Abstract
Turnip is a vegetable that has many health promoting effects. To diversify the usage and increase the consumption of turnip, the effects of hot air drying, infrared drying, explosion puff drying and freeze drying (FD) on the volatiles of turnip chips were studied. The volatiles of fresh turnip and dried turnip chips were isolated by HS-SPME–GC–MS and a total of 67 volatiles were identified. However, the volatiles in turnip chips dried by different methods are quite different. Based on principal component analysis and hierarchical cluster analysis, the volatiles of fresh turnip were distinguished from those of the dried chips and FD was separated from the other drying methods. As the result of orthogonal projection on latent structure-discriminant analysis (OPLS-DA), isothiocyanato-cyclopropane and (2-isothiocyanatoethyl)-benzene were identified as the characteristic volatiles of fresh turnip. While, 2-azido-2,3,3-trimethyl-butane and hexanal were identified as the characteristic volatiles for FD dried chips.
Keywords: Turnip chips, Drying, Volatiles, HS-SPME–GC–MS, OPLS-DA
Introduction
Turnip (Brassica rapa L.) belongs to the Brassicaceae family, which includes many horticultural crops with economic significance, and is grown worldwide for food and/or feed purposes (Klopsch et al. 2017). Turnip has also been traditionally used as health food and/or herbal medicine in Tibet and Xinjiang, China (Zhang et al. 2014; Ma et al. 2016). Recently, turnip received much attention for its bioactive phytochemicals such as phenylpropanoids, glucosinolates, flavonoids, etc., and health promoting effects such as anti-inflammatory, anti-oxidative, anti-cancer, anti-diabetic, etc. (Darwish et al. 2016; Klopsch et al. 2017). However, the methods used for turnip processing are still original and limited. Turnips are mainly used as cooking ingredients in China and some turnips are dried for further processing or storage.
Crisp fruit and vegetable chips have become very prevalent recently in various online platforms for modern customers because of the appealing crispy texture, rich phytonutrients, reduced weight and extended shelf life (Zhang et al. 2010; Zou et al. 2013; Maity et al. 2018). Fried chips (vacuum frying or deep fat frying) are the mainstream in the market place due to the fine taste and crispy texture. However, the high oil content of the chips will cause oxidation and rancidity during the storage, which is also harmful to human health (Ikoko and Kuri 2007; Nunes and Moreira 2009). For freeze dried chips, the nutrients, taste, color and structure are all well-preserved, but the drying process is energy and time consuming (Majid et al. 2018; Wang et al. 2019). Explosion puffing drying (EPD), another efficient chips production technology, is characterized by crispy texture, fast rehydration and well-retained nutrients and porosity (Du et al. 2013). EPD has been experimented on many vegetables, such as mushroom (He et al. 2011), pumpkin (Gao and Zhang 2007), maize (Mrad et al. 2014), pepper (Tellez-Perez et al. 2015), sweetpotato (Jiang et al. 2010), potato (Sullivan et al. 1977), carrot and onion (Louka and Allaf 2002) etc. During the decompression (puffing) process of EPD, a sudden pressure drop results in rapid vaporization of the water within the cells of the vegetable and thus the expansion of internal vapour makes the material undergo a complex alveolation step, resulting in shortened drying time, volume expansion and high porosity of the product (Zou et al. 2013; Yi et al. 2016).
In our previous study, the effects of drying methods on the physicochemical properties of dried turnip chips were studied (Xue et al. 2019). However, the effect of drying methods on the volatile components of dried turnip chips has been scarcely studied, which will definitely affect consumers’ preference for processed vegetables. Therefore, to diversify the usage and increase the consumption of turnip, which shows many health benefits, turnip chips were dried by different methods and then the influence of drying methods on the volatile components of the chips were investigated using multivariate analysis. The findings from this study will deepen our understanding about the processing suitability of turnips.
Materials and methods
Materials
Tubers of turnip (Brassica rapa L.) were collected from Liangshan Prefecture, Sichuan Province, China. All reagents used in the present work were of reagent grade unless stated otherwise.
Sample preparation
Turnips were thoroughly washed by cleaned water and peeled. The peeled tubers were sliced into 4 mm thick slices and blanched in a solution containing 1% sodium chloride and 0.2% citric acid at 100 °C for 1 min. After rapid cooling, the slices were immersed into a 20% sucrose solution (soaking treatment) for 1 h. Then, the turnip slices obtained were frozen at − 18 °C overnight.
Drying process
Hot air drying (AD), infrared drying (ID), explosion puff drying (EPD) and freeze drying (FD) were conducted according to our previous study (Xue et al. 2019).
Volatile components (HS-SPME–GC–MS) analysis
2 g sample powder was transferred into a SPME vial with carboxen-polydimethylsiloxane (CAR/PDMS, 75 μm) fiber (Supelco, Bellefonte, PA, USA). The extraction of volatiles was performed at 50 °C for 1 h.
GC–MS was carried out using a TSQ 8000 Evo system (Thermo Fisher Scientific, Palo Alto, CA, USA). A TG-5MS fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) was used for separation with helium carrier gas at 1.2 mL/min constant flow. The injector was operated in split mode and kept at 250 °C. The oven temperature was programmed as: initial temperature 40 °C, hold 3 min, increased to 80 °C at 3 °C/min, hold 1 min, then raised to 150 °C at 5 °C /min, hold 1 min, and finally ramped to 280 °C at 10 °C /min, and held isothermal for 2 min. Mass spectra was recorded over a range of m/z 33–800 applying electron energy of 70 eV. The temperatures of ion source and transfer line were set to 280 and 300 °C, respectively. Volatile compounds were identified by comparing their mass spectra with those in the Wiley mass spectral library. The relative percentage contents of the volatiles were calculated by peak area normalization method.
