Abstract
Background
Cough is predictive of exacerbations of chronic obstructive pulmonary disease (COPD). Little is known about cough reflex sensitivity during exacerbation of COPD and whether it is associated with exacerbation frequency. This pilot study aimed to investigate cough reflex sensitivity during and following recovery from exacerbation of COPD, and its association with the frequency of future exacerbations. In addition, the repeatability of cough reflex sensitivity in stable COPD was investigated.
Methods
Twenty participants hospitalised with exacerbation of COPD underwent inhaled capsaicin challenge during exacerbation and after 6 weeks of recovery. The frequency of future exacerbations was monitored for 12 months. The repeatability of cough reflex sensitivity was assessed in separate participants with stable COPD, who underwent 2 capsaicin challenge tests, 6 weeks apart.
Results
Cough reflex sensitivity was heightened during exacerbation of COPD. Geometric mean (SD) capsaicin concentration thresholds to elicit 5 coughs (C5) during exacerbation and after 6 weeks of recovery were 1.76 (3.73) vs. 8.09 (6.25) μmol L−1, respectively (p < 0.001). The change in C5 from exacerbation to 6-week recovery was associated with the frequency of future exacerbations (ρ = − 0.687, p = 0.003). C5 was highly repeatable over 6 weeks in stable COPD, and intraclass correlation coefficient was 0.85.
Conclusion
Cough reflex sensitivity is heightened during exacerbation of COPD and reduces after recovery. The persistence of cough reflex hypersensitivity at recovery was associated with the frequency of future exacerbations.
Electronic supplementary material
The online version of this article (10.1007/s00408-020-00366-x) contains supplementary material, which is available to authorised users.
Keywords: Cough reflex sensitivity, Cough, Chronic obstructive pulmonary disease, Exacerbation of chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) exacerbations are acute episodes of sustained deterioration in respiratory symptoms beyond normal daily variation [1]. Such episodes are associated with impaired quality of life [2], accelerated decline in lung function [3] and increased health care utilisation [4]. The identification of individuals with COPD who are more susceptible to exacerbation may lead to targeted preventative therapies. Increased cough and/or sputum volume or purulence are common symptoms of COPD exacerbations [5, 6]. Independent authors have reported that higher levels of both cough symptoms and sputum production in the stable state are associated with exacerbation frequency [2, 7, 8].
The mechanism of cough in general is complex and not fully understood [9, 10]. One potential mechanism is the activation of bronchopulmonary C fibres [9]. C fibres are relatively quiescent in health but can be activated or sensitised by inflammatory mediators [11, 12]. For example, cough reflex sensitivity to capsaicin, a potent activator of C fibres, is heightened during viral upper respiratory tract infection [13, 14]. The changes in cough reflex sensitivity during acute exacerbation of COPD have not previously been investigated.
The aim of this pilot study was, firstly, to determine if cough reflex sensitivity is heightened during exacerbation of COPD and, secondly, to investigate an association between cough reflex sensitivity and exacerbation frequency. Thirdly, we sought to determine the repeatability of cough reflex sensitivity measurements in patients with stable COPD.
Methods
This was a prospective observational study of participants hospitalised with exacerbation of COPD (winter seasons of 2017/2018) and participants with stable COPD (April to August 2017), conducted at a single site (King’s College Hospital, Denmark Hill, London, UK) in accordance with the principles of the Declaration of Helsinki. The study was granted research ethics committee approval (Health Research Authority South East Scotland Research Ethics Service, 16/SS/0189). All participants provided written informed consent.
Participants
COPD Exacerbations
An exacerbation of COPD was defined as an episode of acute deterioration of respiratory symptoms associated with physiological deterioration which required additional therapy [5]. Consecutive patients admitted to hospital with an exacerbation of COPD were recruited prospectively. Initial management was according to local guidelines, with oral corticosteroids, and a combination of antibiotics, nebulised bronchodilator, inhaled meter-dosed bronchodilators and non-invasive ventilation as appropriate. Exclusion criteria were prior exposure to capsaicin challenge test, consolidation on chest radiograph, significant cognitive impairment and any additional causes for hospitalisation.
Stable COPD (Repeatability)
To investigate repeatability, a separate group of consecutive patients with COPD and stable symptoms in the preceding 6 weeks were recruited prospectively from an outpatient specialist clinic. The diagnostic criteria were a ≥ 10 pack-year smoking history and post-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio < 0.70 [15]. Exclusion criteria were previous exposure to capsaicin challenge test and a history suggestive of respiratory tract infection within the previous 6 weeks.
Protocol
All participants underwent investigations at two timepoints, six weeks apart. At timepoint 1, demographic and anthropometric data were collected, and participants underwent spirometry, inhaled capsaicin tussive challenge and 24-h cough frequency monitoring. Participant-reported subjective assessments of COPD health status, cough severity and cough-specific health status were also performed. At timepoint 2, all previous measures from timepoint 1 were repeated, and the participants also underwent body plethysmography and measurements of carbon monoxide transfer factor coefficient.
COPD Exacerbation
Twenty participants underwent investigations on the day of hospital discharge following admission for exacerbation of COPD (TE), and after a 6-week recovery period (TR). Medical fitness for discharge was determined by the senior attending physician [16]. Seventeen participants were then followed up by review of National Health Service electronic patient records for a period of 12 months to measure the frequency of further COPD exacerbations. Exacerbations were defined as episodes of healthcare use for their COPD with a change in medication or hospital admission as per Hurst et al. [17]. The diagnostic criteria used met the definition of moderate and severe exacerbations of COPD, exacerbations requiring a change of medication and to seek medical assistance and exacerbations requiring hospitalisation, respectively [18]. The primary and secondary care clinicians treated patients according to local clinical guidelines. Episodes of subsequent exacerbations of COPD, separated by an exacerbation-free period of ≥ 14 days, were determined from prospective review of the combined local hospital and primary care electronic patient record system [5]. All primary care practices in the local catchment area were linked to accessible electronic local care records.
