Abstract
Several studies demonstrated that protein from whey milk could be a new strategy to reduce energy intake and increase satiety. Sheep whey has high protein content, but it is also rich in lactose. The aim of this study was to screening different ultrafiltration membranes to separate protein and lactose from sheep whey in one step. Protein was recovered in the concentrate feed, and lactose passed through three membranes and was recovered in the permeate feed. Membranes with different chemical composition and molecular weight cut-offs were assayed, and the influence of operating pressure and lactose concentration feed in the permeate flux and lactose rejection coefficients were studied. Lactose separation was not affected by pressure in GR60PP or GR90PP, and 85% and 80%, respectively of the lactose was separated into permeate feed. When the feed concentration increased, lactose separation remained stable in all three membranes, being GR60PP the most efficient, as 90% of the disaccharides were separated. In all cases 100% of the protein was recovered. Finally, the Spiegler–Kedem–Katchalsky model perfectly fitted the results obtained about lactose rejection coefficients.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04350-4) contains supplementary material, which is available to authorized users.
Keywords: Sheep whey, Ultrafiltration membrane, Lactose separation, Protein, SKK model
Introduction
Obesity and overweight have reached alarming levels. It is therefore necessary to develop new strategies focused on obtaining healthy and functional foods that include new ingredients.
In terms of macronutrients, protein has been described as the most satiating (Bendtsen et al. 2013). Milk whey (MW) is known for its excellent functional and nutritional value (Ahmad et al. 2019), and as this protein source is a by-product of cheese manufacturing or casein production, its use as a functional food makes it even more interesting and profitable (Guimarães et al. 2019; Ghanimah 2019). Milk whey contains 70–80% lactose, 9% proteins and 8–20% minerals on a dry basis (Daufin and Aimar 1998). The proteinaceous fraction of milk whey affects satiety by reducing food intake (Froetschel et al. 2001; Pupovac and Anderson 2002). In addition, whey protein and its biopeptides and amino acids released through digestion have many biological functions, including antimicrobial activities and insulinotropic effects, while contributing to the prevention of cancer and cardiovascular diseases, among others (Jakubowicz and Froy 2013; Madureira et al. 2010). In recent years milk whey has been used in formulations that include biotechnological processes such as the production of ethanol in environmental friendly ways (Alves et al. 2019; Trindade et al. 2019; Fangmeier et al. 2019).
The concentration of whey protein depends on several factors such as animal species, breed, lactation period and animal feeding and/or other factors related with the cheese making processes (sweet or acid whey) (Balthazar et al. 2017; Renes et al. 2018). However, one of the most important limitations to the use of whey is the high lactose/protein ratio. Many methods have been used to concentrate WP in a selective manner, and, it is in this context that membrane technology could be considered useful (Ganju and Gogate 2017; Nazir et al. 2019).
Membrane separation constitutes an ecological and economical alternative compared with traditional methods of whey concentration, such as thermal evaporation in dairy processes (Soodam and Guinee 2018). Depending on the membrane cut-off, several processes of whey protein separation have been described, e,g. ultrafiltration, diafiltration and nanofiltration (Baldasso et al. 2011). In this work, ultrafiltration (UF) is since it is capable of separating, in a selective way, molecules with molecular weights from 1000 to 200,000 daltons (Mistry and Maubois 1993). Moreover, UF can provide whey proteins with different degrees of purity (Prazeres et al. 2012), based on the rejection of protein and permeation of lactose, minerals, water and other compounds with lower molar masses (Yadav et al. 2016).
In the case of sheep whey, little research has been carried out into membrane processes, compared with the vast volume of literature that exists on bovine whey processing. Ovine whey has a higher total nitrogen/dry matter ratio than bovine whey; indeed, its protein content is double that of bovine whey, making it an interesting by-product for the functional food industry (Assenat 1985).
Based on the above information, the main aim of this study was to screen ultrafiltration membranes and study the influence of conditions involved to obtain sheep whey protein (lactose-free) that will be serve as a protein-rich functional food able to enhance satiety, and a sugar-free product of interest for diabetic patients. The influence of different ultrafiltration membranes, GR60PP (polysulphone and 25 kDa), and GR80PP and GR90PP (polyethersulphone, 10 and 5 kDa, respectively) and different operational conditions (pressure and lactose concentration) on lactose and protein rejection coefficients was studied. The viability of using the Spiegler–Kedem–Kachalsky model to predict the lactose rejection coefficients in the different membranes was also studied.
Materials and methods
Whey
Three batches of sweet sheep whey samples were obtained from Palancares Alimentación S.L., a local cheese factory from Murcia (Spain). Immediately after reception, ovine whey was defatted by centrifuging at 3000×g (gravity) for 15 min (Eppendorf centrifuge 5804R) and stored at − 80 °C for further analysis. From a nutritional point of view, whey was composed mainly by lactose (44.1 ± 0.7 g L−1) followed by protein (1.8 ± 0.2 g L−1). Mineral content was 4.0 ± 0.01 g L−1 and the pH value of sheep whey was 5.96 ± 0.03.