Statistical analysis
Principal component analysis (PCA) was applied to evaluate the volatile composition of fresh and dried turnip chips. Hierarchical cluster analysis (HCA) and orthogonal projection on latent structure-discriminant analysis (OPLS-DA) were applied to classify the drying methods and identify their characteristic properties. SIMCA 15 Software (Umetrics, MKS Instruments Inc, Sweden) was used for all the PCA, HCA and OPLS-DA analyses.
Results and discussion
Volatile composition of fresh turnip and dried turnip chips
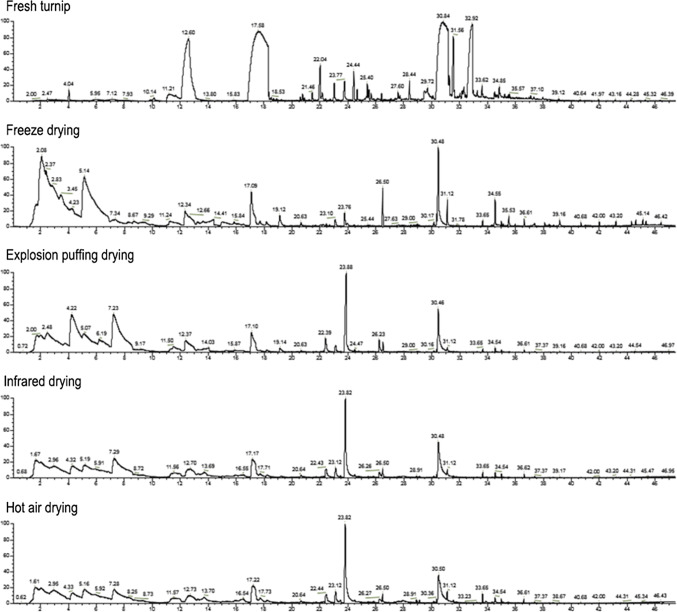
The GC–MS total ion chromatograms of volatile components in fresh turnip and turnip chips dried by different methods are shown in Fig. 1 and the relative contents of the volatile compounds are listed in Table 1. A total of 67 volatile compounds were detected in fresh turnip and turnip chips dried by different methods. There were 22, 22, 17, 15 and 15 kinds of volatile compounds identified in fresh turnip, FD, EPD, VD and AD dried samples, respectively. Esters, the major components of fruit volatile compounds, were the predominant volatile compounds in fresh turnip (92.51%) (El Hadi et al. 2013). Among them, isothiocyanato-cyclopropane (37.30%) and (2-isothiocyanatoethyl)-benzene (28.35%) were the most abundant volatile component in fresh turnip, which was also detected in mustard (Liu et al. 2010; Hu and Wei 2013).
Fig. 1.
GC–MS total ion chromatogram of volatile compounds in fresh turnip and turnip chips dried by different methods
Table 1.
Relative contents of volatile compounds in fresh turnip and turnip chips processed using different drying method
| No. | Category | Compound name | Ri | ID | Relative content (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh | FD | EPD | ID | AD | |||||
| Acids | Total content of acids | 0.39 | – | 8.73 | – | – | |||
| 1 | L-Cysteine sulfinic acid | 582.1 | MS | 0.39 ± 0.04 | – | – | – | – | |
| 2 | 5-Hexynoic acid | 1152.8 | MS | – | – | 8.73 ± 0.52 | – | – | |
| Esters | Total content of esters | 92.51 | 18.31 | 9.50 | 2.59 | 5.00 | |||
| 3 | 4-Isothiocyanato-1-butene | 1020.6 | MS, RI | 16.00 ± 0.87 | 5.89 ± 0.22 | 7.29 ± 0.56 | – | – | |
| 4 | Isothiocyanato-cyclopropane | 1152.5 | MS | 37.30 ± 1.55 | 8.76 ± 0.27 | – | – | – | |
| 5 | 4-Methylpentyl isothiocyanate | 1257.2 | MS, RI | 0.22 ± 0.02 | – | – | – | – | |
| 6 | N-(3-Oxo-4-isoxazolidinyl)-carbamic acid,benzyl ester | 1326.1 | MS | – | 0.75 ± 0.05 | – | – | – | |
| 7 | Benzyl glycolate | 1326.1 | MS | – | – | 1.19 ± 0.13 | – | – | |
| 8 | 1-Isothiocyanato-heptane | 1368.2 | MS | 0.80 ± 0.06 | 0.10 ± 0.02 | – | – | – | |
| 9 | 3-Methylhexyl isothiocyanate | 1374.7 | MS | 0.23 ± 0.03 | – | – | – | – | |
| 10 | 1-Ethyl-cyclohexanecarboxylic acid, methyl ester | 1397.0 | MS | – | 0.13 ± 0.01 | – | – | – | |
| 11 | 2-Methyl-propanoic acid, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl)propyl ester | 1478.7 | MS | – | – | – | 1.87 ± 0.08 | 3.65 ± 0.15 | |
| 12 | Dimethyl phthalate | 1553.8 | MS, RI | – | – | 0.09 ± 0.01 | – | – | |
| 13 | (2-Isothiocyanatoethyl)-benzene | 1576.7 | MS | 28.35 ± 2.03 | – | – | – | – | |
| 14 | 2-Methyl-pentanedioic acid, monomethyl ester | 1650.7 | MS | 8.83 ± 0.74 | – | – | – | – | |