Repeatability of Tussive Challenge Testing in Stable COPD
Ten separate participants with stable COPD underwent investigations over 2 visits separated by 6 weeks: T0 and T6, respectively.
Any participants with either exacerbation of COPD or stable COPD, who had a respiratory tract infection, an exacerbation of COPD or a change in smoking status between the 2 visits, were excluded from data analysis.
Capsaicin Tussive Challenge Test
Cough reflex sensitivity (CRS) was assessed according to the European Respiratory Society (ERS) guidelines [19]. Capsaicin solution (Sigma-Aldrich, Missouri, USA) was delivered in incremental doubling doses (0.49–1000 µmol L−1) at 1-min intervals, as 10-µL single breath inhalations, via an air-powered dosimeter (KoKo Digidoser, nSpire Health Inc, Longmont, CO, USA). To diminish the effect of anticipation, 0.9% saline solution was interspersed randomly [19]. A single characterised nebuliser (DeVilbiss Healthcare, Port Washington, NY, USA) with an output of 1.232 mL min−1 was employed for all participants throughout the study. In addition, a valve limited inspiratory flow to 0.5 L s−1 [19, 20]. The number of coughs elicited by each dose administration was counted for 15 s following each dose inhalation with the aid of an audio recorder (ICD-PX333, Sony Corporation, Tokyo, Japan) [19, 20]. The challenge test continued until ≥ 5 coughs were elicited by a single-dose administration. The threshold concentrations of capsaicin required to elicit 1 (C1), 2 (C2) and 5 (C5) coughs were calculated by interpolation [21].
Cough Frequency Monitoring
Cough frequency was assessed objectively over 24 h with the Leicester Cough Monitor (LCM) [22]. The LCM is a validated ambulatory system, which comprises a MP3 recorder (ICD-PX333, Sony Corporation, Tokyo, Japan), a free-field lapel microphone (LFH9173, Philips, Amsterdam, Netherlands) and a semi-automated cough detection software. Coughs were detected as characteristic sounds, whether occurring in isolation or as part of a bout of prolonged coughing [22]. Total cough count (number of coughs over 24 h), mean cough frequency (coughs h−1 over 24 h), awake cough counts (number of coughs over estimated time spent awake) and awake 24-h cough frequency (coughs h−1) were recorded. Participants recorded and reported their time spent asleep.
Subjective Assessments
COPD-Specific Health Status
COPD health status was assessed with the COPD Assessment Test (CAT), a validated self-administered 8-item questionnaire (range 0–40; lower scores indicating better health status) [23]. The CAT has been demonstrated to be responsive to changes of health status during recovery following an exacerbation of COPD [24].
Cough Severity and Cough-Specific Health Status
Cough severity was self-reported on a visual analogue scale (VAS) (range 0–100 mm; higher scores indicating more severe cough) [19]. The cough-specific health status was assessed with the Leicester Cough Questionnaire (LCQ), a self-administered 19-item assessment for cough-specific health status previously validated in COPD (range 3–21; higher scores indicating better health status) [25].
Lung Function
Spirometry, body plethysmography and diffusing capacity of carbon monoxide (Jaeger MS-PFT Analyser Unit with Sentry Suite software version 2.19.96, Vyaire Medical, Mettawa, IL, USA) were measured as per guidelines by the ERS and the American Thoracic Society [26–28].
Statistical Analysis
The distribution of data was assessed using the D’Agostino-Pearson test. Parametric data were summarised with means (standard deviation, SD), whilst non-parametric data with medians (interquartile range, IQR). Capsaicin challenge and cough frequency data were presented as geometric mean (geometric standard deviation, SD). Parametrically and non-parametrically distributed paired data were analysed with paired t test and the Wilcoxon matched-pairs signed rank test, respectively. Fisher’s exact test and Chi-squared test were used for categorical data. Correlations between variables were assessed with Pearson product-moment correlation coefficient (r) for parametric data, and Spearman’s rank-order correlation coefficient (ρ) for non-parametric data. Repeatability was analysed with the Bland–Altman method, and with intraclass correlation coefficients (ICC) based on a single-rater, absolute agreement, two-way mixed-effects model. p values < 0.05 were considered statistically significant with the exception for the analysis of factors associated with future exacerbation frequency when a Bonferroni correction was applied, whereby p values < 0.007 were considered statistically significant. The threshold concentrations of capsaicin required to elicit 1, 2 and 5 coughs (C1, C2 and C5, respectively) were calculated by interpolation of the log dose–response curve [21]. A value of 1000 µmol L−1 was assigned to any interpolated values which were > 1000 µmol L−1 [21].
A previous study has reported a significant reduction in capsaicin-evoked cough reflex sensitivity in 14 participants following recovery from an upper respiratory tract infection [13]. A study by Dicpinigaitis reported the reproducibility of the capsaicin cough challenge in a sample size of 10 subjects [20]. We therefore aimed to recruit 10 participants to investigate the repeatability of CRS in COPD, and 15 participants to investigate CRS during and following an exacerbation of COPD. All analyses were performed using Prism® version 8.1.1 (GraphPad Software, San Diego, California, USA), except the Bland–Altman and intraclass correlation correlations analyses which were performed with R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) for macOS version 10.14.5.
Results
Participant Characteristics
A total of 20 participants with exacerbation of COPD were recruited; demographics, anthropometrics and clinical characteristics are summarised in Table 1. The median (IQR) duration of hospital admission was 6 (3–8) days. Two participants required acute non-invasive ventilation; no participant required invasive mechanical ventilation or organ support. All participants were discharged from hospital to their usual place of residence, and had completed their course of oral corticosteroid before the second visit at 6 weeks. No participant took oral azithromycin during the course of this study. Seventeen out of 20 (90%) participants agreed to participate in the 12-month period of observation to determine subsequent exacerbation frequency (3 participants declined to participate). Ten separate participants with stable COPD were recruited to investigate the repeatability of cough reflex sensitivity testing and objective cough frequency (Table 2).
Table 1.
