Abstract
Tomato field wastes and industrial by-products represents a valuable source of compounds with nutraceutical potential, and therefore of raw material to obtain food ingredients and additives. The objective of this study was to obtain a flour from tomato industrial by-product and from tomato field waste, dried by a conventional method, that allows to remain important nutraceutical compounds, which in the future, can be used for biotechnological purposes. We found that the drying procedure that allowed to reach an adequate water activity (0.4–0.6) in a forced convection oven were: 55 °C during 120 min. Both, the by-product and the field waste are potential sources for the extraction of phenolic and carotenoid compounds, getting up 11.26 μg/mg dry extract of lycopene and 162.82 μg/mg dry extract of phenolic compounds, highlighting the flavonoids: naringenin, catechin, and rutin. On the other hand, antioxidant analysis showed that oven dried by-product exhibits an inhibition around 80% against hydroxyl and peroxyl radicals, and a positive correlation of both lycopene and β-carotene with myoglobin protection ratio against these radicals. We concluded that the flour from tomato industrial by-products and field waste have nutraceutical properties attractive to the food industry.
Keywords: Industrial by-products, Field waste, Water activity, Nutraceutical compounds, Antioxidant capacity
Introduction
Nowadays, there is a growing demand for healthy ingredients and additives for food industry. The search for alternative sources of natural additives or food ingredients is necessary; as well use of ecological and low-cost process to obtain them (Poore and Nemecek 2018). Among these alternative sources, agri-food by-products or wastes are being explored strongly, some of them are tomato industrial by-products.
In 2018, the worldwide production of tomato was around 182,256,458 t, and Mexico contribute with around 2.5% (FAO-STAT 2019); Sinaloa state is the main producer, in there, where more than 10,000 ha of tomato are grown; and high amounts of tomato field waste and industrial by-products are generated, reaching up to 30 t/ha and around of 3750 t/year, respectively (SIAP, 2019; Personal communication). Although previous works indicate that as well as whole tomato fruit, the industrial by-products are an attractive source of nutraceuticals; highlighting carotenoids, especially lycopene and β-carotene, and a great variety of phenolic compounds, these are not being exploited biotechnologically (Valdez-Morales et al. 2014; Zhao et al. 2017; Perea-Domínguez et al. 2018; Vela-Hinojosa et al. 2018).
Drying is a practical process to preserve material extending their shelf life based on an adequate water activity (aw). Different reports indicate that using the appropriate drying conditions results in preservation of the content of nutraceutical compounds significantly (Kong et al. 2010; M’hiri et al. 2018).
The objective of this work was to obtain a flour from tomato industrial by-product or tomato field waste, dried by a conventional method, that allows to reach a aw that remains important nutraceutical compounds. Subsequently we used eco-friendly and food-grade solvents to obtain polar (rich in phenolic compounds) and no polar (rich in carotenoids) extracts, then HPLC quantifications and antioxidant capacity by myoglobin protection ratio method analysis of both fractions, were performed. We hypothesize that flour from tomato industrial by-products or tomato field waste, dried in forced convection oven, has similar content of some nutraceuticals and antioxidant activity than lyophilized flour from tomato industrial by-product.
Material and methods
Industrial tomato by-product (pomace) was provided by the Food Company La Costeña®, Guasave, Sinaloa, Mexico, the excess of water was eliminated using a homemade hydraulic press (supported by a bottle jack press of 4 t). The field waste was provided by local farmer association, this consist of tomato fruit in the late stage of production, even when the fruit is free of mechanical or biotic damage is not harvested, due to it does not meet quality criteria, and usually is incorporated as organic matter into soil. After remove any strange material from field waste, a scalding was carried out at 80 °C during 3 min, these conditions correspond to a scalding process in the industry (Personal communication, January 15th 2018 La Costeña), subsequently skin and seeds were manually separated from the pulp. Both, industrial by-product and field waste material were stored at − 20 °C until use.
Drying conditions determination
Drying process was done using a forced convection oven (D-20 Food Dehydrator Stainless Steel Shelves, #32750, USA), and tomato industrial by-product was used to establish drying conditions. 200 g of starting material were spread in 50 cm2 on metal racks per run. Three temperatures were evaluated: 45, 50, and 55 °C, and dry process was extended up to 140 min; aw (Rotronic HP23-AW-SET) and total carotenoids (AOAC 970.64) were monitored every 20 min. As reference a lyophilized by-product control was included.
