Shah N.J. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front. Hum. Neurosci. 2014;8:362. doi: 10.3389/fnhum.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Bermpohl F. Cortical midline structures and the self. Trends Cogn. Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Parent A., Hazrati L.-N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-C. [DOI] [PubMed] [Google Scholar]
- Peters S.K., Dunlop K., Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016;10:104. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi C.L., Duff M.C., Denburg N.L., Tranel D., Rudrauf D. Medial PFC Damage Abolishes the Self-reference Effect. J. Cogn. Neurosci. 2012;24:475–481. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Duncan N., Northoff G. Why and How is the Self-Related to the Brain Midline Regions? Front. Hum. Neurosci. 2013;7:909. doi: 10.3389/fnhum.2013.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rogers T.B., Kuiper N.A., Kirker W.S. Self-reference and the encoding of personal information. J. Pers. Soc. Psychol. 1977;35:677–688. doi: 10.1037/0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rüsch N., Lieb K., Göttler I., Hermann C., Schramm E., Richter H., Jacob G.A., Corrigan P.W., Bohus M. Shame and Implicit Self-Concept in Women With Borderline Personality Disorder. Am. J. Psychiatry. 2007;164:500–508. doi: 10.1176/ajp.2007.164.3.500. [DOI] [PubMed] [Google Scholar]
- Sansone R.A., Sansone L.A. Gender patterns in borderline personality disorder. Innov. Clin. Neurosci. 2011;8:16–20. [PMC free article] [PubMed] [Google Scholar]
- Sarkheil P., Goik N., Ibrahim C.N., Schneider F. Effect of negative valence on assessment of self-relevance in female patients with borderline personality disorder. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer C., Arens E.A., Stopsack M., Spitzer C., Barnow S. Emotional hyper-reactivity in borderline personality disorder is related to trauma and interpersonal themes. Psychiatry Res. 2014;220:468–476. doi: 10.1016/j.psychres.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Schmidtke D.S., Schröder T., Jacobs A.M., Conrad M. ANGST: Affective norms for German sentiment terms, derived from the affective norms for English words. Behav. Res. Methods. 2014;46:1108–1118. doi: 10.3758/s13428-013-0426-y. [DOI] [PubMed] [Google Scholar]
- Schmitz T.W., Johnson S.C. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci. Biobehav. Rev. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger C.A. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neurosci. Biobehav. Rev. 2008;32:265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzi S., Giannini G., Zapparoli L. Neurofunctional correlates of body-ownership and sense of agency: A meta-analytical account of self-consciousness. Cortex. 2019;121:169–178. doi: 10.1016/j.cortex.2019.08.018. [DOI] [PubMed] [Google Scholar]
- Soeteman D.I., Verheul R., Busschbach J.J.V. The Burden of Disease in Personality Disorders: Diagnosis-Specific Quality of Life. J. Pers. Disord. 2008;22:259–268. doi: 10.1521/pedi.2008.22.3.259. [DOI] [PubMed] [Google Scholar]
- Sui J., Humphreys G.W. The Integrative Self: How Self-Reference Integrates Perception and Memory. Trends Cogn. Sci. 2015;19:719–728. doi: 10.1016/j.tics.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Li N. Neural mechanisms of movement planning: motor cortex and beyond. Curr. Opin. Neurobiol. 2018;49:33–41. doi: 10.1016/j.conb.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Torgersen S., Kringlen E., Cramer V. The Prevalence of Personality Disorders in a Community Sample. Arch. Gen. Psychiatry. 2001;58:590. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Iacoboni M., Lange C., Keenan J.P. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn. Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Vater A., Schröder-Abé M., Weißgerber S., Roepke S., Schütz A. Self-concept structure and borderline personality disorder: Evidence for negative compartmentalization. J. Behav. Ther. Exp. Psychiatry. 2015;46:50–58. doi: 10.1016/j.jbtep.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Wagner D.D., Haxby J.V., Heatherton T.F. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3:451–470. doi: 10.1002/wcs.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn : A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Winter D., Bohus M., Lis S. Understanding Negative Self-Evaluations in Borderline Personality Disorder—a Review of Self-Related Cognitions, Emotions, and Motives. Curr. Psychiatry Rep. 2017;19:17. doi: 10.1007/s11920-017-0771-0. [DOI] [PubMed] [Google Scholar]
- Winter D., Herbert C., Koplin K., Schmahl C., Bohus M., Lis S. Negative Evaluation Bias for Positive Self-Referential Information in Borderline Personality Disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D., Koplin K., Lis S. Can’t stand the look in the mirror? Self-awareness avoidance in borderline personality disorder. Borderline Personal. Disord. Emot. Dysregulation. 2015;2:13. doi: 10.1186/s40479-015-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R.C., Sambataro F., Vasic N., Schmid M., Thomann P.A., Bienentreu S.D., Wolf N.D. Aberrant connectivity of resting-state networks in borderline personality disorder. J. Psychiatry Neurosci. 2011 doi: 10.1503/jpn.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon L., Somerville L.H., Kim H. Development of MPFC function mediates shifts in self-protective behavior provoked by social feedback. Nat. Commun. 2018;9:3086. doi: 10.1038/s41467-018-05553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Luo L., Li Q., Kendrick K.M. What Can Psychiatric Disorders Tell Us about Neural Processing of the Self? Front. Hum. Neurosci. 2013;7:485. doi: 10.3389/fnhum.2013.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Jiang X., Ji W. The Mechanism of Cortico-Striato-Thalamo-Cortical Neurocircuitry in Response Inhibition and Emotional Responding in Attention Deficit Hyperactivity Disorder with Comorbid Disruptive Behavior Disorder. Neurosci. Bull. 2018;34:566–572. doi: 10.1007/s12264-018-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.