| 15 | 2-Methyl-propanoic acid, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester | 1676.7 | MS | – | 0.30 ± 0.03 | 0.21 ± 0.02 | 0.72 ± 0.04 | 1.27 ± 0.11 | |
| 16 | 2-(Prop-2-enoyloxy)pentadecane | 1707.9 | MS | 0.24 ± 0.02 | – | – | – | – | |
| 17 | 2,6-Difluoro-3-methylbenzoic acid, decyl ester | 1709.1 | MS | – | 2.39 ± 0.16 | 0.72 ± 0.09 | – | – | |
| 18 | Chloromethyl 2-chlorodecanoate | 1720.8 | MS | 0.41 ± 0.02 | – | – | – | – | |
| 19 | Andrographolide | 1743.1 | MS | – | – | – | – | 0.08 ± 0.01 | |
| 20 | 7-Methyl-Z-tetradecen-1-ol acetate | 1806.0 | MS | 0.08 ± 0.02 | – | – | – | – | |
| 21 | 1,2-Benzenedicarboxylic acid, butyl decyl ester | 1841.3 | MS | 0.07 ± 0.01 | – | – | – | – | |
| Sulfones | Total content of sulfones | 0.52 | 1.71 | – | – | 10.43 | |||
| 22 | [(Methylsulfonyl)methyl]-benzene | 1326.8 | MS | 0.52 ± 0.04 | – | – | – | 10.43 ± 1.01 | |
| 23 | 2-Chloroethyl benzyl sulfone | 1346.8 | MS | – | 1.71 ± 0.27 | – | – | – | |
| Nitriles | Total content of nitriles | 0.79 | – | 72.32 | 64.24 | 50.03 | |||
| 24 | Cyclopropylacetonitrile | 738.8 | MS | – | – | 19.80 ± 1.77 | – | – | |
| 25 | 5-Cyano-1-pentene | 869.1 | MS, RI | – | – | 34.64 ± 3.59 | 30.50 ± 3.24 | 16.04 ± 1.19 | |
| 26 | 5-(Methylthio)-pentanenitrile | 1304.5 | MS | – | – | 3.02 ± 0.11 | 4.58 ± 0.27 | – | |
| 27 | Benzenepropanenitrile | 1348.7 | MS | 0.79 ± 0.07 | – | 14.87 ± 0.19 | 29.16 ± 2.13 | 33.99 ± 2.55 | |
| Aldehydes | Total content of aldehydes | 1.18 | 26.02 | – | – | 22.82 | |||
| 28 | Hexanal | 785.6 | MS, RI | – | 25.47 ± 2.44 | – | – | – | |
| 29 | Benzaldehyde | 987.3 | MS, RI | 1.18 ± 0.18 | – | – | – | – | |
| 30 | (E,Z)-2,6-Nonadienal | 1156.2 | MS, RI | – | – | – | – | 22.82 ± 2.24 | |
| 31 | Dodecanal | 1305.2 | MS, RI | – | 0.55 ± 0.06 | – | – | – | |
| Ketones | Total content of ketones | 1.02 | 0.41 | 0.02 | 20.77 | 5.96 | |||
| 32 | 5-Methyl-1-phenyl-1-hexanone | 1136.9 | MS | – | – | – | – | 2.75 ± 0.21 | |
| 33 | 3,4,4-Trimethyl-isoxazol-5(4H)-one | 1154.8 | MS | – | – | – | 19.06 ± 1.03 | – | |
| 34 | 2-Undecanone | 1395.9 | MS | 1.02 ± 0.08 | – | – | – | – | |
| 35 | 2,6-Bis(1,1-dimethylethyl)-2,5-cyclohexadiene-1,4-dione | 1595.9 | MS, RI | – | – | – | – | 0.49 ± 0.03 | |
| 36 | [1aR-(1aα,4aβ,8aS*)]-1,1a,5,6,7,8-Hexahydro-4a,8,8-trimethyl-cyclopropa[d]naphthalen-2(4aH)-one | 1700.9 | MS | – | – | – | – | 0.11 ± 0.01 | |
| 37 | 3,5-Bis(1,1-dimethylethyl)-4-hydroxy-2,4-cyclohexadien-1-one | 1708.4 | MS | – | 0.62 ± 0.05 | – | – | – | |
| 38 | 7-(Dydroxymethyl)-1,4-dioxaspiro[4.5]decan-8-one | 1708.8 | MS | – | – | – | 1.66 ± 0.07 | 2.04 ± 0.09 | |
| 39 | 2,6-Bis(1,1-dimethylethyl)-4-(1-oxopropyl)phenol | 1858.8 | MS | – | 0.17 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | – | |
| 40 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 1869.0 | MS, RI | – | – | – | 0.02 ± 0.00 | 0.10 ± 0.01 | |
| 41 | 9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione | 1967.7 | MS | – | 0.25 ± 0.02 | – | – | – | |
| Alcohols | Total content of alcohols | – | 1.01 | 1.94 | 4.01 | 0.05 | |||
| 42 | 5-Methyl-5-hexen-2-ol | 1058.4 | MS | – | – | – | 3.84 ± 0.28 | – | |
| 43 | 3-Methyl-4-heptanol | 1067.8 | MS, RI | – | – | 1.92 ± 0.17 | – | – | |
| 44 | 2-Nonen-1-ol | 1181.8 | MS, RI | – | 0.60 ± 0.04 | – | – | – | |
| 45 | [R-(R*,R*)]-α,4-Dimethyl-α-(4-methyl-3-pentenyl)-3-cyclohexene-1-methanol | 1613.3 | MS | – | – | – | – | 0.46 ± 0.03 | |