Total carotenoid contents changes by processing
An official method (AOAC 970.64) for carotenoids quantification was followed (AOAC 1990). Absorbance was determined at 436 nm in a spectrophotometer (Model GENESYS 10 UV, Serie 2H7G229001, USA) using 96-well microplates. The results were compared with a calibration curve of β-carotene and a correlation analysis between aw and total carotenoids was performed using Microsoft Excel 2013.
Antioxidant compounds extraction
To extract the phenolic compounds, we follow the method described by Ferreres et al. (2010) with modifications by Valdez-Morales et al. (2014). For the carotenoids extraction we followed the method reported by Silva et al. (2019). We use food grade ethyl acetate for lipophilic extraction, trying to make this process not only more efficient, but also eco-friendly.
Both extracts (phenolic compounds and carotenoids) were taken to dryness using a rotary evaporator; subsequently they were stored in 15 mL plastic tubes (at constant weight) at − 20 °C protected from light.
Bioactive compounds quantification (HPLC)
Both phenolic compounds and carotenoids quantification were performed by HPLC, based in the previously reporting standards.
The profile of phenolic compounds was determined by the method reported by Espinosa-Alonso et al. (2006) and modified by Valdez-Morales et al. (2014), they were identified and quantified on a Dionex Ultimate 3000 model (Dionex Corporation, USA) chromatograph, with automatic injection, equipped with a quaternary pump, and a diode array detector. As stationary phase we use a Dionex Acclaim 120 column, C18 4.6 mm × 250 mm, 5 µm (Dionex Corporation, USA). The mobile phase used consists of: A = acidified water with at pH 2.8 h acetic acid and B = acetonitrile; gradient started with 95% of A and 5% of B until 2.5 min, gradually increasing the percentage of B: 12% at 6 min, 23% at 18 min, 35% at 24 min, 95% at 30 min and returned returning to the initial conditions at a final time of 40 min. The flow rate was 0.6 mL/min, and the fixed wavelengths were 260, 280, 320, and 360 nm. Commercial analytical phenolic standards were used (Sigma-Aldrich, Pennsylvania, USA) to construct standard curves.
For carotenoids quantification in lipophilic extracts, 800 µL of filtered extract was injected into the Dionex Ultimate 3000 chromatograph (Dionex Corporation, USA) described before, using an Acclaim Polar Advantage II C18 column 4.6 mm × 150 mm, 5 µm (Sunnyvale, CA, USA). The mobile phase was an isocratic flow composed of: acetonitrile:methanol:dichloromethane (43:43:14 v/v), at a flow rate of 0.3 mL/min, and elution time was 20 min. For the identification and quantification of lycopene and β-carotene, we used commercial analytical standards (Sigma-Aldrich, Pennsylvania, USA) (López-Vidal et al. 2014).
Antioxidant capacity (myoglobin protection ratio)
Antioxidant activity of both phenolic and carotenoid extracts were measured following the method report by Terashima et al. (2012) confronting the extracts against the reactive oxygen species peroxyl and hydroxyl radicals and hypochlorite ion.
For the evaluation of carotenoid-rich extracts, we made modifications to the original methodology of Terashima et al. (2012). The preparation of the solutions and the plate filling volume were modified, considering the final well volume of each reagent. To allow the integration of the carotenoid-rich extracts into the analysis, it was performed a solubility tests, the extracts were dried to total dryness with gaseous nitrogen, subsequently dry extract was suspended in a mixture of methanol:acetone (1:1) (v/v) evaluating the maximum solubility capacity to be subsequently suspended in the phosphate buffer. The final concentration was 10 mg/mL. We used a 96-well microplate (353,072, Costar, USA) for all measurements, 10 mg of dry extract in 200 µL of the methanol:acetone (1:1) (v/v) mixture and subsequently adding 800 µL of PBS buffer (8.10 mM Na2HPO4, 1.47 mM KH2PO4, 273 mM NaCl, 26.8 mM KCl), 100 mg of myoglobin were dissolved in 50 mL of PBS buffer (8.10 mM Na2HPO4, 1.47 mM KH2PO4, 273 mM NaCl, 26.8 mM KCl).