COPD exacerbation group: demographics and clinical characteristics of participants during exacerbation and 6 weeks following recovery
| Participants with exacerbation of COPD (n = 20) | |||
|---|---|---|---|
| Admission | 6-week recovery | p values | |
| Age (years) | 67.5 (6.3) | N/A | |
| Gender (female) | 12 (60) | N/A | |
| BMI (kg m−2) | 27.4 (7.3) | N/A | |
| Smoking status | > 0.999c | ||
| Ex | 15 (75) | 15 (75) | |
| Current | 5 (25) | 5 (25) | |
| MRC dyspnoea scale (stable state) | N/A | 4 (3–5) | |
| CAT | 30 (26–33) | 27 (21–29) | 0.039d |
| Spirometry | |||
| FEV1% predicted | 34.0 (10.1) | 43.5 (15.0) | 0.004d |
| FVC % predicted | 65.4 (15.5) | 83.8 (15.0) | < 0.001d |
| Body plethysmographya | N/A | ||
| TLC % predicted | 122.9 (108.0–130.4) | ||
| RV % predicted | 155.5 (39.1) | ||
| Diffusion capacityb | N/A | ||
| TLCO % predicted | 52.3 (16.7) | ||
| KCO % predicted | 59.9 (23.9) | ||
| GOLD stage* | N/A | ||
| GOLD 1 | 0 (0) | ||
| GOLD 2 | 5 (25) | ||
| GOLD 3 | 11 (55) | ||
| GOLD 4 | 3 (15) | ||
| Co-morbidities | N/A | ||
| Ischaemic heart disease | 5 (25) | ||
| Cerebrovascular disease | 2 (10) | ||
| Hypertension | 11 (55) | ||
| Diabetes mellitus | 4 (20) | ||
| Index exacerbation | N/A | ||
| Duration of exacerbation symptom pre-admission (days) | 3 (2–9) | ||
| Systemic steroids use pre-admission | 9 (45) | ||
| Antibiotics use pre-admission | 9 (45) | ||
| Admission vital signs | N/A | ||
| Temperature (°C) | 37.0 (0.5) | ||
| Heart rate (min−1) | 96 (13) | ||
| Respiratory rate (min−1) | 21 (3) | ||
| SpO2 (%) | 94 (89–95) | ||
| Routine laboratory test on admission | N/A | ||
| Leucocytes (× 109 L−1) | 10.89 (3.96) | ||
| Neutrophils (× 109 L−1) | 8.06 (3.65) | ||
| Eosinophils (× 109 L−1) | 0.24 (0.21) | ||
| Haemoglobin (g L−1) | 140 (15) | ||
| C-reactive protein (mg L−1)* | 40 (69) | ||
| Duration of admission (days) | 6 (3–8) | N/A | |
Data presented as mean (SD), median (IQR) or absolute values (percentage)
BMI body mass index, MRC Medical Research Council, CAT COPD Assessment Tool, FEV1 forced expiratory volume in 1 s, FVC = forced vital capacity, TLC total lung capacity, RV residual volume, TLCO carbon monoxide transfer factor, KCO carbon monoxide transfer coefficient, GOLD Global Initiative for Chronic Obstructive Pulmonary Disease, SpO2 transcutaneous oxygen saturation
*n = 19
an = 13
bn = 16
cFisher’s exact test
dPaired t test
Table 2.
Stable COPD group: demographics and clinical characteristics of participants undergoing repeatability of cough measurements
| Participants with chronic obstructive pulmonary disease (n = 10) | |
|---|---|
| Age (years) | 64.0 (60.5–71.8) |
| Gender (female) | 3 (30%) |
| BMI (kg m−2) | 28.3 (19.8–34.4) |
| Smoking status | |
| Ex | 7 (70) |
| Current | 4 (40) |
| MRC dyspnoea scale | 4 (3–4) |
| GOLD stage | |
| GOLD 1 | 2 (20) |
| GOLD 2 | 1 (10) |
| GOLD 3 | 6 (60) |
| GOLD 4 | 1 (10) |
| Lung function | |
| FEV1% predicted | 45.7 (38.9–77.3) |
| FVC % predicted | 97.1 (66.9–108.2) |
| FEV1/FVC (%) | 38.5 (33.8–57.4) |
| TLC % predicted* | 130.0 (102.2–138.6) |
| RV % predicted* | 130.4 (92.3–183.9) |
| TLCO % predicteda | 43.5 (30.5–57.9) |
| KCO % predicteda | 49.5 (39.8–71.5) |
| CAT | 25 (17–29) |
| LCQ | |
| Physical | 5.5 (4.3–6.4) |
| Psychological | 5.3 (4.5–7.0) |
| Social | 5.4 (4.7–7.0) |
| Total | 16.2 (13.4–20.5) |
Data presented as median (IQR) or absolute value (percentage) unless otherwise stated
BMI body mass index, MRC Medical Research Council, GOLD Global Initiative for Chronic Obstructive Pulmonary Disease, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, TLC total lung capacity, RV residual volume, TLCO carbon monoxide transfer factor, KCO carbon monoxide transfer coefficient, CAT COPD Assessment Test, LCQ Leicester Cough Questionnaire
*n = 7
an = 9
Exacerbation of COPD
Cough Reflex Sensitivity
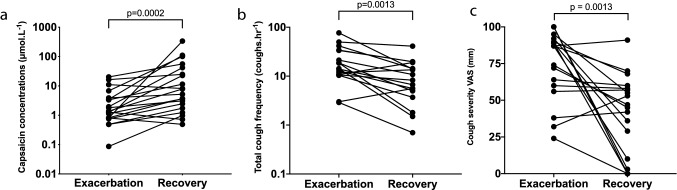
Cough reflex sensitivity was heightened during exacerbation compared to that following recovery. The capsaicin cough thresholds were significantly lower during exacerbation (TE) than at 6 weeks after recovery (TR) (Table 3). The geometric mean (SD) C5 at TE and TR were 1.76 (3.73) vs. 8.09 (6.25) μmol L−1, respectively, mean difference (95% CI) 2.20 (1.01–3.14) doubling doses (p < 0.001) (Fig. 1). The mean difference in C2 between TE and TR was 1.46 (p = 0.001) doubling doses (Table 3). There were no adverse events observed or reported by patients associated with capsaicin cough challenge testing during acute exacerbation of COPD. There was no significant difference in the change in C1, C2 and C5 doubling dose from TE to TR between gender (p = 0.392, 0.640 and 0.624, respectively). In addition, there was no association between the change in C5 doubling dose from TE to TR, and age (p = 0.684), FEV1 (p = 0.333) and history of exacerbation of COPD prior to the index exacerbation (p = 0.211). There were no adverse incidents associated with capsaicin challenge tests performed during acute exacerbations in participants with COPD.