Membranes
Three ultrafiltration membranes (GR60PP, GR80PP and GR90PP) were supplied by Alfa Laval and the specifications are shown in Supplementary Table 1.
Ultrafiltration equipment
The experimental ultrafiltration unit consisted of a feed tank (capacity of 0.8 L), a flat sheet membrane module (specific area 8.48 cm2), and a pump to drive the sheep whey solution through the membrane.
Experimental procedure
The experiment was carried out similar as described in Hidalgo et al. (2016). Water and sweet ovine whey were treated in the ultrafiltration test module. Rejection percentages and permeate fluxes were calculated as an average value of the last three measurements. All the experiments were run in duplicate, obtaining standard deviation values of about 2.8% for the whole set of data. The following experimental conditions were maintained unchanged throughout the experimental series: membrane area 8 × 10−3 m2, temperature 22 ± 0.5 °C and assay time 30 min.
Solutions of sweet ovine whey (protein concentrations ranging between 1.27 kg m−3 and 2.34 kg m−3) were treated in the test module at operating pressures varying from 4 to 10 bar for ultrafiltration; and varying the feed lactose concentration from 1.5 to 6.15%.
Membrane characterization
The water permeability coefficients (Aw) were obtained as an initial characterisation of the membranes. For this, distilled water was used as feed, and fluxes were measured using pressures ranging from 4 to 10 bar for the three ultrafiltration membranes tested.
The water flux (Jw) depends on the hydraulic pressure applied across the membrane, ΔP, according Eq. (1) (Bhattacharya and Ghosh 2004).
| 1 |
The water permeability coefficient was measured in the native membrane (prior to each experiment), as well as at the end of all assays for each condition and membrane tested.
Analytical methods/analysis
Lactose content was measured by high-performance liquid chromatography (HPLC) with refractive index detector (RID) (VWR-Hitachi Elite LaChrom® HPLC system, USA). Chromatographic separation was achieved with a CARBOSep CHO-682 column, with 7 µm particle size; 200 mm × 7.8 mm i.d. (Teknokroma, Barcelona, Spain) at 70 °C. The mobile phase was milliQ water with an isocratic elution with a flow-rate of 0.4 mL min−1. The volume of the sample (previously filtered through a 0.22 µm-pore nylon filter) injected was 20 µL in triplicate. Peaks were identified comparing rejection times with lactose standard. The peak area corresponding to lactose was used for the quantitative analysis. Calibration curves for lactose were prepared for 0.1, 0.5, 1, 1.5, 2.5, 5, 10% concentrations. Data analysis was performed using Agilent EZChrom Elite software.
Protein was measured using the colorimetric Coomasie (Bradford) protein assay kit (Pierce Biotechnology, USA) based on protein–dye binding using the microplate protocol (Bradford 1976). A standard curve was made for a range of 100–1500 µg mL−1. Absorbance was measured at 595 nm using a UV/Vis spectrophotometer FLUOstar Omega (BMG Labtech, Germany) and the software Fluostar to analyse data.
The ash content was determined using a muffle furnace and heating the samples at 526 °C for 24 h (Case 1985). After skimming the whey, the fat content was determined using the Gerber method (International Dairy Federation 1991). The pH was measured using a digital pH-meter Crison MicropH 2001 (Crison, Germany).
The analysis of data obtained was made using SPSSv.19 statistical program.
Application of the model
Membrane performance was evaluated using two criteria: membrane rejection coefficient, R (%), and permeate flux, Jp.
Lactose and protein rejection coefficients (R) reflect the membrane's ability to separate protein and lactose in the feed solution, and is given as a percentage:
| 2 |
where Cp and Cf are lactose and protein concentration in the permeate and feed streams, respectively (Chandrapala et al. 2016; Galanakis et al. 2014; Koros et al. 1996). The permeate flux was calculated by measuring the quantity of permeate collected over a certain time period and dividing it by the membrane area used for filtration. Since lactose passed through membrane and was recovered into permeate feed, the lactose separation was defined as:
| 3 |
Transport phenomena and the properties of solvent and solute are a problem in osmosis, nanofiltration and ultrafiltration, and several models have been proposed to quantify them (Wang et al. 2014). Pressure-driven membrane processes (osmosis, nanofiltration and ultrafiltration) can be described by irreversible thermodynamics. In general, the transport equation for the solutes pass through a membrane is defined by two phenomena: diffusion and convection.