| 46 | 6-Tridecanol | 1631.3 | MS, RI | – | 0.04 ± 0.01 | – | – | – | |
| 47 | Octahydro-3,8,8-trimethyl-6-methylene-1H-3a,7-methanoazulen-5-ol | 1685.5 | MS | – | – | – | 0.14 ± 0.02 | – | |
| 48 | Octahydro-4a(2H)-naphthalenemethanol | 1731.6 | MS | – | 0.20 ± 0.01 | – | – | – | |
| 49 | 3-Hydroxy-7,8-dihydro-β-ionol | 1858.8 | MS | – | – | – | – | 0.05 ± 0.01 | |
| 50 | 2,3-Dihydro-1,1,3,3-tetramethyl-4,6-bis(1-methylethyl)-1H-inden-5-ol | 1871.8 | MS | – | 0.17 ± 0.02 | 0.03 ± 0.00 | 0.03 ± 0.00 | – | |
| Amines | Total content of amines | – | – | 3.20 | 3.07 | – | |||
| 51 | 1-Benzamido-N-benzyl-1-[α-(2-pyridylthio)benzylidene]acetamide | 994.9 | MS | – | – | 3.20 ± 0.22 | – | – | |
| 52 | Benzenemethanesulfonamide | 1326.7 | MS | – | – | – | 3.07 ± 0.21 | – | |
| Ethers | Total content of ethers | 1.70 | 2.39 | 0.94 | – | – | |||
| 53 | 3-Buten-1-yl ethenyl sulfide | 1208.4 | MS | – | 1.69 ± 0.12 | 0.94 ± 0.03 | – | – | |
| 54 | 9-Oxabicyclo[6.1.0]nonane | 1293.1 | MS | 1.70 ± 0.12 | – | – | – | – | |
| 55 | 3,4,5-Trimethoxydiphenyl ether | 1763.9 | MS | – | 0.07 ± 0.01 | – | – | – | |
| Heteroatoms | Total content of heteroatoms | – | 44.24 | 2.48 | – | – | |||
| 56 | 2-Azido-2,3,3-trimethyl-butane | 571.2 | MS | – | 44.24 ± 4.32 | – | – | – | |
| 57 | 1-(2-Methyl-1-propenyl)-aziridine | 1423.3 | MS | – | – | 2.47 ± 0.20 | – | – | |
| 58 | 3-Chloro-5,6,9,10-tetrahydro-5,7,8,9-tetramethyl-5,9-methanobenzocyclooctene | 1763.9 | MS | – | – | 0.01 ± 0.00 | – | – | |
| Alkanes | Total content of alkanes | 1.20 | 0.76 | – | 0.34 | 1.28 | |||
| 59 | 1-Methylene-1H-indene | 1277.1 | MS | 0.20 ± 0.02 | – | – | – | – | |
| 60 | 2,6,6-Trimethyl-decane | 1467.9 | MS | 0.33 ± 0.03 | – | – | – | – | |
| 61 | 2-Methyl-decane | 1494.3 | MS | 0.38 ± 0.05 | – | – | – | – | |
| 62 | 2,6-Dimethyl-decane | 1496.1 | MS | – | 0.07 ± 0.01 | – | – | – | |
| 63 | Cedrene | 1510.2 | MS, RI | – | – | – | 0.27 ± 0.02 | 1.28 ± 0.09 | |
| 64 | Nonadecane | 1675.6 | MS | 0.29 ± 0.01 | – | – | – | – | |
| 65 | 1,2,3,4,4a,5,8,9,12,12a-Decahydro-1,4-methanobenzocy clodecene | 1700.9 | MS | – | – | – | 0.07 ± 0.00 | – | |
| 66 | (5α)-Cholest-14-ene | 2121.6 | MS | – | 0.69 ± 0.01 | – | – | – | |
| Oximes | Total content of oximes | 0.09 | – | – | – | – | |||
| 67 | Methoxy-phenyl-oxime | 958.6 | MS | 0.09 ± 0.01 | – | – | – | – | |
| Total content | 99.41 | 94.86 | 99.14 | 95.03 | 95.57 | ||||
| Number of compounds | 22 | 22 | 17 | 15 | 15 | ||||
On the other hand, interestingly, the volatile compounds in turnip chips dried by different methods are quite different from each other. There is only one common volatile compound identified in all the chips: 2-Methyl-propanoic acid, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester (No. 15 in Table 1), but with low relative contents.
Nitriles, the characteristic volatile components of Cruciferaes, were the most abundant volatile compounds in EPD (72.32%), ID (64.24%) and AD (50.03%) dried chips (Liu et al. 2009). While, heterocyclics (44.24%), aldehydes (26.02%) and esters (18.31%) were identified as the major volatile compounds in FD dried chips. Besides, aldehydes (22.82%) and sulfones (10.43%) were also found abundant in AD dried chips, and ketones (20.77%) were found rich in ID dried chips.