Table 3.
Cough reflex sensitivity, objective cough frequency and subjective assessments during and 6 weeks after exacerbation of chronic obstructive pulmonary disease
| Admission | 6-week recovery | p values | |
|---|---|---|---|
| Cough reflex sensitivity* | |||
| C1 (μmol L−1) | 0.90 (3.48) | 2.08 (3.51) | 0.002b |
| C2 (μmol L−1) | 1.06 (3.47) | 2.92 (3.64) | 0.001b |
| C5 (μmol L−1) | 1.76 (3.73) | 8.09 (6.25) | < 0.001b |
| 24-h cough monitoring*,a | |||
| Total cough count (coughs) | 391.2 (2.4) | 176.3 (2.9) | 0.001b |
| Total cough frequency (coughs h−1) | 16.3 (2.4) | 7.4 (2.9) | 0.001b |
| Awake cough count (coughs) | 322.1 (2.3) | 135.0 (3.2) | 0.002b |
| Awake cough frequency (coughs h−1) | 19.2 (2.2) | 8.7 (3.2) | 0.003b |
| Cough severity VAS (mm)c | 74 (24) | 40 (28) | 0.001d |
| LCQ | |||
| Physical | 3.8 (1.2) | 4.6 (1.3) | 0.088d |
| Psychological | 4.1 (1.6) | 4.7 (1.4) | 0.145d |
| Social | 4.2 (1.8) | 4.8 (1.7) | 0.342d |
| Total | 12.1 (4.3) | 14.1 (4.1) | 0.162d |
Data presented as mean (SD) or median (IQR) unless otherwise stated
C1 capsaicin concentration required to elicit 1 cough, C2 capsaicin concentration required to elicit 2 coughs, C5 capsaicin concentration required to elicit 5 coughs, VAS visual analogue scale, LCQ Leicester Cough Questionnaire
*Geometric mean (SD)
an = 17
bWilcoxon matched-pairs signed rank test
cn = 18
dPaired t test
Fig. 1.
Capsaicin concentrations required to elicit 5 coughs (C5) during capsaicin challenge test, objective 24-h cough frequency and cough severity during and 6 weeks after exacerbation of chronic obstructive pulmonary disease. a C5 during and 6 weeks after exacerbation of COPD. b Total 24-h cough frequency during and 6 weeks after exacerbation of COPD. c Cough severity VAS during and 6 weeks after exacerbation of COPD
Objective Cough Frequency
Seventeen participants with exacerbation of COPD agreed to undergo 24-h cough monitoring. The geometric mean (SD) total 24-h cough frequency and awake cough frequency at TE were 16.3 (2.4) and 19.3 (2.3) coughs h−1, respectively (Table 3). There was a significant reduction in total 24-h cough frequency between TE and TR, mean difference (95% CI) 2.22 (1.43–3.44) fold difference (p = 0.001) (Fig. 1 and Table 3). There was no significant association between the changes in total 24-h cough frequency and changes in cough reflex sensitivity values from TE to TR (C1: ρ = 0.321, p = 0.209; C2: ρ = 0.355, p = 0.162 and C5: ρ = 0.436, p = 0.082). Neither was there a significant association between total 24-h cough counts and cough reflex sensitivity (C1, C2 and C5) at TE (ρ = 0.147, 0.147 and 0.011, respectively, all p > 0.50).
Subjective Measures
There was a significant reduction in median (IQR) CAT symptom scores at TR compared to TE: 27 (21–29) vs. 30 (26–33), respectively (p = 0.039). There was no significant association between CAT scores and cough reflex sensitivity (C1, C2 and C5) at TE (Table E1), or between the changes in CAT scores and cough reflex sensitivity (C1, C2 and C5) from TE to TR (p = 0.569–0.953).
There was a significant reduction in cough severity at TR compared to TE (mean (SD) cough severity VAS scores 74 (24) vs. 40 (28) mm, respectively (p = 0.001); Fig. 1) and a trend towards improvement in LCQ cough health status between these timepoints (mean (SD) LCQ total scores 14.1 (4.1) vs. 12.1 (4.3), respectively, p = 0.162). There was no significant association between cough reflex sensitivity (C1, C2 and C5) and either cough severity or LCQ cough-specific health status scores at TE (Table E1), or between change in cough reflex sensitivity (C1, C2 and C5) and change in either cough severity or LCQ cough health status from TE to TR (p = 0.549–0.814, and p = 0.528–0.956, respectively).
Association Between Cough Reflex Sensitivity and Future Exacerbation Frequency
Subsequent exacerbations of COPD occurred in 15 of the 17 individuals who were followed up for 12 months following the initial hospitalisation. The mean (SD) number of further COPD exacerbations per participant during follow-up was 2.5 (2.0) episodes. There were a total of 42 exacerbation episodes, of which 30 led to utilisation of primary care services, 12 to presentation to the emergency department, and 9 to hospital admission. The median (IQR) time to the next exacerbation following recruitment at TE was 135 (78–238) days.
