Abstract
Pearl millet, a nutritionally remarkable cereal with a sustainable yield in the grey regions of India, is not consumed much. Consumption of Nutrition bars has gained momentum in recent years and considering this, in the present study pearl millet-based protein bars are formulated to increase its consumption rate and establish it as a reliable source of protein and other nutrients. The proximate and mineral composition of the three variants of pearl millet incorporated (25, 27.5, 30%) protein bars were analyzed using standard protocols. The acceptability of the bars was assessed using the 9-point hedonic scale among twenty panelists. The textural parameters were measured by Perten TVT 6700 Texture Analyzer. The in-vitro digestibility of protein (IVPD) and starch (IVSD) of the best variant was also estimated. The bars provide 15.74–18.32 g of protein, 332–379 kcal energy, 74.53–83.87 mg calcium, and 555.93–603.80 mg phosphorous per 100 g. The results showed that the organoleptic parameters of the bars were not affected by the proportion of ingredients. Whereas the increase in pearl millet incorporation marginally increased textural properties such as hardness, cohesiveness, and chewiness. The IVPD of the selected variant is 75.65 ± 0.02% and IVSD revealed 252.00 ± 10.00 mg of maltose is released per 100 g of the sample. The protein bars are nutritionally beneficial and appealing. This study gives scope for the production of pearl millet-based convenience foods that will raise the consumption pattern of pearl millet at the household level.
Keywords: Convenience food, Nutrition bar, Food security, Sensory analysis, Whey protein concentrate, Intermediate-moisture foods
Introduction
Nutritious and safe food is always in demand due to the ever-increasing population worldwide (Marques et al. 2014). Millets, a heterogeneous group of small-seeded cereal crops which are known for their small coarse grains (Weber and Fuller 2008), had a role in strengthening food security (Kumar et al. 2018). The dry regions of India have an adverse environment for vegetation, but millet has ensured sustaining crop yield in the grey areas due to their ability to withstand extreme climatic conditions, pH, the salinity of the soil, in a short cultivation period (Saxena et al. 2018).
Among the millets, pearl millet cultivation and yield were predominant in the grey areas. In the third world countries, for most parts of Africa and Asia, Pearl millet (Pennisetum glaucum) is considered as the future crop that ensures nutritional security for people. The nutritional quality of pearl millet makes it an essential source of dietary macronutrients, soluble and insoluble fibers, presence of resistant starch is also noted and provides an economical solution in combating micronutrient deficiency, as it is rich in iron, magnesium, phosphorus, zinc, folic acid, and riboflavin (Ragaee et al. 2006). The Food and Agriculture Organization Corporate Statistical Database (FAOSTAT) reported an upsurge in millet yield over the decades following the green revolution. In the late ’80s in India, the production of pearl millet was stable and soon high yielding hybrids were introduced, steadily increasing the production but per capita consumption reduced by 50–75% by 2000 (Yadav 2011). Since 2013 domestic supply quantity reduced drastically (FAOSTAT 2016). The usage of millets is curbed, mainly due to the lack of advanced technologies for its efficient processing and utilization (Sangeetha and Devi 2012).
Ready-to-eat products have garnered more attention among consumers since it is convenient and suits the palatability of the majority. Nutrition bars meet this trend as it is generally prepared from cereal grains, dried fruits, and nuts, encompassing sweetness and delightful flavor, which makes it more appealing. These nutrition bars are splendid sources of nutrients like vitamins, minerals, fiber, protein, and complex carbohydrates (Izzo and Niness 2001), further, these products use a variety of ingredients that cater to various segments of consumers (Palazzolo 2003). The production of bars gained momentum in recent years, and the global market has widened the diversity of these bars based on customer inclination. In 2007, the consumption rate of nutrition bars was increased to 11% globally, which is about USD 4 billion in markets (Sharma et al. 2014). TechSci Research (2019) published a report on nutrition bars market in India, stated that the Compound Annual Growth Rate (CAGR) is expected to grow at a rate of more than 30% in 2024, taking into consideration the growth of the working population, alarming increase of lifestyle diseases, better awareness of health and nutrition, rising per capita expenditure, and soaring youth population (Radhakrishnan 2005). The young consumers are also showing a keen interest in indigenously made nutrition bars (Padmashree et al. 2011). Keeping this in view the present research is carried out to formulate a protein bar from an underutilized protein source- pearl millet, which is economical as well as nutritious. Its nutritional parameters are enriched by the incorporation of whey protein and other commonly consumed protein sources. The protein bars’ nutritional composition, consumer preference, and measure of in-vitro digestibility of protein and starch are analyzed.