Hypochlorite ion
For measurements of protection ratio against the hypochlorite ion, 82.5 µL of myoglobin solution (1 mg/mL), 80 µL of the sample and 30 µL of the hypochlorite solution (0.02%) were added. After mixing by pipetting.
Peroxyl radical
For the peroxyl radical, we added 70 µL of the myoglobin solution, 70 µL of sample and 55 µL of 2,2-azobis-2-methylpropylamidine dichlorated solution (70 mM), before measuring the absorbance, the microplate with the solution was incubated at 60 °C for 15 min in a water bath, in order to generate the peroxyl radical.
Hydroxyl radical
In the case of the hydroxyl radical, 82.5 µL of the myoglobin solution (1 mg/mL) and 100 µL of the sample were added and mixed by pipetting, in a test tube, 200 µL of FeSO4 solution (10 mM) and 2.0 mL of H2O2 (13 mM) were placed in order to generate the hydroxyl radical by the Fenton reaction. Immediately, 5.5 µL of the reaction mixture was added to the microplate and mixed by pipetting.
The absorbance was measured at 405 nm and the results were calculated using the following equation:
means: abs0 = absorbance of myoglobin solution; absrad = myoglobin absorbance with radical; a.o. = antioxidant.
A correlation analysis was made between myoglobin protection ratio and the amount of the principal quantified metabolites, using Microsoft Excel 2013.
Results and discussion
Drying conditions determination
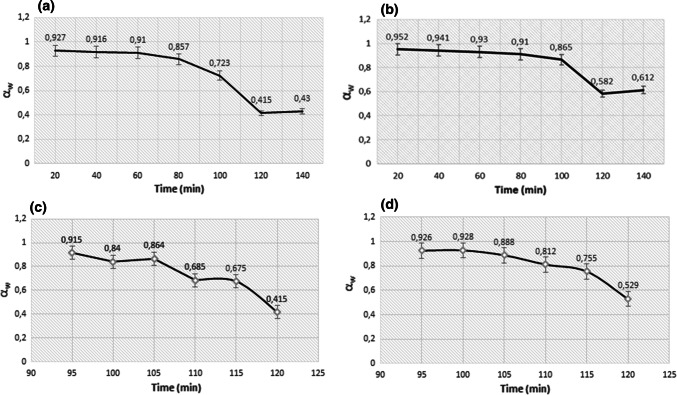
The results of αw obtained are shown in Fig. 1, it was sought to find the combination of temperature and time that allows to obtain a water activity that allowed to preserve the raw material for long periods of time, while facilitating the extraction of bioactive compounds, without significantly affecting the metabolites content. Drying conditions were stablished using the tomato industrial by-product due to technical facilities, because this material was first available for its study.
Fig. 1.
Water activity (αw) during the oven drying from 0 to 140 min a 55 °C and b 50 °C, and from 90 to 125 min c 55 °C and d 50 °C. Starting material (200 g) were spread in 50 cm2 on metal racks per run (n = 3)
During 140 min of oven dry, the αw of the samples decrease from 0.915 to 0.43 at 55 °C and from 0952 to 0.61 at 50 °C (Fig. 1a, b). To preserve the raw material for long periods of time, and facilitating the extraction of bioactive compounds without significantly metabolites content affectation, an adequate value of αw is between 0.4 and 0.6, specifically for carotenoids stability has been reported a αw = 0.44 (Lavelli et al. 2007; Lavelli and Torresani 2011). The adequate interval of αw was found in a range between 95 to 120 min (Fig. 1c, d), when the αw was measured every 5 min, resulting that after 115 min the αw was more closer to the target value (0.4–0.6) under both tested temperatures (50 and 55 °C).
Drying conditions were established considering the maximum preservation of bioactive compounds, based on the total carotenoid content, since these compounds tend to be more degraded than phenolic compounds, due to heat effect. The highest value of total carotenoids was found when αw < 0.6, at both drying temperatures (50 and 55 °C). It was established 55 °C during 120 min, αw = 0.415 ± 0.006 as the best drying condition to preserve total carotenoids (2.74 ± 0.5 mg/100 g of dry extract), which is until ten times higher than a lyophilized control (0.21 ± 0.9 mg/100 g, at a αw 0.324 ± 0.004). This αw value coincides with that reported by Jorge et al. (2018) to preserve different tomato puree metabolites exposed to different drying operations; as well as, the αw range established for Lavelli et al. (2007) equal to 0.31–0.54, where they found the maximum carotenoids content after the application of different drying treatments. Those results shown that carotenoids have a close relationship with αw, this variable was not monitored during the lyophilization process; and, it has been reported that low levels of water activity induces autocatalytic oxidation, phenomenon described by Lavelli et al. (2007), when they evaluate the effect of αw on the carotenoid content in dehydrated carrots.