Furthermore, isothiocyanates are abundant in cruciferous vegetables, which showed antibacterial and anticancer activities (Lin et al. 2000; Yuan et al. 2008). There were six isothiocyanate/isothiocyanato containing volatiles (3, 4, 5, 8, 9 and 13 in Table 1) detected in fresh turnip, but only three (3, 4 and 8) were maintained in FD dried chips and one (3) was left in EPD dried chips. Thus, FD is the best drying method for maintaining the bioactive volatiles.
PCA analyses
The results shown above indicate that the volatiles of turnip chips were affected by drying methods. However, mere visual inspection cannot properly detect differences between drying methods. Then, the GC–MS spectra were analyzed using principal component analysis (PCA) to explore the relative variability within the different turnip samples.
As shown in Fig. 2a, two principle components were generated in the PCA (based on the 67 identified volatile compounds in Table 1), accounting for 68% (36% for PC1 and 32% for PC2) of the total variance. Fresh turnip had positive scores along PCA axis 1 and can be separated from those dried turnip chips. Although all dried chips exhibited similar negative PC1 scores, FD (negative PC2 scores) was found different from the other drying methods (positive PC2 scores) along PC2. As results, EPD, ID and AD were tightly clustered together, and fresh turnip and FD were separated from those three, respectively. The loading plot of PCA (Fig. 2b) illustrated that the volatile compounds that contributed most for the scattering pattern of fresh turnip and the drying methods. Volatiles detected only in fresh turnip (1, 5, 9, 13, 14, 16, 18, 20, 21, 29, 34, 54, 59, 60, 61, 64 and 47 in Table 1) positioned most positively along PC1, whereas volatile No. 15 contributed most negatively, which was detected in all the dried chips. Volatiles detected only in FD dried chips (6, 10, 23, 28, 31, 37, 41, 44, 46, 48, 55, 56, 62 and 66 in Table 1) contributed most negatively along PC2, whereas volatile No. 27 contributed most positively, which was detected in fresh turnip and the other dried chips. Based on the PCA score plot (Fig. 2a), attempts were made to separate the volatiles. However, although all the exclusive volatiles were included in one group, there were several volatiles mixed in the wrong groups (such as 3, 4 and 8 in the fresh turnip group, and 53 in the FD group). Thus, the volatiles from fresh turnip and dried chips cannot be separated properly using PCA.
Fig. 2.
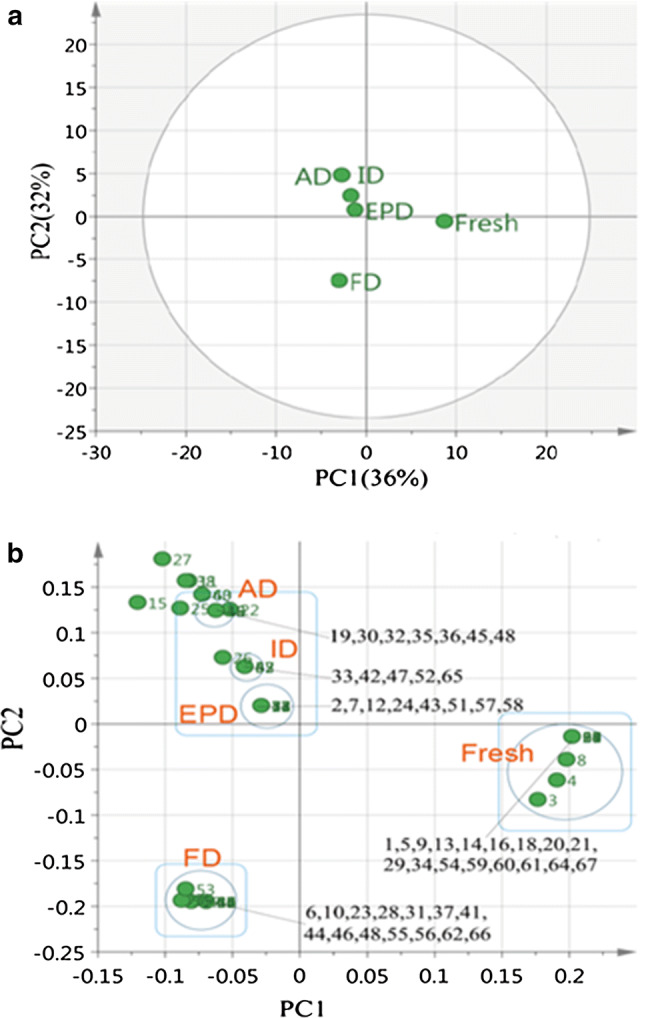
Principal component analysis of GC–MS data of fresh turnip and turnip chips dried by different methods. a Score plot of PC1 versus PC2 scores. b Loading plot for PC1 and PC2 contributing mass peaks and their assignments, with each volatile denoted by its number in Table 1
HCA analysis
Then, the data after PCA was applied to HCA to explore the heterogeneity of the samples. As illustrated in Fig. 3, the samples can be divided into two clear clusters: groups A (fresh turnip) and B (dried turnip chips). In addition, two sub-clusters were detected under group B: B1 (FD) and B2 (AD, EPD and ID). The grouping results are similar with the results of PCA (Fig. 2a), which illustrates that fresh turnip differ with their dried chips, and FD is different from the other drying methods. However, the characteristic volatiles could not be identified for each cluster using merely PCA and HCA.