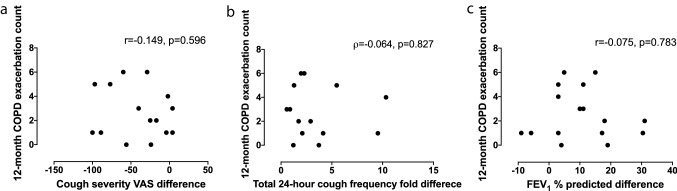
There was a significant association between the change in C5 from TE to TR and the rate of acute exacerbation of COPD in the following 12 months (ρ = − 0.687, p = 0.003) (Table 4 and Fig. 2). In addition, there was no significant association between the rate of exacerbation of COPD in the following 12 months and the changes from TE to TR in either objective cough frequency, CAT scores (p = 0.116), cough severity VAS (p = 0.596), LCQ or FEV1% predicted (Table 4 and Fig. 3). Neither at time TE or TR was there an association between the rate of exacerbation of COPD in the following 12 months and CRS, objective cough frequency, CAT scores, cough severity VAS, LCQ or FEV1% predicted (all p > 0.06).
Table 4.
Relationship between cough and 12-month prospective frequency of exacerbation of chronic obstructive pulmonary disease
| Future 12-month exacerbation count | ||
|---|---|---|
| Correlation coefficients | p values | |
| Admission vs. 6-week recovery | ||
| Cough reflex sensitivity (C5)* | − 0.687 | 0.003 |
| Total cough count (coughs)a,b | − 0.064 | 0.827 |
| Awake cough count (coughs)a,b | 0.033 | 0.912 |
| LCQ | 0.244c | 0.345 |
| FEV1% predicted | − 0.075c | 0.783 |
All correlation coefficients are Spearman’s rank-order correlations unless stated otherwise. Bonferroni correction was applied and p values < 0.007 were considered significant
Significant p values are given in bold
C5 capsaicin concentration required to elicit 5 coughs, VAS visual analogue scale, LCQ Leicester Cough Questionnaire, CAT COPD Assessment Test, FEV1 forced expiratory volume in 1 s
*Doubling dose change
an = 15
bFold difference
cPearson product-moment correlation
Fig. 2.
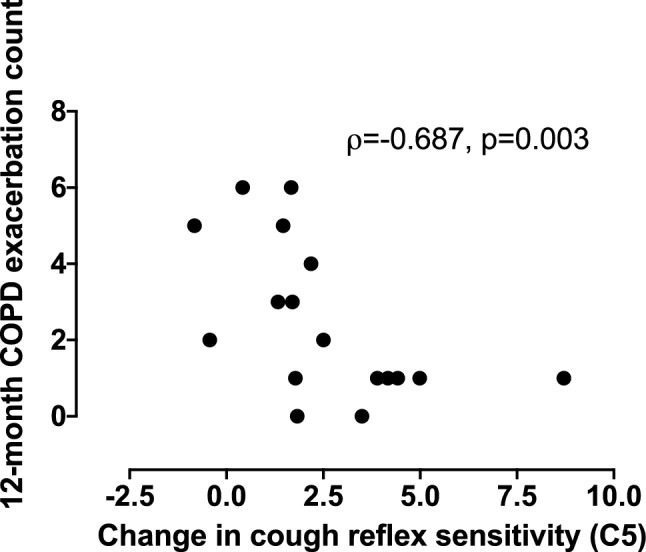
The relationship between the change in cough reflex sensitivity following exacerbation of chronic obstructive pulmonary disease and future exacerbations
Fig. 3.
The relationship between COPD exacerbation frequency and change between exacerbation and 6-week recovery visit in cough severity visual analogue scale score, objective cough frequency and forced expiratory volume in 1 s. a Cough severity visual analogue scale. b Total (24-h) cough frequency. c Forced expiratory volume in 1 s
Repeatability of Cough Reflex Sensitivity and Objective Cough Frequency in Stable COPD
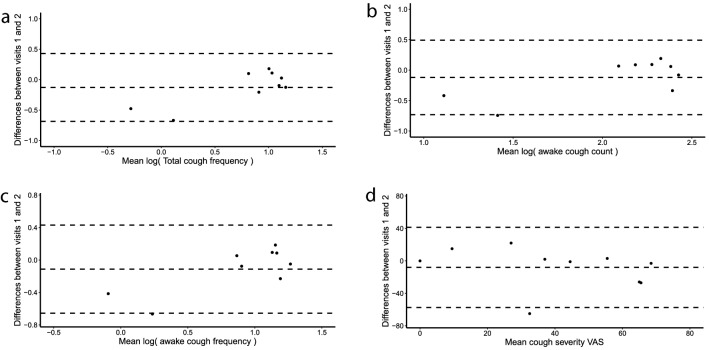
No participant was excluded from the study of CRS repeatability. Intraclass correlation coefficients to determine repeatability of the cough reflex sensitivity measures log(C1), log(C2) and log(C5) were 0.37, 0.54 and 0.85, respectively, in participants with stable COPD (Table 5). The mean differences (95% CI) in C1, C2 and C5 over 6 weeks were 0.34 (− 0.13–2.40), 0.31 (− 0.11–2.16) and 0.21 (− 0.40–1.79) doubling doses, respectively (Fig. 4). C5 was the most repeatable capsaicin challenge test threshold and was therefore used for the main analysis.
Table 5.
Repeatability of capsaicin challenge test, objective cough frequency and cough severity over 6 weeks in participants with stable chronic obstructive pulmonary disease
| Week 1 | Week 6 | ICC | |
|---|---|---|---|
| Cough reflex sensitivity | |||
| C1 (μmol L−1) | 0.93 (2.89) | 2.04 (3.90) | 0.37 |
| C2 (μmol L−1) | 1.50 (3.14) | 3.05 (4.13) | 0.54 |
| C5 (μmol L−1) | 6.29 (8.44) | 10.20 (7.45) | 0.85 |
| 24-h cough monitoring* | |||
| Total cough count (coughs) | 124.7 (4.1) | 165.0 (2.6) | 0.86 |
| Total cough frequency (coughs h−1) | 5.1 (4.2) | 6.9 (2.5) | 0.85 |
| Awake cough count (coughs) | 101.5 (3.9) | 134.0 (2.4) | 0.70 |
| Awake cough frequency (coughs h−1) | 6.5 (3.9) | 8.4 (2.4) | 0.84 |
| Cough severity VAS (mm) | 37 (23) | 45 (30) | 0.56 |
Data presented as geometric mean (SD) or mean (SD)
ICC intraclass correlation coefficient, C1capsaicin concentration required to elicit 1 cough, C2 capsaicin concentration required to elicit 2 coughs, C5 capsaicin concentrations required to elicit 5 coughs, VAS visual analogue scale
*n = 9
Fig. 4.