Bioactive compounds quantification (HPLC)
The phenolic compounds profile found in the phenolic rich extract is shown in Table 1. The higher content of the total phenolic compounds (sum of each individual compound) corresponds to the control lyophilized industrial by-product used as control (508.3 µg/100 g of dry extract), it represented twice and 3.8 times more than industrial by-product and field waste, respectively, and is similar to the free fraction phenolic compounds (522.2 µg/100 g) reported previously for industrial tomato by-product (Perea-Domínguez et al. 2018). On the other hand, these results are superior to those reported by Jorge et al. (2018) of 339 µg/100 g in a similar tomato sample.
Table 1.
Phenolic acid, flavonoid, and carotenoid content from tomato field waste and industrial by-products
| Oven dry field waste | Oven dry by-product | Liophylized by-product | |
|---|---|---|---|
| Flavonoids (μg/mg dry extract) | |||
| Catechin | 23.59 ± 2.36c | 55.96 ± 2.44b | 255.03 ± 25.5a |
| Quercetin | 7.02 ± 0.39b | 6.92 ± 0.94b | 14.54 ± 0.06a |
| Naringenin | 19.79 ± 3.67b | 90.04 ± 6.39a | 79.66 ± 7.7a |
| Rutin | 27.42 ± 3.63b | 33.28 ± 2.73b | 46.52 ± 1.65a |
| Kaempferol | 1.09 ± 0.00a | 0.56 ± 0.02b | 0.5 ± 0.03b |
| Phenolic acids (μg/mg dry extract) | |||
| Gallic | 3.88 ± 1.55c | 9.85 ± 0.33b | 34.68 ± 1.01a |
| Caffeic | 14.13 ± 3.47ab | 11.27 ± 1.11b | 17.85 ± 0.26a |
| Ferulic | 6.38 ± 1.12a | 5.19 ± 0.68a | 6.82 ± 0.34a |
| Vanillic | 5.54 ± 1.15a | 9.46 ± 3.11a | 8.70 ± 2.04a |
| o-Coumaric | 23.97 ± 4.54b | 17.37 ± 2.61b | 35.10 ± 4.10a |
| p-Coumaric | 1.47 ± 0.20b | 2.35 ± 0.26b | 8.90 ± 0.12a |
| Carotenoids (μg/mg dry extract) | |||
| β-Carotene | 6.12 ± 0.09a | 1.69 ± 0.68c | 2.88 ± 0.09b |
| Lycopene | 11.26 ± 1.41a | 2.96 ± 1.17c | 5.13 ± 0.17b |
Data corresponds to the average ± standard deviation of 3 repetitions. Different superscript letters between columns indicate significant difference (Tukey, p ≤ 0.05)
The main compounds were flavonoids, naringenin value reached up to 90.04 ± 6.39 µg/mg of dry extract from oven-dried by-product, and up to 255.03 ± 25.5 µg/mg of dry extract of catechin from extract from lyophilized by-product. Fattore et al. (2016) reported both compounds as the main phenolic compounds in fresh tomato. And these compounds previously showed bioactive potential as ROs scavenging, as well as cytotoxic and antimutagenic activities. Kaempferol was higher in extract from tomato field waste flour; and, the content of ferulic, vanillic, and o-coumaric acids did not showed significant difference between the two samples and the lyophilized flour.