Fig. 3.
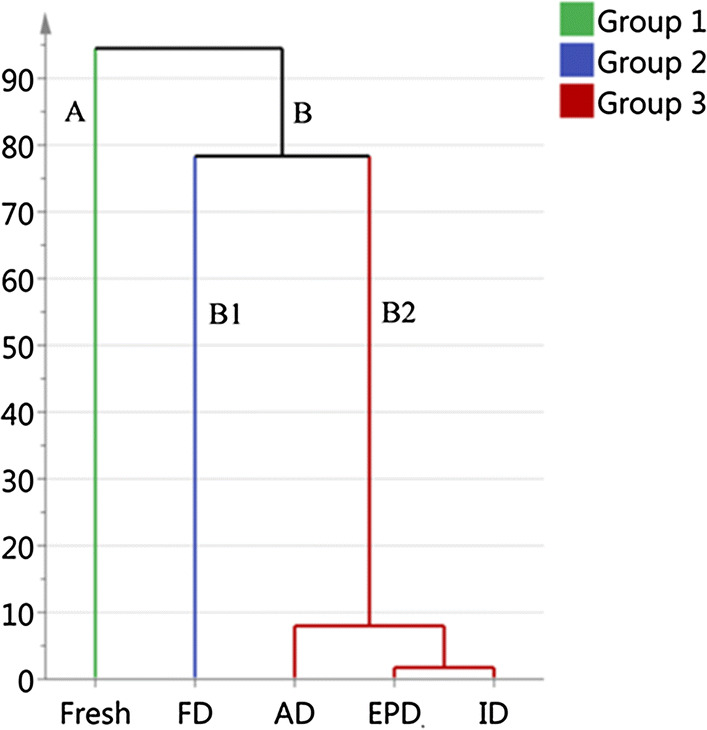
Hierarchical dendrogram for volatile compounds in fresh turnip and turnip chips dried by different methods
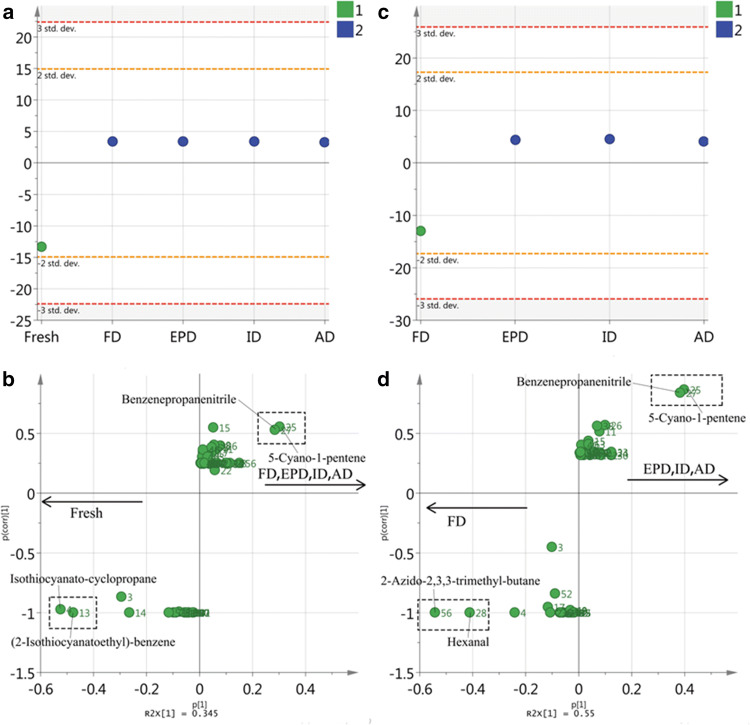
OPLS-DA analysis
OPLS-DA is a supervised multivariate analysis tool that can separate the predictive variation from orthogonal variation and sign characteristic markers for each cluster (Khalil et al. 2017), which was applied to try to identify the characteristic volatiles for each cluster in Fig. 3. As shown in the first OPLS-DA score plot (Fig. 4a), fresh turnip could be separated from the dried turnip chips. Total percentages of the variance (R2) explained by the constructed model is 84.4% with a 99.8% prediction goodness parameter (Q2). According to the S-plot (Fig. 4b), isothiocyanato-cyclopropane (4) and (2-isothiocyanatoethyl)-benzene (13) were identified as the characteristic volatiles of fresh turnip, which positioned to the most negative side of p[1] and was also detected in mustard (Liu et al. 2010; Hu and Wei 2013). Furthermore, in order to identify the characteristic volatiles of the chips dried by different methods, OPLS-DA was performed again, and to avoid the influence of fresh turnip volatiles, the data was deleted before the 2nd OPLS-DA. As shown in Fig. 4c, FD could be distinguished from the other drying methods with R2 = 79.3% and Q2 = 99.6%. According to the 2nd S-plot (Fig. 4d), 2-azido-2,3,3-trimethyl-butane (56) and hexanal (28) were identified as the characteristic volatiles of FD dried chips, while 5-cyano-1-pentene (25) and benzenepropanenitrile (27) were identified as the characteristic volatiles of EPD, ID and AD dried turnip chips. Among them, hexanal, which is a product of autoxidation of linoleic acid and a contributor to green aroma (Erten and Cadwallader 2017), is a common aroma volatile in fruits, such as apple, pear, kiwifruit, etc. (Musetti and Fava 2012). 5-cyano-1-pentene was also detected in the leaves of wheat (Shibamoto et al. 2007) and benzenepropanenitrile is a characteristic volatile component of Tai-tsai and Chinese cabbage (Wu et al. 2009; Song et al. 2010).