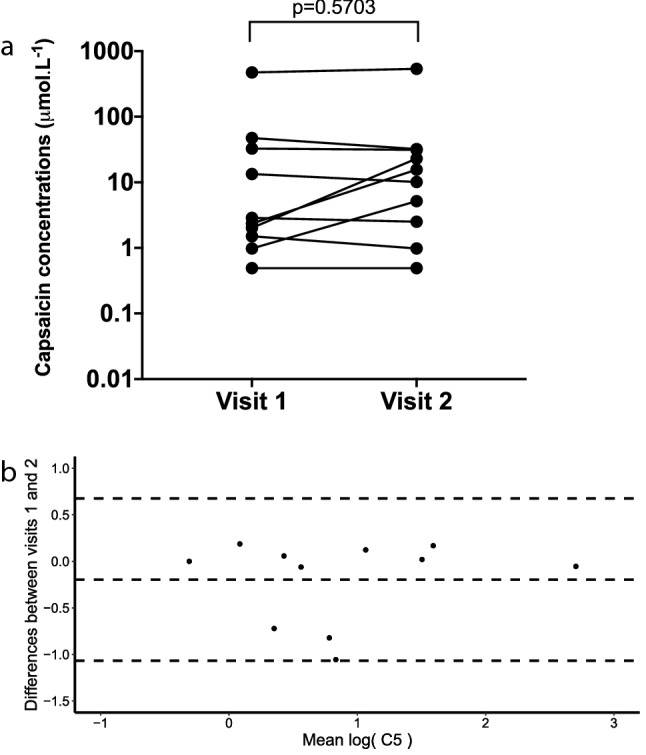
Repeatability of capsaicin cough challenge test threshold to elicit 5 coughs (C5) during capsaicin challenge test in stable chronic obstructive pulmonary disease. a C5 (6 weeks apart) in stable COPD. b Bland–Altman plot of C5 (6 weeks apart) in stable COPD
For objective cough frequency, the ICC coefficients for repeatability of log(total 24-h cough count), log(total cough frequency), log(awake cough count) and log(awake cough frequency) over 6 weeks were 0.86, 0.85, 0.70 and 0.84, respectively (Table 5). The mean differences (95% CI) in total cough frequency, awake cough count and awake cough frequency were 0.76 (0.47–1.22), 1.32 (0.76–2.29) and 0.11 (0.79–2.12) fold differences, respectively (Fig. 5). The ICC for repeatability of cough severity VAS scores over 6 weeks was 0.56 (Table 5 and Fig. 5).
Fig. 5.
Bland–Altman plot assessing the repeatability of threshold capsaicin concentrations during capsaicin challenge test, objective cough frequency and cough severity visual analogue scale over 6 weeks in participants with stable chronic obstructive pulmonary disease. a Total cough frequency. b Awake cough count. c Awake cough frequency. d Cough severity visual analogue scale
Discussion
We investigated the sensitivity of the cough reflex during exacerbations of COPD and its relationship with the frequency of future exacerbations. Cough reflex sensitivity (CRS) was heightened during exacerbations of COPD. The magnitude of change in cough reflex sensitivity 6 weeks after hospitalisation for exacerbation was associated with the frequency of future exacerbations. There was no association between subjective cough severity, objective cough frequency or FEV1 and the frequency of exacerbations. In stable COPD patients, C5 was the most repeatable measure of CRS.
To the best of our knowledge, our study is the first to investigate CRS during exacerbations of COPD. CRS to capsaicin was heightened during exacerbation. Viral upper respiratory tract infections are commonly associated with exacerbations of COPD [29]. There are studies which have investigated CRS in healthy subjects with acute cough associated with upper respiratory tract infection [13, 14]. O’Connell et al. demonstrated that CRS was heightened during upper respiratory tract infection, and reduced following 4 weeks of recovery in healthy participants with non-productive cough [13]. Dicpinigaitis et al. also reported that CRS to capsaicin was heightened during upper respiratory tract infection, and reduced following 4 weeks of recovery [14]. Capsaicin is a selective agonist of transient receptor potential vanilloid 1 ion channel receptors (TRPV1), which are expressed on C-fibre sensory nerves [30]. Bronchopulmonary C fibres are relatively quiescent in health and can be sensitised or activated by inflammatory mediators [11]. Exposure to ozone [12], lactic acid [12], cationic proteins [31] and prostaglandin-E2 [11] are known to enhance pulmonary C-fibre responses to mechanical and chemical stimuli in a reversible fashion. Airway inflammation in exacerbation of COPD could therefore lead to sensitisation or activation of C fibres.
A key finding from our study was that the magnitude of change in CRS following an exacerbation was associated with prospective frequency of exacerbations. The frequency of exacerbation in our study was comparable to those in previous studies [2, 17]. Further exacerbations were more frequent in individuals demonstrating a lower reduction in cough reflex sensitivity at six weeks after the index episode. Previous studies have investigated the predictive factors for exacerbations of COPD, including exacerbation frequency in the preceding 12 months, disease severity, gastro-oesophageal reflux and health status [17, 32]. A failure of CRS to change during recovery from exacerbation has not been previously studied as a potential marker of future exacerbation risk. Terada et al. conducted the only study of CRS and its association with exacerbations, and investigated CRS in patients with stable COPD [33]. In contrast to our findings, Terada et al. did not find an association between CRS and exacerbation of COPD defined by a change of medication [33]. There are several key differences between the studies that may explain the contrasting findings. Terada et al. assessed a single measure of CRS during a stable phase and did not assess it longitudinally for the persistence of CRS following exacerbation of COPD [33]. Terada et al. did however report a weak correlation with frequency of exacerbations of COPD when defined on the basis of a change of symptoms rather than a change in therapy for exacerbation of COPD [33].