Initially, the Spiegler–Kedem–Katchalsky model (SKK model) was developed to be applied in reverse osmosis. However, several authors have used it successfully in nanofiltration processes, and it has been suggested, but not tested, for ultrafiltration technology (Bhattacharya and Ghosh 2004).
Basically, the Spiegler–Kedem–Katchalsky model (Katchalsky and Kedem 1962; Spiegler and Kedem 1966) shows a relation between solvent flux (Jw) and solute flux (Js) through the membrane.
The observed rejections can be explained by SKK theory as follows:
| 4 |
where Robs is the observed rejection and F is a parameter that depends on the solvent flux, the rejection coefficient and solute permeability coefficient according to the equation:
| 5 |
The Spiegler–Kedem–Katchalsky model was applied and the values of the reflection coefficient (σ) and the solute permeability (Ps) coefficient were obtained by the regression analysis of real rejection and flux data (Hidalgo et al. 2016).
Results and discussion
Membrane characterization
Membrane characterizations were performed with distilled water. Figure 1 shows the water permeate fluxes for the three membrane assays used as functions of operating pressure. It can be observed that the increase of water flux was directly proportional to the pressure applied. The values of permeability coefficients depend on molecular weight cut off (MWCO) and chemical composition of the membranes.
Fig. 1.
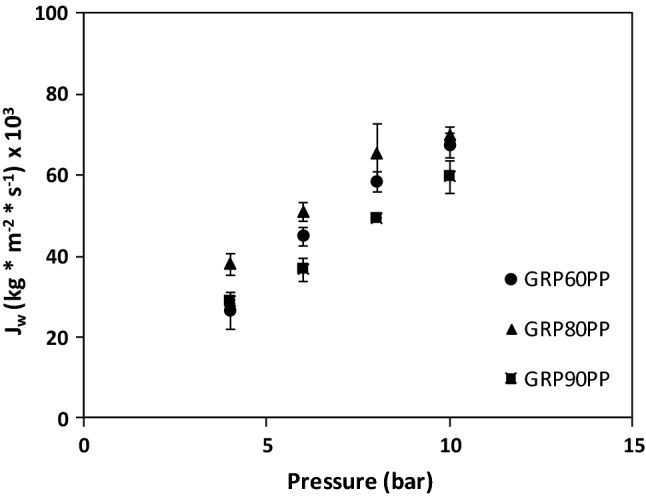
Water flux vs operating pressure. (●) GR60PP, (▲) GR80PP and (■) GR90PP
Table 1 shows the initial Awo coefficients for the three ultrafiltration membrane assays. The main differences in permeability coefficients were observed between the membranes with different MWCO, (GR90PP 5 kDa and GR80PP 10 kDa) and the same chemical composition. Moreover, differences in permeability coefficient of the three membranes according to chemical compositions: GR60PP (polysulphone) versus GR80PP and GR90PP (polyethersulphone) were also found.
Table 1.
Water permeability coefficients obtained at the beginning and end of experiments for the different membrane
| Membranes | |||
|---|---|---|---|
| GR60PP | GR80PP | GR90PP | |
| Awo (m s−1) (initial) | 6.69 × 10−8 | 5.38 × 10−8 | 5.06 × 10−8 |
| Awf (m s−1) (final) | 5.71 × 10−8 | 4.29 × 10−8 | 4.83 × 10−8 |
| fw (%) | 14.75 | 20.18 | 4.52 |
A comparison was made between the initial and final experiments, and a fouling parameter, fw, based on water permeability coefficients, was obtained as:
| 6 |
where subscript 0 is the initial value and subscript f is the final value.
The final permeability coefficients (Awf) decreased for the three ultrafiltration membranes, presumably due to fouling phenomena on the membrane surface (Macedo et al. 2011). The GR80PP membrane showed the highest fouling parameter, which could be due to interactions between membrane and whey solution, and concentration of polarization phenomena.
Influence of pressure in the ovine whey treatment and lactose separation
The effect of operating pressure on lactose separation in ovine whey was also performed for the three studied membranes; as it can be seen in Fig. 2a. As expected, a higher pressure induced a higher permeate flux, and the values obtained for GR60PP were recorded higher than for GR80PP and GR90PP. The two membranes made with polyethersulphone showed a similar trend between them. The higher permeate flux of the GR60PP membrane may be explained due to its higher molecular weight cut-off, and secondary to its chemical composition (GR60PP > GR80PP > GR90PP, 20 kDa > 10 kDa > 5 kDa, respectively).
Fig. 2.