For carotenoid-rich extracts, the field waste has higher lycopene (11.26 ± 1.41 µg/mg or 1126 ± 141 mg/100 g) than the lyophilized control sample (5.13 ± 1.41 µg/mg or 513 ± 170 mg/100 g) and the by-product oven dried (2. 96 ± 1.17 µg/mg or 296 ± 117 mg/100 g); these values were superior than those reported by Jorge et al. (2018), these authors reported 404.3 ± 60.29 mg lycopene/100 g for tomato powders oven dried. At the same way, the field waste extract, registered the highest content of β-carotene (6.12 ± 0.09 µg/mg or 612 ± 9 mg/100 g) compared with the lyophilized control (5.13 ± 0.17 µg/mg or 513 ± 17 mg/100 g) and by-product oven dried (296 ± 117 mg/100 g), all superior to previously reported in tomato powders oven dried by Jorge et al. (2018) (211.7 ± 60.03 mg β-carotene/100 g). On the other hand, Silva et al. (2019) reported the optimized extraction conditions for recover lycopene from tomato by-product, using a combination of ethyl acetate and ethyl lactate as solvents, obtaining up to 1334.8 ± 83.9 mg/100 g of lycopene, similar value registered in this work from the extract obtained from flour of tomato field waste. Based on these results, it is suggested that carotenoid rich extracts, recovered using an eco-friendly solvent from industrial by-product or tomato field waste flours, may have a high bioactive potential.
When we quantified total carotenoids spectrophotometrically, it was observed that total carotenoids content decreased considerably in the lyophilized industrial by-product with respect to industrial by-product oven dried. And, when we run the analysis by HPLC, a different behavior was observed as was described before. Fratianni et al. (2013) reported in apricot fruits that although the content of most of the carotenoids decreases due to the effect of drying, some compounds such as: 9-cis-β-carotene and 13-cis-β-carotene remain stables depending on the drying method and time of drying. Also, Ghafoor et al. (2020) reported a better content of carotenoids in samples of ginger (Zingiber officinale) freeze drying than in the drying oven ones.
It should be noted that lycopene has been linked to the attenuation of oxidative stress by inducing the Nrf2/NF-kB transcriptional pathway, on the other hand, in vitro studies have been carried out in which it has shown potential to treat prostate and ovarian cancer, suppressing proliferation and inducing apoptosis (Zhao et al. 2017; Paur et al. 2017; Xu et al. 2019).
Antioxidant capacity (myoglobin protection ratio)
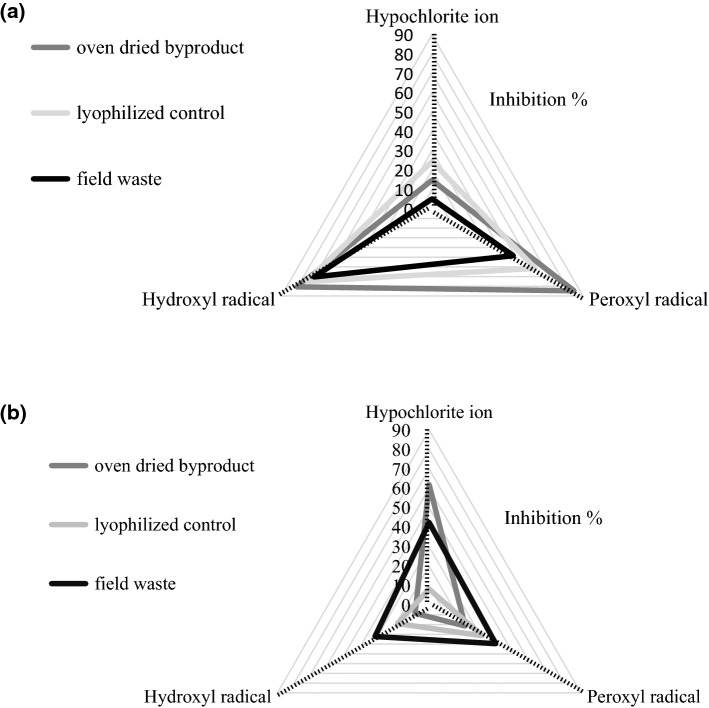
The myoglobin protection ratio was evaluated and is presented in Fig. 2 as the summed-area of the percentage of inhibition against three different radicals. The phenolic extract from oven dried by-product showed inhibitions of 14.78% against the hypochlorite radical, 80.64% against the hydroxyl radical, and 84.74% against the peroxyl radical (Fig. 2a). The area observed for oven dried by-product sample was higher than the other two samples: lyophilized control and field waste. Terashima et al. (2012) performed the evaluation of antioxidant activity by this method, testing different flavonoids: rutin, quercetin, and kaempferol, finding around 30% inhibition against the hypochlorite ion, and 100% inhibition against hydroxyl and peroxyl radicals, using around 300–500 µg of antioxidants per reaction mix, and considering that in that study they use pure chemical, it could be suggested that our extracts have an acceptable potential. A positive correlation was established between the inhibition of the radicals evaluated and the presence of the flavonoids catechin and naringenin, with (r) values greater than 0.95.