A strength of the current study is that measurement of the change in CRS within the same individual at two time points controls for much of the large variation in cough reflex sensitivity that we have confirmed to exist between subjects in the stable state in COPD. This large inter-individual variation in CRS might explain the apparent lack of association between absolute values of C5 and COPD exacerbation frequency.
The mechanism by which a persistently heightened cough reflex sensitivity may predispose to future exacerbations is not clear. An associated persistence of airway inflammation following an exacerbation may be important but this has seldom been studied. Airway inflammation in patients with stable COPD however has been reported to predispose to exacerbations. Bhowmik et al. demonstrated that raised levels of cytokines, IL-6 and 8, in sputum were associated with frequent exacerbations [34]. Gompertz et al. reported reduced levels of sputum protease inhibitors in those with frequent COPD exacerbations [35]. Another possible mechanism for persistent cough reflex sensitivity, and predisposition for future exacerbation and symptoms, is changes in central neural pathways and cognitive influences, such as those that have been reported in inflammatory airway diseases such as asthma using functional neuroimaging [36].
The assessment of CRS may be a tool to identify patients at high risk of future exacerbations. The existing cough challenge methodology is not practical for routine clinical use and simpler protocols would need to be developed. The capsaicin challenge test was however well tolerated by severe COPD patients suffering from acute exacerbation study. This is the first study to assess cough reflex sensitivity during an exacerbation and may facilitate future research investigating the mechanisms involved in exacerbation of COPD. It is not possible to elucidate the mechanism between the magnitude of change in CRS following exacerbation and the frequency of future exacerbations from our study. A better understanding of this mechanism may lead to targeted therapy for the prevention of exacerbations.
Previous studies have investigated whether other measures of cough predict COPD exacerbation frequency, with conflicting findings. Burgel et al. and Seemungal et al. reported that the presence of self-reported cough is associated with exacerbation frequency in stable COPD [2, 7]. In contrast, Hurst et al.[17], in common with this current study, did not find an association between self-reported cough and numbers of exacerbation of COPD. Neither did we observe an association between objective cough counts and exacerbation frequency. Objective CRS, subjective symptom scales and objective cough counts assess very different dimensions of cough, and a poor association between them is well reported [37]. It is likely that CRS reflects the interaction between airway inflammation and sensory nerves.
There are limitations to our study. We studied a relatively small sample size and were therefore unable to assess the predictors of exacerbations of COPD with multivariate regression analysis. Our patients had severe COPD and therefore our findings should be interpreted with caution for those with mild disease. We were also unable to assess the effects of factors such as smoking status that may influence CRS due to the small sample size. To standardise the timing of the cough challenge test during the hospital admission, patients were assessed on the first day that they were deemed fit for a challenge test by the clinical and research teams. It is possible that the variability of the timing of the cough challenge test between the patients may have impacted the cough reflex sensitivity assessment. The assessment of CRS in the recovery phase may have been too soon after the exacerbation. We did, however, demonstrate that CRS is highly repeatable over 6 weeks in stable COPD with no order effect. Furthermore, the CRS values following recovery from exacerbation in our study were similar to those reported previously in stable COPD by Sumner et al.[38]. FEV1% predicted during exacerbation, following recovery and the change between 2 occasions were not associated with the frequency of future exacerbations despite previous reports of an association between severity of COPD according to FEV1% predicted and exacerbation frequency [17, 39]. This may be due to the small sample size and the relatively limited range of disease severity in our study. Patients with more severe disease were more likely to have exacerbations, and therefore be included in our study. The subjective cough measures were not associated with CRS, which may be due to the small sample size in our study. This is not unexpected as health status and CRS assess very different dimensions of cough, and is consistent with previous reports [37, 40]. We did not find an association between cough reflex sensitivity and objective cough frequency, and this is consistent with a previous study reporting a poor association [38].
The technology for measuring cough frequency in the current study has not yet been widely used in COPD, but is well established in chronic cough in other contexts [40]. Validity for our approach though is suggested by the similar magnitude and range of cough frequencies we report in stable COPD compared to those of a study by Sumner et al. using an alternative method to determine cough frequency [38]. Whilst there was a slight excess of female participants in our cohort, there was no significant difference in C5 between gender. A slight excess of female participants is consistent with other studies of patients hospitalised for exacerbation of COPD [16, 41]. Whilst C5 generally appeared a repeatable measure of cough reflex sensitivity, this observation was not universal; some participants with stable COPD demonstrated marked differences in C5 between the two timepoints. This may therefore impact the utility of the C5 test in future studies. We did not investigate the cause of COPD exacerbation or markers of inflammation in sputum and serum, and these should be assessed in future studies. We only assessed CRS with inhaled capsaicin. It is possible that COPD patients may respond differently to other tussive agents, and produce different results [42, 43].
In conclusion, cough reflex sensitivity is heightened during acute exacerbation of COPD and is associated with the frequency of future exacerbations. Further studies are needed to replicate our findings in a larger study, and to investigate the mechanisms for persistent cough reflex hypersensitivity as this may lead to targeted therapy for the prevention of COPD exacerbations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the patients for their participation in the study. We would like to thank the integrated respiratory team, Chest Unit and the staff of the COPD clinic at King’s College Hospital for their assistance in characterising the patients.
Author Contributions
Conception and design: SB, CJJ, PC, RT, HF and IP; Participants screening: PC; Study recruitment: PC; Data analysis: PC; Interpretation of data: PC, SB and RT; Drafting manuscript: PC, SB, RT, HF, CJJ and IP; Revised manuscript: SB, PC, RT, HF, CJJ and IP.
Funding
PC has received grant funding from Merck. SB has received grant funding from Merck and personal fees for consulting from Merck, Bayer, GSK, Shionogi, Bellus, NeRRe.
Compliance with Ethical Standards
Conflict of interest
HF, RT, IP and CJ declare no conflicts of interest.