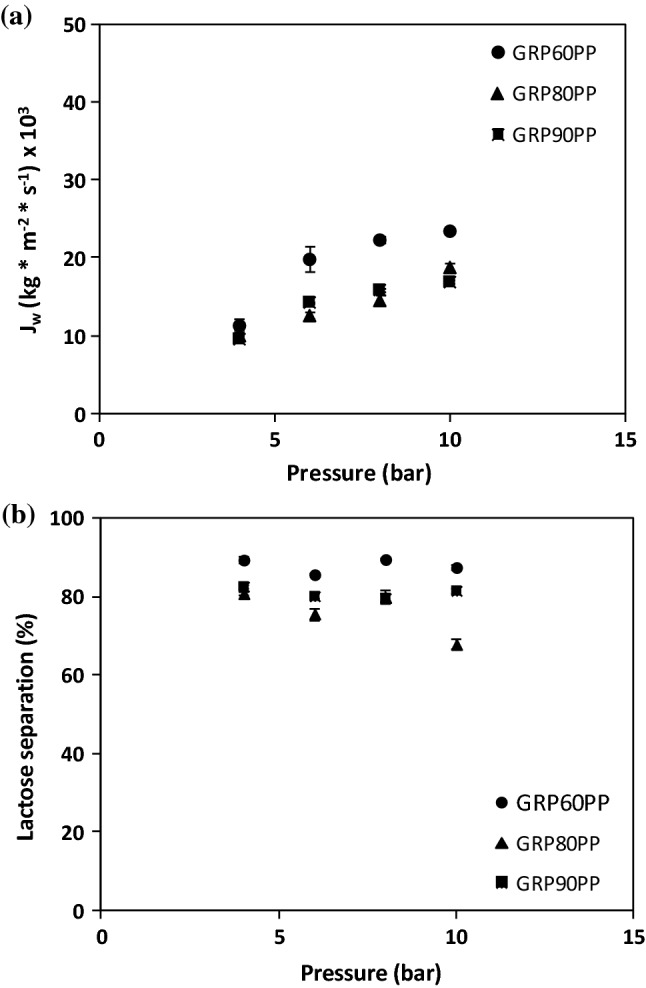
Variation in permeate flux (A) and lactose separation coefficient (B) with pressure for: Lactose concentration (3.5 ± 0.05 %) and pressure (4 to 10 bar). GR60PP membrane (●), GR80PP membrane (▲) and GR90PP membrane (■)
The permeate flux increased with pressure for all three membranes, which means that the fouling or polarization effects were less intense than what it might be expected (Galanakis et al. 2014; Van der Bruggen et al. 1999). Of note, is that the whey-permeate flux was smaller than the water flux, suggesting that the adsorptive fouling and pore blocking effect was very significant (especially in the case of GR80PP and GR90PP) for whey at the initial concentration and that this effect increased with operating pressure (Corbatón-Báguena et al. 2015; Brink and Romijn 1990).
Similar results were found in the literature, where the whey permeate flux was lower than the water flux at all pressures (Atra et al. 2005; Baldasso et al. 2011; Butylina et al. 2006; Galanakis et al. 2014; Macedo et al. 2011; Rektor and Vatai 2004). According to literature, when the solute molecules are smaller than or similar to the membrane pore size, these molecules can penetrate inside membrane pores, reducing their effective radius gradually (adsorptive fouling) or causing the entire pore to be completely blocked (pore blocking mechanism). Corbatón-Báguena et al. demonstrate that fouling is more severe when the difference between the membrane MWCO and the molecular weight of solute molecules is lower (Corbatón-Báguena et al. 2014; Qu et al. 2014).
When the effect of operating pressure on lactose separation was studied, no statistically significant differences were found in the case of GRP60PP and GRP90PP, which gave 85 and 80% disaccharide separation from the whole whey, respectively, at all the pressures tested. However, the lactose separation capacity of GRP80PP decreased when higher pressures were applied (Fig. 2b). It is important to mention that none of the membranes nor the different pressures tested affected protein separation, since this macronutrient is absent in any whey permeate, so the protein recovery was 100%. All these results were better than those described in the literature by Baldasso et al. (2011) and Slukova et al. (2016). Baldaso et al. that used a UF-6001 membrane made of polyethersulphone with a MWCO of 10 kDa, obtaining, with the best strategy used, a protein concentrate of around 70%. Meanwhile, Sluková et al. used a tubular ceramic ultrafiltration membrane of 50 kDa, obtaining a high recovery of protein (81%) and good reduction of lactose during the ultrafiltration step. The same authors obtained a 37% level of lactose remaining in the rejected fraction in a single-step, which was reduced to 14% by diafiltration. Similar results were obtained by Galanakis et al. (2014) when using the scenery combined process (20 kDa polysulphone, GR70PP, and 2 kDa polyethersulphone, GR95PP, membranes). Their recovery of proteins (87–90%) and the rejection of non-reducing sugars (39–32%) were quite lower than the obtained in present study. In our research, when the GR60PP membrane was used at a similar pressure, only 13% lactose remained in the protein after a single-step.