Materials and methods
Procurement of raw material
Pearl millet grains, peanut, soy nuts, raisins, honey, and sugar powder purchased from the local markets of Vellore, Tamilnadu. 80% Whey protein concentrate (with added digestive enzymes) was purchased online from the manufacturer.
Preparation of pearl millet protein bars
The ingredients: pearl millet (steamed and oven-dried), whey protein, roasted peanuts, roasted soy nuts, raisins, honey, and sugar powder were mixed in a bowl, poured into rectangle silicone molds and kept in the refrigerator for 2 h. The bars were then baked at 180 °C for 30 min. Each bar was 3.5 cm in width, 2.5 cm in height, and 6.5 cm in length and weighed approximately 50 g. It was packed individually in aluminum foil and stored in the refrigerator until further use. Three variants of the pearl millet protein bars (PMPB) were formulated: V1, V2, and V3 with 25%, 27.5%, and 30% incorporation of pearl millet respectively.
Nutritional analysis
The proximate composition and mineral composition of all the variants were analyzed using standard protocol.
Proximate composition
The moisture content of each sample was determined by drying 5 g of the sample in an oven at 105 °C for 16 h and weighed AOAC Method No: 934.01 (2005). The ash content in the different samples estimated by incinerating the samples in a muffle furnace at a temperature of 550 °C by following the procedure of AOAC Method No: 923.03 (2005). The carbohydrate content was determined using the Anthrone method as described by Sadasivam and Manickam (2005). The protein content was determined using the Kjeldahl method as described in AACC Method No: 46-10.01 (2010). Fat content was determined by hydrolyzing samples with diluted acid and extracted with petroleum ether using the Soxhlet apparatus according to AOAC Method No: 920.39 (2005). The total fiber estimated by digesting the fat-free samples in 1.25% sulfuric acid followed by 1.25% sodium hydroxide solution as mentioned in AACC Method No: 32-10.01 (2010). Atwater factor method was used to determine the energy value of each variant (Chima and Igyor 2007).
Mineral composition
Sodium, potassium, and iron in each sample were determined using the standard procedure as described by Sadasivam and Manickam (2005). Calcium was estimated using titrimetric method (AACC Method No: 40-20.01) (2010) and Zinc was estimated by Atomic Absorption Spectrophotometry (AACC Method No: 40-70.01) (2010). The phosphorus content of each sample was estimated by ashing a weighed amount of sample at 550 °C. The phosphorus was estimated through colorimetry by developing color with ammonium molybdate solution according to the phosphovanado-molybdate colorimetric method (AOAC Method No: 995.11) (2005).
Organoleptic attributes
The organoleptic properties of the pearl millet protein bars were evaluated at room temperature in a well- lit, clean, and properly ventilated room. A panel of twenty untrained judges examined and evaluated the product using a 9-point hedonic rating scale. The verbal degree of likeness is expressed on a numerical scale of 1–9, where 1 signifies dislike extremely, 5 denotes the neutral choice (neither like nor dislike), and 9 indicates like extremely. The participants were requested to evaluate the protein bars based on appearance, color, flavor, taste, texture, and overall acceptability. An explanation was given to assess these variables precisely before starting the evaluation.
Texture analysis
Instrument texture measurement is a cost-effective and novel approach to determine the textural characterization of a food product. The texture analyses of the three variants were carried out using Perten TVT 6700 Texture Analyzer for parameters such as Hardness (N), Springiness, Stickiness (N), Cohesiveness, Gumminess (N), and Chewiness (N). Calibration settings used were the 5 kg load cell and a 25 mm cylinder probe. The measurement mode settings for double-cycle compression were set to test speed of 5.0 mm/s and the probe height was adjusted to 5.0 mm from the sample. A trigger force of 50 mN was selected and the samples were compressed to 50%.