Fig. 2.
Antioxidant activity of a phenolic–rich extracts and b carotenoid-rich extracts, expressed by inhibition % by myoglobin ratio protection method
About the antioxidant activity analyzed through the myoglobin protection of the carotenoid-rich extracts (Fig. 2b), the extract from field waste showed the highest inhibition against hydroxyl (32.13%) and peroxyl (39.53%) radicals, and a 41.5% against the hypochlorite radical, but against hypochlorite the highest inhibition was exhibited by the extract from oven dried by-product. The results of antioxidant activity of no polar extracts, had in general, a similar behavior than that observed in the quantification of β-carotene and lycopene, best results were found in the following order: field waste > oven dried by-product > lyophilized by-product.
Despite of this method has been reported for polar compounds, in this study has been observed a high correlation between the carotenoids content and the inhibition of the peroxyl and hydroxyl radicals, with r values higher than 0.98, which indicates that the bioactive potential of non-polar terpenes was actually measured under this method. However, it was not possible to establish a relationship with the hypochlorite ion, it is known that the hypochlorite ion is stabilized by donating hydrogen groups, giving as product its corresponding oxacid hypochlorous acid, meanwhile the antioxidant activity of carotenoids is attributed to their conjugated double bonds (Fiedor and Burda 2014), which act as sequestrants of the unstable molecule, suggesting incompatible antioxidant activity mechanisms.
Conclusion
It was possible to establish drying conditions with a traditional and easily scalable method to dry tomato industrial by-product or field waste. The hydrophilic (80% ethanolic) and lipophilic (food grade ethyl acetate) agro-industrial by-product extractions preserves a good content of bioactive compounds: carotenoids and phenolics. The myoglobin protection ratio against peroxyl and hydroxyl radicals could evaluate the antioxidant carotenoid rich extract. Based upon the metabolites content and antioxidant capacity, tomato by-products and tomato field waste, are potential candidates for the extraction of phenolic and carotenoid compounds, the extracts could be used as a food additive, or even the flours could be added by themselves into a new food product.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Segoviano-León Juan Paulino, Email: juan_paulino_29@hotmail.com.
Ávila-Torres Germán Adrián, Email: german_avilatorres@hotmail.com.
Espinosa-Alonso Laura Gabriela, Email: lespinosaa@ipn.mx.
Valdez-Morales Maribel, Email: mvaldezmo@conacyt.mx.
Medina-Godoy Sergio, Email: smedinam@ipn.mx.
References
- AOAC . Official methods of analysis. 15. Washington, D.C.: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Espinosa-Alonso LG, Lygin A, Widholm JM, et al. Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.) J Agric Food Chem. 2006;54:4436–4444. doi: 10.1021/jf060185e. [DOI] [PubMed] [Google Scholar]
- FAO-STAT (2019) Statistics Division. Food Balance Sheets: Balance as Domestic Supply/Utilization. Food and Agriculture Organization of the United Nations, Rome
- Fattore M, Montesano D, Pagano E, Teta R, Borrelli F, et al. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino vesuviano” tomatoes. J Food Compos Anal. 2016;53:61–68. doi: 10.1016/j.jfca.2016.08.008. [DOI] [Google Scholar]
- Ferreres F, Taveira M, Pereira DM, et al. Tomato (Lycopersicon esculentum) Seeds: new flavonols and cytotoxic effect. J Agric Food Chem. 2010;58:2854–2861. doi: 10.1021/jf904015f. [DOI] [PubMed] [Google Scholar]
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratianni A, Albanese D, Mignogna R, et al. Degradation of carotenoids in apricot (Prunus armeniaca L.) during drying process. Plant Foods Hum Nutr. 2013;68:241–246. doi: 10.1007/s11130-013-0369-6. [DOI] [PubMed] [Google Scholar]