Influence of lactose concentration in the whey protein separation treatment
During ultrafiltration, the concentration of lactose and proteins increased, changing the environment of the liquid to be filtered. The effect of lactose concentration on the permeate flux for the different ultrafiltration membranes is shown in Fig. 3a. As it can be observed, the permeate flux decreased when lactose concentration increased, but the membranes showed significant variations on this phenomenon. The decrease of permeate flux when using GRP80PP was inversely proportional, while the permeate flux when using GRP90PP was stable and only decreased at higher concentrations of lactose. In the case of the polysulphone membrane, a different trend was observed. The lowest flux values were with the highest lactose concentration. Those variations could be explained by the sum of two effects: the increase in solute flux due to the increase in feed concentration, and the decrease in water flux as a consequence of the increased osmotic pressure.
Fig. 3.

Variation in permeate flux (A) and lactose separation coefficient (B) with lactose concentration for pressure = 6 ± 0.5 bar. Range of whey concentration between 1.5 to 6.15 % of lactose. GR60PP membrane (●), GR80PP membrane (▲) and GR90PP membrane (■)
Similar results have been obtained by researchers working with other types of membranes (Atra et al. 2005; Baldasso et al. 2011; Galanakis et al. 2014; Smit 2003). In addition, other authors concluded that the higher the protein concentration, the lower the permeate flux, mainly due to the higher osmotic pressure and the greater accumulation of solute molecules in the polarized layer, increasing its thickness and, consequently its resistance to permeation (Bacchin et al. 2006; Rektor and Vatai 2004).
Several authors have investigated the separation of whey components using ultrafiltration in association with discontinuous diafiltration to concentrate and to purify the whey proteins (Baldasso et al. 2011; Sluková et al. 2016). However, none of them took into account the effect of sugar concentration on the separation of proteins and sugars (mainly lactose). In this study, an increase in the lactose concentration in the feed liquid was noticed, so the effect of this increase on protein rejection was assayed. No statistically significant effect of the lactose concentration in the feed phase on rejection was observed, since the separation of lactose from the whole whey remained constant independently of the concentration in the feed liquid, with around an 80% of lactose being recovered (Fig. 3b). When the lactose feed concentration increased, the permeate concentration also increased in the case of GR60PP membrane, so that there was no significant change in lactose rejection coefficients. With the GR90PP membrane, no changes were found, while GR80PP showed a slightly decrease in permeate concentration. These results agreed with Galanakis et al. (2014) when using GR70PP (polysulphone) and GR95PP (polyethersulphone).
Fitting the Spiegler–Kedem–Katchalsky model
For each experiment using different experimental conditions and different compounds, the reflection coefficient (σ) and the solute permeability coefficient (Ps) were obtained. Average values were calculated as the mathematical mean using (SPSSv.19) statistical program. The average values of the reflection coefficient (σ) and the solute permeability coefficient (Ps) obtained for the different membranes are shown in Table 2. According to the bibliography, Cuartas-Uribe et al. (2010) applied the Kedem–Spiegler model to predict lactose rejection using a nanofiltration membrane (Desal 5 DL) and obtained a high reflection coefficient, and low solute permeability coefficient. The different results obtained are explained by the different chemical composition of membrane and MWCO.
Table 2.
Model constants for the three membranes studied
| Membranes | |||
|---|---|---|---|
| GR60PP | GR80PP | GR90PP | |
| σ | 0.271 ± 8.00 × 10−3 | 0.811 ± 10.0 × 10−3 | 0.762 ± 1.75 × 10−3 |
| Ps (m s−1) | 2.72 × 10−2 ± 8.03 × 10−4 | 2.98 × 10−2 ± 3.85 × 10−4 | 3.20 × 10−2 ± 7.37 × 10−5 |
| r2 | 0.9418 | 0.9743 | 0.9954 |
Figure 4 shows the theoretical and experimental rejection coefficients for the different membranes. As it can be observed, real and hypothetical values were quite similar and perfectly fit the diagonal.
Fig. 4.
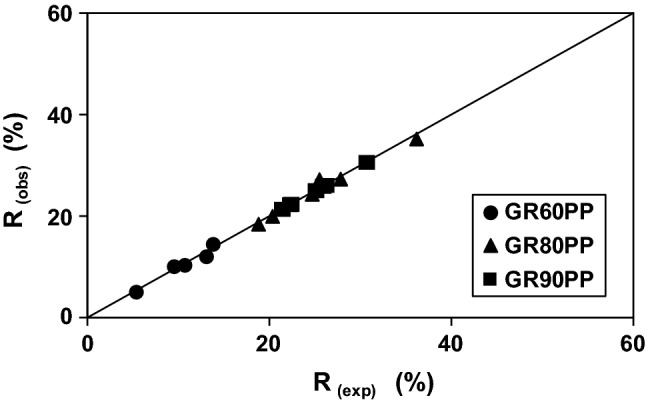
Experimental and model retention coefficient for GR60PP membrane (●), GR80PP membrane (▲) and GR90PP membrane (■)
Considering all the results for the different membranes tested, the final correlation coefficient between observed and predicted values (with SKK model) of r2 = 0.9892 was obtained. Although this model is usually used in nanofiltration, it also fitted perfectly in our study, and it can be considered a very interesting approximation for predicting different lactose rejection coefficients using ultrafiltration membranes, at least in the studied range of pressure and lactose feed concentrations.