Ethical Approval
Health Research Authority South East Scotland Research Ethics Service, 16/SS/0189.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The National Institute for Health and Care Excellence (NICE) (2018) Chronic obstructiv obstructive pulmonary disease in over 16s: diagnosis and managment. London [PubMed]
- 2.Seemungal T, Donaldson G, Paul E, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2009;117:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 5.Seemungal TAR, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 6.Crooks MG, Hayman Y, Innes A, et al. Objective measurement of cough frequency during COPD exacerbation convalescence. Lung. 2016;194:117–120. doi: 10.1007/s00408-015-9782-y. [DOI] [PubMed] [Google Scholar]
- 7.Burgel PR, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975–982. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 8.Foreman MG, DeMeo DL, Hersh CP, et al. Clinical determinants of exacerbations in severe, early-onset COPD. Eur Respir J. 2007;30:1124–1130. doi: 10.1183/09031936.00009307. [DOI] [PubMed] [Google Scholar]
- 9.Mazzone SB, Farrell MJ. Heterogeneity of cough neurobiology: clinical implications. Pulm Pharmacol Ther. 2019;55:62–66. doi: 10.1016/j.pupt.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Chung KF, McGarvey L, Mazzone SS. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013;1:414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 11.Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E2 enhances chemical and mechanical sensitivities of pulmonary C fibers the rat. Am J Respir Crit Care Med. 2000;162:528–533. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- 12.Ho C, Lee L. Ozone enhances excitabilities of pulmonary C fibers to chemical and mechanical stimuli in anesthetized rats. J Appl Physiol. 1998;85:1509–1515. doi: 10.1152/jappl.1998.85.4.1509. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell F, Thomas VE, Studham JM, et al. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med. 1996;90:279–286. doi: 10.1016/s0954-6111(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 14.Dicpinigaitis PV, Tibb AS, Ramsey DL, et al. Stability of cough reflex sensitivity during viral upper respiratory tract infection (common cold) Pulm Pharmacol Ther. 2014;28:154–157. doi: 10.1016/j.pupt.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence (2010) CG101 Chronic obstructive pulmonary disease (update): full guideline. London
- 16.Suh E-S, Mandal S, Harding R, et al. Neural respiratory drive predicts clinical deterioration and safe discharge in exacerbations of COPD. Thorax. 2015;70:1123–1130. doi: 10.1136/thoraxjnl-2015-207188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 19.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 20.Dicpinigaitis PV. Short- and long-term reproducibility of capsaicin cough challenge testing. Pulm Pharmacol Ther. 2003;16:61–65. doi: 10.1016/S1094-5539(02)00149-9. [DOI] [PubMed] [Google Scholar]
- 21.Prudon B, Birring SS, Vara DD, et al. Cough and glottic-stop reflex sensitivity in health and disease. Chest. 2005;127:550–557. doi: 10.1378/chest.127.2.550. [DOI] [PubMed] [Google Scholar]
- 22.Birring SS, Fleming T, Matos S, et al. The leicester cough monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J. 2008;31:1013–1018. doi: 10.1183/09031936.00057407. [DOI] [PubMed] [Google Scholar]
- 23.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 24.Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142:134–140. doi: 10.1378/chest.11-0309. [DOI] [PubMed] [Google Scholar]
- 25.Berkhof FF, Boom LN, ten Hertog NE, et al. The validity and precision of the leicester cough questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes. 2012;10:4. doi: 10.1186/1477-7525-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 27.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1600016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 28.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 29.Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:245–251. doi: 10.1513/pats.200512-125SF. [DOI] [PubMed] [Google Scholar]
- 30.Lalloo UG, Fox AJ, Belvisi MG, et al. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 31.Gu Q, Lee L. Hypersensitivity of pulmonary C fibre afferents induced by cationic proteins in the rat. J Physiol. 2001;537:887–897. doi: 10.1111/j.1469-7793.2001.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quint JK, Donaldson GC, Hurst JR, et al. Predictive accuracy of patient-reported exacerbation frequency in COPD. Eur Respir J. 2011;37:501–507. doi: 10.1183/09031936.00035909. [DOI] [PubMed] [Google Scholar]
- 33.Terada K, Muro S, Ohara T, et al. Cough-reflex sensitivity to inhaled capsaicin in COPD associated with increased exacerbation frequency. Respirology. 2009;14:1151–1155. doi: 10.1111/j.1440-1843.2009.01620.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gompertz S, Bayley DL, Hill SL, Stockley RA. Relationship between airway inflammation and the frequency of exacerbations in patients with smoking related COPD. Thorax. 2001;56:36–41. doi: 10.1136/thorax.56.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenkranz MA, Busse WW, Johnstone T, et al. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci USA. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faruqi S, Thompson R, Wright C, et al. Quantifying chronic cough: Objective versus subjective measurements. Respirology. 2011;16:314–320. doi: 10.1111/j.1440-1843.2010.01893.x. [DOI] [PubMed] [Google Scholar]
- 38.Sumner H, Woodcock A, Kolsum U, et al. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:943–949. doi: 10.1164/rccm.201211-2000OC. [DOI] [PubMed] [Google Scholar]
- 39.Müllerová H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4:e006171. doi: 10.1136/bmjopen-2014-006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birring SS, Spinou A. How best to measure cough clinically. Curr Opin Pharmacol. 2015;22:37–40. doi: 10.1016/j.coph.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Murphy PB, Kumar A, Reilly C, et al. Neural respiratory drive as a physiological biomarker to monitor change during acute exacerbations of COPD. Thorax. 2011;66:602–608. doi: 10.1136/thx.2010.151332. [DOI] [PubMed] [Google Scholar]
- 42.Long L, Yao H, Tian J, et al. Heterogeneity of cough hypersensitivity mediated by TRPV1 and TRPA1 in patients with chronic refractory cough. Respir Res. 2019;20:1–9. doi: 10.1186/s12931-019-1077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belvisi MG, Birrell MA, Khalid S, et al. Neurophenotypes in airway diseases: Insights from translational cough studies. Am J Respir Crit Care Med. 2016;193:1364–1372. doi: 10.1164/rccm.201508-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.