Fig. 4.
OPLS-DA score plot (a) and loading S-plots (b) derived from grouping fresh turnip modelled against dried chips in a separate group; OPLS-DA score plot (c) and loading S-plots (d) derived from grouping FD modelled against EPD, ID and AD in a separate group
As results, OPLS-DA properly separated fresh turnip from the dried chips, and FD was also distinguished from the other three drying methods. Furthermore, the characteristic volatiles of each class were identified.
Conclusion
Turnip chips were processed by AD, ID, EPD and FD, respectively. According to the results of HS-SPME–GC–MS, a total of 67 volatile compounds were isolated and identified from the turnip samples, and esters were the predominant volatiles in fresh turnip. The turnip chips dried by different methods differed greatly from each other in constituents and relative abundance of volatiles, and nitriles were the most abundant volatile compounds in AD, ID and EPD dried chips. After PCA, HCA and OPLS-DA analyses, isothiocyanato-cyclopropane and (2-isothiocyanatoethyl)-benzene were identified as the characteristic volatiles of fresh turnip; 2-azido-2,3,3-trimethyl-butane and hexanal were identified as the characteristic volatiles of FD dried chips; 5-cyano-1-pentene and benzenepropanenitrile were identified as the characteristic volatiles of ID, AD and EPD dried chips.
Acknowledgements
The authors would like to thank Dr. Jiangkuo Li from National Engineering and Technology Research Center for Preservation of Agricultural Products (Tianjin) for kindly sharing the SIMCA Software. This work was supported by the Special Fund for Agro-scientific Research in the Public Interest, Ministry of Agriculture (201503142), the China Postdoctoral Science Foundation (2017M611752), the Liaoning Revitalization Talents Program (XLYC1807270) and the Science and Technology Support Program from Shenyang City (17136800).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
You-lin Xue, Email: xueyoulin@lnu.edu.cn.
Chun-quan Liu, Email: liuchunquan2009@163.com.
References
- Darwish MM, Osman NN, Mfs F, Yonies BM. Turnip (Brassica rapa L.) tenuate liver and kidney damage induced by gamma irradiation in rats. BAOJ Pharm Sci. 2016;2(3):28–33. [Google Scholar]
- Du LJ, Gao QH, Ji XL, Ma YJ, Xu FY, Wang M. Comparison of flavonoids, phenolic acids, and antioxidant activity of explosion-puffed and sun-dried jujubes (Ziziphus jujuba mill.) J Agric Food Chem. 2013;61:11840–11847. doi: 10.1021/jf401744c. [DOI] [PubMed] [Google Scholar]
- El HadiZhang MFJ, Wu FF, Zhou CH, Tao J. Advances in fruit aroma volatile research. Molecules. 2013;18:8200–8229. doi: 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erten ES, Cadwallader KR. Identification of predominant aroma components of raw, dry roasted and oil roasted almonds. Food Chem. 2017;217:244–253. doi: 10.1016/j.foodchem.2016.08.091. [DOI] [PubMed] [Google Scholar]
- Gao W, Zhang PZ. Study on technology of puffing explosion for pumpkin chips. Sci Technol Food Ind. 2007;28:164–166. [Google Scholar]
- He XY, Liu JF, Cheng LL (2011) Effects of drying methods on the quality perpoties of dehydration mushroom. In: International conference on new technology of agricultural engineering, pp 941–944 (in Chinese)
- Hu SQ, Wei W. Study on extraction of wasabi plant material bio-activity substances and anti-cancer activities. Adv Mater Res. 2013;690–693:1395–1399. doi: 10.4028/www.scientific.net/AMR.690-693.1395. [DOI] [Google Scholar]
- Ikoko J, Kuri V. Osmotic pre-treatment effect on fat intake reduction and eating quality of deep-fried plantain. Food Chem. 2007;102:523–531. doi: 10.1016/j.foodchem.2006.06.008. [DOI] [Google Scholar]
- Jiang N, Liu CQ, Li DJ, Jin BQ. Explosion puffing technology optimization for sweet potato chips. Trans Chin Soc Agric Eng. 2010;26:361–367. [Google Scholar]
- Khalil MN, Fekry MI, Farag MA. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC-MS and chemometrics. Food Chem. 2017;217:171–181. doi: 10.1016/j.foodchem.2016.08.089. [DOI] [PubMed] [Google Scholar]
- Klopsch R, Witzel K, Börner A, Schreiner M, Hanschen FS. Metabolic profiling of glucosinolates and their hydrolysis products in a germplasm collection of Brassica rapa turnips. Food Res Int. 2017;100:392–403. doi: 10.1016/j.foodres.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Liu MC, Zheng-Guo L, Wang XY, Yang YW, Wang GM, Deng W. Analysis of volatile flavor compounds of pickled mustard tuber. Sci Technol Food Ind. 2010;31:118–121. [Google Scholar]