Regarding the solute permeability coefficient (Ps), an inversely proportional relation was found with the molecular weight cut-off of the membrane tested—the higher the MWCO, the lower the Ps.
These findings, along with the predicted value of SKK model, could be a useful tool for cheese industry to obtain an optimum performance to improve whey utilization. Furthermore, these ultrafiltration membranes may help dairy industry to develop environmental friendly and economically profitable processes (Alves et al. 2019).
Conclusion
The results using three specific membranes (GRP60PP, GRP80PP and GRP90PP), different pressure conditions and concentrations of feed solution suggest that the ultrafiltration of sheep whey could be a suitable alternative for its problematic separation, contributing to the little knowledge that exists on the sheep whey separation. The GR60PP membrane showed the best lactose separation using a feed sheep whey solution with a lactose concentration ranging from 2 to 6%, and an operating pressure from 4 to 8 bar.
Taking the results into account, the main conclusions of this work were that the range of pressure used had no significant effect on lactose separation in the case of GRP60PP and GRP90PP. However, GRP80PP membrane showed a decreased permeation pattern when higher pressures were assessed. Furthermore, increasing the volume and concentration of the feed phase did not affect lactose permeation, which remained constant during the ultrafiltration process. The separation of protein in the concentrate feed was not affected by the type of membrane tested or range of operating pressures assessed, 100% recovery being obtained in all cases.
The ultrafiltration process itself led to a reduction of permeates flux when lactose concentration increased, as the GRP80PP membrane was the most affected in this aspect. Cleaning the membranes with water after the ultrafiltration assays restored initial conditions, and the same membrane could be used several times.
Finally, the Spiegler–Kedem–Katchalsky model (applied to different membranes) can be used to predict, accurately (r2 = 0.9892), the effect of different experimental conditions on the separation of lactose and protein from sheep whey using ultrafiltration membranes. This model represents a suitable tool for predicting the permeation pattern for a given membrane and sheep whey and illustrates the effect of different process conditions. In this way, the by-product can be optimized for use as a functional food.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank to Ministerio de Economía y Competitividad (Spain) through the Project AGL2016-78125-R. We also extend our gratitude to Ministerio de Educación, Cultura y Deporte (Spain), for the Ph.D. Grant to Teresa Sánchez-Moya (FPU 13/02380). We also wish to thank Palancares S.L., Murcia. Spain, for providing the milk whey samples used in the present study.
List of symbols
- Aw
Solvent permeability (s/m)
- Awo
Initial solvent permeability (s/m)
- Awf
Final solvent permeability (s/m)
- Cf
Lactose concentration in the feed stream (kg/m3)
- Cp
Lactose concentration in the permeate (kg/m3)
- fw
Fouling parameter (%)
- F
Parameter depending on the solvent flux, the rejection coefficient and solute permeability coefficient
- Jp
Permeate flux (kg/m2 s)
- Js
Solute flux (kg/m2 s)
- Jw
Solvent flux (kg/m2 s)
- Ps
Solute permeability (s/m)
- r2
Statistical correlation coefficient
- R
Membrane rejection (%)
- Rexp
Experimental lactose rejection (%)
- Robs
Observed rejection
- σ
Reflection coefficient (dimensionless)
- ΔP
Hydraulic pressure across the membrane (Nw/m2)
- ΔΠ
Osmotic pressure (Nw/m2
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Teresa Sánchez-Moya, Email: tsm09382@um.es.
Asunción M. Hidalgo, Email: ahidalgo@um.es
Gaspar Ros-Berruezo, Email: gros@um.es.
Rubén López-Nicolás, Email: rubenln@um.es.