In vitro protein digestibility test (IVPD)
In vitro protein digestibility test was carried out for the sample with the highest protein content among the three variants by the method of pepsin/pancreatin digest followed by Prakash and Prakash (1999). 200 mg of the weighed sample was suspended in 15 ml of HCl (0.1 N) containing 1.5 mg pepsin and incubated at 37 °C for 3 h. The suspension was then treated with 4 mg of pancreatin in 7.5 ml of phosphate buffer (0.2 M, pH 8.0) after neutralizing with NaOH (0.5 N). The mixture was incubated at 37 °C for 24 h, treated with 10 ml of 10% TCA and centrifuged. Nitrogen in the supernatant was estimated by the Kjeldahl method. Suitable enzyme blanks were also run. Digestibility was calculated as percent protein digested. All the estimations were done in triplicate of samples individually.
In vitro starch digestibility
50 mg of the selected variant was mixed with 1 ml of Phosphate buffer (0.2 M, pH 6.9). In brief, 100 µl of the test samples were allowed to react with 200 µl of pancreatic α-amylase enzyme and incubated for 5 min. 500 µl of C7H4N2O7 (dinitrosalicylic acid) reagent was added to the test tubes and kept in a boiling water-bath for 10 min. After which 250 µl of C4H4O6KNa·4H2O (sodium potassium tartrate) solution was added. The test tube was to cooled and made up to 3 ml with distilled water. The absorbance was read at 540 nm using a spectrophotometer. A blank containing water and C7H4N2O7 reagent was run simultaneously (Dhami and Devi 2017). Standard maltose was used for calibration. The values were expressed in terms of mg maltose liberated/g of food bar.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 23 Software package. The analyses were done in triplicates and the data are subjected to descriptive statistics such as mean and standard deviation. The analysis of variance (ANOVA) followed by Post Hoc Duncan’s test (p < 0.05) is adopted to determine the nutritionally, organoleptically, and texturally superior variant. Pearson’s Correlation Coefficient test is done to establish the relationship between pearl millet concentration and nutrient content.
Results and discussions
Proximate composition
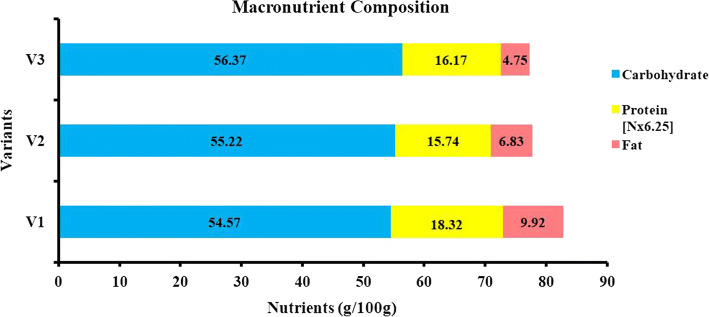
The proximate analysis of the PMPB revealed that the variant with 25% pearl millet (V1) had less carbohydrate and more protein and fat than the other variants (p < 0.05) (Fig. 1). It is important to highlight the fact that the findings of the analysis prove that the PMPB variants are high protein bars (HPB) with a protein content of 15.74–18.32% (Lu and Zhou 2019; Loveday et al. 2009). The distribution of calories in PMPB shows that 58–68% of the energy liberated from carbohydrates, 18–20% from protein, and 13–24% calories from fat per 100 g. Whereas, in HPB more calories from protein (31–35%) and fat (27–48%) and lesser energy yield from carbohydrates (21–40%) are preferred (the distribution is calculated based on percent weight of macronutrients in HPB) (Lu and Zhou 2019; Zhu and Labuza 2010). The crude fiber and total ash were low in all variants. The moisture content in PMPB (Table 1) is in agreement that high protein bars are intermediate moisture foods (10–40% moisture) (Banach et al. 2016).