- Ghafoor K, Al Juhaimi F, Özcan MM, et al. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. Lwt. 2020 doi: 10.1016/j.lwt.2020.109354. [DOI] [Google Scholar]
- Jorge A, Sauer Leal E, Sequinel R, et al. Changes in the composition of tomato powder (Lycopersicon esculentum Mill) resulting from different drying methods. J Food Process Preserv. 2018;42:e13595. doi: 10.1111/jfpp.13595. [DOI] [Google Scholar]
- Kong KW, Ismail A, Tan CP, Rajab NF. Optimization of oven drying conditions for lycopene content and lipophilic antioxidant capacity in a by-product of the pink guava puree industry using response surface methodology. LWT Food Sci Technol. 2010;43:729–735. doi: 10.1016/j.lwt.2009.10.011. [DOI] [Google Scholar]
- Lavelli V, Torresani MC. Modelling the stability of lycopene-rich by-products of tomato processing. Food Chem. 2011;125:529–535. doi: 10.1016/j.foodchem.2010.09.044. [DOI] [Google Scholar]
- Lavelli V, Zanoni B, Zaniboni A. Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chem. 2007;104:1705–1711. doi: 10.1016/j.foodchem.2007.03.033. [DOI] [Google Scholar]
- López-Vidal O, Escalona-Buendía H, Pelayo-Zaldívar C, et al. Carotenoid content, antioxidant capacity and volatile compounds of the aroma during tomato ripening | Carotenoides, capacidad antioxidante y compuestos volátiles del aroma durante la maduración de jitomate. Phyton (B Aires) 2014;83:185–192. [Google Scholar]
- M’hiri N, Ghali R, Ben Nasr I, Boudhrioua N. Effect of different drying processes on functional properties of industrial lemon byproduct. Process Saf Environ Prot. 2018;116:450–460. doi: 10.1016/j.psep.2018.03.004. [DOI] [Google Scholar]
- Paur I, Lilleby W, Bøhn SK, et al. Tomato-based randomized controlled trial in prostate cancer patients: effect on PSA. Clin Nutr. 2017;36:672–679. doi: 10.1016/J.CLNU.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Perea-Domínguez XP, Hernández-Gastelum LZ, Olivas-Olguin HR, et al. Phenolic composition of tomato varieties and an industrial tomato by-product: free, conjugated and bound phenolics and antioxidant activity. J Food Sci Technol. 2018 doi: 10.1007/s13197-018-3269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore J, Nemecek T. Reducing food’s environmental impacts through producers and consumers. Science (80- ) 2018;360:987LP–992LP. doi: 10.1126/science.aaq0216. [DOI] [PubMed] [Google Scholar]
- SIAP (2019) Servicio de Información Agroalimentaria y Pesquera. Cierre de la producción agrícola por cultivo. Secretaría de Agricultura y Desarrollo Rural. https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 Accessed 15 May 2020.
- Silva YPA, Ferreira TAPC, Celli GB, Brooks MS. Optimization of lycopene extraction from tomato processing waste using an eco-friendly ethyl lactate–ethyl acetate solvent: a green valorization approach. Waste Biomass Valoriz. 2019;10:2851–2861. doi: 10.1007/s12649-018-0317-7. [DOI] [Google Scholar]
- Terashima M, Kakuno Y, Kitano N, et al. Antioxidant activity of flavonoids evaluated with myoglobin method. Plant Cell Rep. 2012;31:291–298. doi: 10.1007/s00299-011-1163-2. [DOI] [PubMed] [Google Scholar]
- Valdez-Morales M, Espinosa-Alonso LG, Espinoza-Torres LC, et al. Phenolic content and antioxidant and antimutagenic activities in tomato peel, seeds, and byproducts. J Agric Food Chem. 2014;62:5281–5289. doi: 10.1021/jf5012374. [DOI] [PubMed] [Google Scholar]
- Vela-Hinojosa C, Escalona-Buendía HB, Mendoza-Espinoza JA et al (2018) Chemical and sensory analysis of native genotypes and experimental lines of tomato (Solanum lycopersicum L.). Fruits 73:60–71. 10.17660/th2018/73.1.7
- Xu J, Li Y, Hu H. Effects of lycopene on ovarian cancer cell line SKOV3 in vitro: Suppressed proliferation and enhanced apoptosis. Mol Cell Probes. 2019;46:101419. doi: 10.1016/j.mcp.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Zhao B, Ren B, Guo R, et al. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem Toxicol. 2017;109:505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]