References
- Ahmad T, Aadil RM, Ahmed H, Rahman U, Soares BCV, Souza SLQ, Pimentel TC, Scudino H, Guimarães JT, Esmerino EA, Freitas MQ, Almada RB, Vendramel SMR, Silva MC, Cruz AG. Treatment and utilization of dairy industrial waste: a review. Trends Food Sci Technol. 2019;88:361–372. [Google Scholar]
- Alves EDP, Morioka LRI, Suguimoto HH. Comparison of bioethanol and beta-galactosidase production by Kluyveromyces and Saccharomyces strains grown in cheese whey. Int J Dairy Technol. 2019;72:409–415. [Google Scholar]
- Assenat L. Le lait de brebis: composition et propriétés. In Technique et Documentation Lavoisier, Paris. In: Luquet FM, editor. Laits et Produits Laitiers: Vache. Paris: Brebis, Chèvre, Technique et Documentation Lavoisier; 1985. pp. 335–373. [Google Scholar]
- Atra R, Vatai G, Bekassy-Molnar E, Balint A. Investigation of ultra- and nanofiltration for utilization of whey protein and lactose. J Food Eng. 2005;67:325–332. [Google Scholar]
- Bacchin P, Aimar P, Field RW. Critical and sustainable fluxes: theory, experiments and applications. J Membr Sci. 2006;281:42–69. [Google Scholar]
- Baldasso C, Barros TC, Tessaro IC. Concentration and purification of whey proteins by ultrafiltration. Desalination. 2011;278:381–386. [Google Scholar]
- Balthazar CF, Pimentel TC, Ferrão LL, Almada CN, Santillo A, Albenzio M, Mollakhalili N, Mortazavian AM, Nascimento JS, Silva MC, Freitas MQ, Sant' Ana AS, Granato D, Cruz AG. Sheep milk: physicochemical characteristics and relevance for functional food development. Compr Rev Food Sci Food Saf. 2017;16:247–262. doi: 10.1111/1541-4337.12250. [DOI] [PubMed] [Google Scholar]
- Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr. 2013;4:418–438. doi: 10.3945/an.113.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Ghosh P. Nanofiltration and reverse osmosis membranes: theory and application in separation of electrolytes. Rev Chem Eng. 2004;20:111–173. [Google Scholar]
- Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brink LES, Romijn DJ. Reducing the protein fouling of polysulfone surfaces and polysulfone ultrafiltration membranes: optimization of the type of presorbed layer. Desalination. 1990;78:209–233. [Google Scholar]
- Butylina S, Luque S, Nystrom M. Fractionation of whey-derived peptides using a combination of ultrafiltration and nanofiltration. J Membr Sci. 2006;280:418–426. [Google Scholar]
- Case RA. Chemical and physical methods. In: Richardson GH, editor. Standard methods for the examination of dairy products. Washington: American Public Health Association; 1985. [Google Scholar]
- Chandrapala J, Duke MC, Gray SR, Weeks M, Palmer M, Vasiljevic T. Nanofiltration and nanodiafiltration of acid whey as a function of pH and temperature. Sep Purif Technol. 2016;160:18–27. [Google Scholar]
- Corbatón-Báguena MJ, Álvarez-Blanco S, Vicent-Vela MC. Cleaning of ultrafiltration membranes fouled with BSA by means of saline solutions. Sep Purif Technol. 2014;125:1–10. [Google Scholar]
- Corbatón-Báguena MJ, Álvarez-Blanco S, Vicent-Vela MC. Fouling mechanisms of ultrafiltration membranes fouled with whey model solutions. Desalination. 2015;360:87–96. [Google Scholar]
- Cuartas-Uribe B, Vincent-Vela MC, Alvarez-Blanco S, Alcaina-Miranda MI, Soriano-Costa E. Application of nanofiltration models for the prediction of lactose retention using three modes of operation. J Food Eng. 2010;99:373–376. [Google Scholar]
- Daufin GRF, Aimar P (1998) Les Separations par Membrane Dans les Procédés de Líndustrie Alimentaire. Collection Sciences et Techniques Agroalimentaires
- Fangmeier M, Kemerich GT, Machado BL, Maciel MJ, Souza CFV. Effects of cow, goat, and buffalo milk on the characteristics of cream cheese with whey. Food Sci Technol. 2019;39:122–128. [Google Scholar]
- Froetschel MA, Azain MJ, Edwards GL, Barb CR, Amos HE. Opioid and cholecystokinin antagonists alleviate gastric inhibition of food intake by premeal loads of casein in meal-fed rats. J Nutr. 2001;131:3270–3276. doi: 10.1093/jn/131.12.3270. [DOI] [PubMed] [Google Scholar]
- Galanakis CM, Chasiotis S, Botsaris G, Gekas V. Separation and recovery of proteins and sugars from Halloumi cheese whey. Food Res Int. 2014;65:477–483. [Google Scholar]
- Ganju S, Gogate PR. A review on approaches for efficient recovery of whey proteins from dairy industry effluents. J Food Eng. 2017;215:84–96. [Google Scholar]
- Ghanimah MA. Functional and technological aspects of whey powder and whey protein products. Int J Dairy Technol. 2019;71:454–459. [Google Scholar]