Fig. 1.
Macronutrient composition of pearl millet protein bars
Table 1.
Proximate and mineral composition of pearl millet protein bars
| Nutritional composition | V1 | V2 | V3 | p value |
|---|---|---|---|---|
| Energy (kcal/100 g) | 379.33 ± 3.05c | 345.33 ± 1.52b | 332.66 ± 1.53a | 0.000 |
| Crude fibre (g/100 g) | 1.86 ± 0.04b | 1.53 ± 0.03a | 1.82 ± 0.03b | 0.000 |
| Moisture (g/100 g) | 16.52 ± 0.11a | 19.22 ± 0.22c | 18.46 ± 0.16b | 0.000 |
| Total ash (g/100 g) | 1.92 ± 0.03c | 1.74 ± 0.02b | 1.64 ± 0.01a | 0.000 |
| Phosphorous (mg/kg) | 603.80 ± 0.26c | 555.93 ± 0.473a | 595.30 ± 1.31b | 0.000 |
| Sodium (mg/kg) | 138.37 ± 0.67a | 144.87 ± 0.40b | 188.67 ± 0.86c | 0.000 |
| Potassium (mg/kg) | 397.47 ± 0.98c | 391.33 ± 0.85b | 349.20 ± 0.36a | 0.000 |
| Calcium (mg/kg) | 74.53 ± 0.75a | 83.87 ± 1.66c | 78.17 ± 0.64b | 0.000 |
| Iron (mg/kg) | 4.41 ± 0.04a | 6.36 ± 0.07b | 4.36 ± 0.02a | 0.000 |
| Zinc (mg/kg) | 1.53 ± 0.01a | 1.59 ± 0.01c | 1.57 ± 0.01b | 0.001 |
Values are expressed as mean and standard deviation (n = 3). Statistically significant at p < 0.05, where a,b,c indicate significance difference in each row
V1 Variant 1, V2 Variant 2, V3 Variant 3
The studies on millet-based nutrition bars (Vahini 2018; Bukya et al. 2018; Sobana 2017) have comparatively similar findings. The PMPB variants have higher protein content when compared with the results of previous studies (Vahini 2018; Sobana 2017). Bukya et al. (2018) developed a wheat-millet-legume nutrition bar with a higher fat (40%) and lesser carbohydrate content (29%) compared to PMPB. Since the nutrient profile of a food product is a manifestation of the quality and proportion of ingredients used. The relationship between quantity of pearl millet incorporated, and nutritional value of the bar is assessed (Table 2). Though pearl millet is the key ingredient, a significant relationship between pearl millet concentration and nutrient content couldn’t be established statistically.
Table 2.
The correlation between pearl millet concentration and nutrient content
| Variables | Pearl millet | Energy | Carbohydrates | Protein | Fat | Fiber | Moisture | Total ash |
|---|---|---|---|---|---|---|---|---|
| Pearl millet | 1 | |||||||
| Energy | − 0.97ns | 1 | ||||||
| Carbohydrates | 0.98ns | − 0.92ns | 1 | |||||
| Protein | − 0.78ns | 0.91ns | − 0.67ns | 1 | ||||
| Fat | − 0.99ns | 0.99ns | − 0.96ns | 0.85ns | 1 | |||
| Fiber | − 0.12ns | 0.37ns | 0.04ns | 0.71ns | 0.23 | 1 | ||
| Moisture | 0.69ns | − 0.86ns | 0.58ns | − 0.99ns | − 0.77ns | − 0.79ns | 1 | |
| Total ash | − 0.98ns | − 0.99* | − 0.94ns | 0.88ns | 0.99* | 0.30ns | − 0.82ns | 1 |
ns Non significant
*Significant at p < 0.05
Mineral composition
The mineral composition of the PMPB was significantly different among the variants (p < 0.05) (Table 1). The variant V1 is rich in potassium and phosphorous. V2 has a higher concentration of iron and zinc. Calcium and sodium is predominant in V3. It is evident that the proportion of pearl millet in the bars does not affect the mineral content of the bars. The amount of phosphorous, potassium, sodium, and calcium in the variants is notable when compared to the quantity of zinc and iron. The result conforms to previously published data (Vahini 2018; Sobana 2017; Padmashree et al. 2011).