- Liu MC, Li ZG, Deng W, Wang GM, Yang YW. Changes in volatile compounds of pickled mustard tuber (Brassica juncea var. tsatsai) during the pickling process. Int J Food Sci Technol. 2009;44:2278–2286. doi: 10.1111/j.1365-2621.2009.02070.x. [DOI] [Google Scholar]
- Lin CM, Perston JF, Wei CI. Antibacterial mechanism of allyl Isothiocyanate. J Food Prot. 2000;63(6):727–734. doi: 10.4315/0362-028X-63.6.727. [DOI] [PubMed] [Google Scholar]
- Louka N, Allaf K. New process for texturizing partially dehydrated biological products using controlled sudden decompression to the vacuum: application on potatoes. J Food Sci. 2002;67:3033–3038. doi: 10.1111/j.1365-2621.2002.tb08855.x. [DOI] [Google Scholar]
- Ma G, Wang Y, Xuan Z. Analysis and comparison of nutritional compositions in Xinjiang turnip (Brassica rapa L.) Sci Technol Food Ind. 2016;37:360–364. [Google Scholar]
- Maity T, Bawa AS, Raju PS. Effect of preconditioning on physicochemical, microstructural, and sensory quality of vacuum-fried jackfruit chips. Dry Technol. 2018;36:63–71. doi: 10.1080/07373937.2017.1300590. [DOI] [Google Scholar]
- Majid I, Dar BN, Nanda V. Rheological, thermal, micro structural and functional properties of freeze dried onion powders as affected by sprouting. Food Biosci. 2018;22:105–112. doi: 10.1016/j.fbio.2018.01.012. [DOI] [Google Scholar]
- Mrad R, Rouphael M, Maroun RG, Louka N. Effect of expansion by “Intensification of Vaporization by Decompression to the Vacuum” (IVDV) on polyphenol content, expansion ratio, texture and colour changes of Australian chickpea. LWT Food Sci Technol. 2014;59:874–882. doi: 10.1016/j.lwt.2014.07.026. [DOI] [Google Scholar]
- Musetti A, Fava P. Sensory effects of hexanal vapor on fresh-cut slices of golden delicious apples. J Food Sci. 2012;77:S314–S318. doi: 10.1111/j.1750-3841.2012.02836.x. [DOI] [PubMed] [Google Scholar]
- Nunes Y, Moreira RG. Effect of osmotic dehydration and vacuum-frying parameters to produce high-quality mango chips. J Food Sci. 2009;74:E355–362. doi: 10.1111/j.1750-3841.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- Shibamoto T, Horiuchi M, Umano K. Composition of the young green barley and wheat leaves. J Essent Oil Res. 2007;19:134–137. doi: 10.1080/10412905.2007.9699245. [DOI] [Google Scholar]
- Song TY, Wu CY, Hou XL, He QW, Xu YF. SPME and GC-MS analysis of volatile components in three Tai-tsai cultivars. Food Sci. 2010;31:185–188. [Google Scholar]
- Sullivan JF, Konstance RP, Aceto NC, Heiland WK, Craig JC. Continuous explosion-puffing of potatoes. J Food Sci. 1977;42:1462–1463. doi: 10.1111/j.1365-2621.1977.tb08400.x. [DOI] [Google Scholar]
- Tellez-Perez C, Sobolik V, Abdulla G, Allaf K. Impact of swell-drying process on water activity and dry-ing kinetics of Moroccan pepper (Capsicum annum) Dry Technol. 2015;33:131–142. doi: 10.1080/07373937.2014.936556. [DOI] [Google Scholar]
- Wang Y, Sun JC, Ma D, Li X, Gao XX, Miao J, Gao WY. Improving the contents of the active components and bioactivities of Chrysanthemum morifolium Ramat.: the effects of drying methods. Food Biosci. 2019;29:9–16. doi: 10.1016/j.fbio.2019.03.003. [DOI] [Google Scholar]
- Wu CY, He QW, Song TY, Deng YL, Wang CH, Xu WL, Mu JH. GC–MS analysis of volatile components in Chinese cabbages. Food Sci. 2009;30:116–119. [Google Scholar]
- Xue YL, Chen JN, Han HT, Liu CJ, Gao Q, Li JH, Li DJ, Tanokura M, Liu CQ. Multivariate analyses of the physicochemical properties of turnip (Brassica rapa L.) chips dried using different methods. Dry Technol. 2019 doi: 10.1080/07373937.2019.1578971. [DOI] [Google Scholar]
- Yi J, Zhou L, Bi J, Chen Q, Liu X, Wu X. Influence of pre-drying treatments on physicochemical and organoleptic properties of explosion puff dried jackfruit chips. J Food Sci Technol. 2016;53:1120–1129. doi: 10.1007/s13197-015-2127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Chen BA, Liu DL. Anticancer mechanisms and researches of isothiocyanates. Chin J Nat Med. 2008;6(5):325–0332. [Google Scholar]
- Zhang M, Jiang H, Lim RX. Recent developments in microwave-assisted drying of vegetables, fruits, and aquatic products—drying kinetics and quality considerations. Dry Technol. 2010;28:1307–1316. doi: 10.1080/07373937.2010.524591. [DOI] [Google Scholar]
- Zhang Y, Tang W, Ni M, Li J, Nima D, Gong L, Chen X, Liu Y, Bian B, Wu X. Effects of Tibetan turnip and its pocessed products on human tolerance to hypoxia. Food Sci. 2014;35:178–182. [Google Scholar]
- Zou K, Teng J, Huang L, Dai X, Wei B. Effect of osmotic pretreat-ment on quality of mango chips by explosion puffing drying. LWT Food Sci Technol. 2013;51:253–259. doi: 10.1016/j.lwt.2012.11.005. [DOI] [Google Scholar]