- Guimarães JT, Silva EK, Ranadheera CS, Moraes J, Raices RSL, Silva MC, Ferreira MS, Freitas MQ, Meireles MA, Cruz AG. Effect of high-intensity ultrasound on the nutritional profile and volatile compounds of a prebiotic soursop whey beverage. Ultrason Sonochem. 2019;55:157–164. doi: 10.1016/j.ultsonch.2019.02.025. [DOI] [PubMed] [Google Scholar]
- Hidalgo AM, Gomez M, Murcia MD, Gomez E, Leon G, Sanchez A. Removal of anilinic compounds using the NF-97 membrane: application of the solution–diffusion and SKK models. Sep Sci Technol. 2016;51:2429–2439. [Google Scholar]
- International Dairy Federation (1991) International provisional IDF standard “milk and milk products determination of fat content”. In: International dairy federation (ed) General guidance on the use of butyrometric methods. Brussels, Belgium
- Jakubowicz D, Froy O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J Nutr Biochem. 2013;24:1–5. doi: 10.1016/j.jnutbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Katchalsky A, Kedem O. Thermodynamics of flow processes in biological systems. Biophys J. 1962;2:53–78. doi: 10.1016/s0006-3495(62)86948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koros WJ, Ma YH, Shimidzu T. Terminology for membranes and membrane processes. Pure Appl Chem. 1996;68:1479–1489. [Google Scholar]
- Macedo A, Duarte E, Pinho M. The role of concentration polarization in ultrafiltration of ovine cheese whey. J Membr Sci. 2011;381:34–40. [Google Scholar]
- Madureira AR, Tavares T, Gomes AMP, Pintado ME, Malcata FX. Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci. 2010;93:437–455. doi: 10.3168/jds.2009-2566. [DOI] [PubMed] [Google Scholar]
- Mistry VV, Maubois JL. Application of membrane separation technology to cheese production. In: Fox PF, editor. Cheese: chemistry, physics ans microbiology. Boston: Springer; 1993. pp. 493–522. [Google Scholar]
- Nazir A, Khan K, Maan A, Zia R, Giorno L, Schroën K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci Technol. 2019;86:426–438. [Google Scholar]
- Prazeres AR, Carvalho F, Rivas J. Cheese whey management: a review. J Environ Manag. 2012;110:48–68. doi: 10.1016/j.jenvman.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Pupovac J, Anderson GH. Dietary peptides induce satiety via cholecystokinin-A and peripheral opioid receptors in rats. J Nutr. 2002;132:2775–2780. doi: 10.1093/jn/132.9.2775. [DOI] [PubMed] [Google Scholar]
- Qu F, Liang H, Zhou J, Nan J, Shao S, Zhang J, Li G. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Microcystis aeruginosa: effects of membrane pore size and surface hydrophobicity. J Membr Sci. 2014;449:58–66. [Google Scholar]
- Rektor A, Vatai G. Membrane filtration of Mozzarella whey. Desalination. 2004;162:279–286. [Google Scholar]
- Renes E, De la Fuente F, Fernández D, Tornadijo ME, Fresno JM. Effect of feeding regimen on the fatty acid profile of sheep bulk tank milk. Int J Dairy Technol. 2018;71:857–867. [Google Scholar]
- Slukova M, Hinkova A, Henke S, Smrz F, Lukacikova M, Pour V, Bubnik Z. Cheese whey treated by membrane separation as a valuable ingredient for barley sourdough preparation. J Food Eng. 2016;172:38–47. [Google Scholar]
- Smit G. Dairy processing: improvement quality. New York: CRC Press; 2003. [Google Scholar]
- Soodam K, Guinee TP. The case for milk protein standardisation using membrane filtration for improving cheese consistency and quality. Int J Dairy Technol. 2018;71:277–291. [Google Scholar]
- Spiegler KS, Kedem O. Thermodynamics of hyperfiltration (reverse-osmosis)—criteria for efficient membranes. Desalination. 1966;1:311–326. [Google Scholar]
- Trindade MB, Soares BCV, Scudino H, Guimarães JT, Esmerino EA, Freitas MQ, Pimentel TC, Silva MC, Souza SLQ, Almada RB, Cruz AG. Cheese whey exploitation in Brazil: a questionnaire survey. Food Sci Technol. 2019;39:788–791. [Google Scholar]
- Van der Bruggen B, Schaep J, Wilms D, Vandecasteele C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J Membr Sci. 1999;156:29–41. [Google Scholar]
- Wang J, Dlamini DS, Mishra AK, Pendergast MTM, Wong MCY, Mamba BB, Freger V, Verliefde ARD, Hoek EMV. A critical review of transport through osmotic membranes. J Membr Sci. 2014;454:516–537. [Google Scholar]
- Yadav JSS, Yan S, Ajila CM, Bezawada J, Tyagi RD, Surampalli RY. Food-grade single-cell protein production, characterization and ultrafiltration recovery of residual fermented whey proteins from whey. Food Bioprod Process. 2016;99:156–165. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