Organoleptic attributes
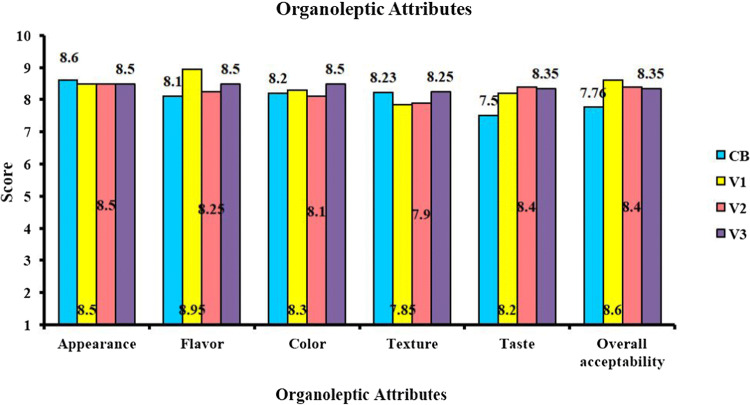
The organoleptic evaluation conducted to judge consumer preference of the variants indicated that the pearl millet nutrition bars were generally well accepted by the panelists for all sensory attributes evaluated (Fig. 2). Though the formulation influences the sensory qualities of the protein bars, there was no significant difference in the organoleptic score among the variants. When compared to the commercial standard, the pearl millet protein bars had a higher preference in terms of taste, flavor, color, and overall acceptability among the panelist. The participants commented on the after-taste and dry texture of the commercial bar.
Fig. 2.
Organoleptic attributes of pearl millet protein bars. CB commercial bar, V1 Variant 1, V2 Variant 2, V3 Variant 3
As Stefan (2003) had suggested, protein bars harden during storage resulting in lower consumer acceptance. The protein in nutrition bars tends to produce off-flavors and after-taste during prolonged storage (Lu and Zhou 2019). Since commercial bar used for the sensory evaluation had a much prolonged time gap from manufacture to consumption when compared to the pearl millet protein bars. The time frame influenced the sensorial properties of the bars. Moreover, whey protein (WPC) and soy protein (soy nuts) give a sweeter aroma and smoother texture to the pearl millet protein bars (Childs et al. 2007). The panelist remarked on the no after tastes and easily chewable, moist nature of the pearl millet protein bars. Nevertheless, the appearance and texture of the commercial standard had marginally better scores.
Texture analysis
The texture of processed foods is a salient sensory quality, which can be modified to achieve a desirable attribute by monitoring the processing technology and formulation. The texture analyzers adopt the force–deformation mechanism to mimic the mouthfeel, and the double cycle compression mode is a simulation of mastication (two bites) (Lu and Tipper 2009). The variants have almost similar textural characteristics (p < 0.05) (Table 3). The instrumental result corresponds with the organoleptic evaluation of the bars (Fig. 2). The sensory perception by consumers as well showed an insignificant difference among the variants. Interestingly, the increase in pearl millet (25–30%) and WPC (10–15%) content in the bars, marginally increase textural properties such as hardness, cohesiveness, and chewiness. Compared to the commercial bar, the PMPB bars are softer and easy to chew. There’s a significant difference between the textural attributes of the variants and the commercial bar (Table 3).
Table 3.
Textural characteristics of pearl millet protein bars
| Texture parameters | CB | V1 | V2 | V3 | p value |
|---|---|---|---|---|---|
| Hardness (N) | 34.41 ± 21.10a | 3.08 ± 00.65b | 4.62 ± 02.37b | 4.93 ± 02.97b | 0.019 |
| Springiness | 0.42 ± 0.27b | 0.92 ± 00.15a | 0.65 ± 00.34a | 0.93 ± 0.08a | 0.075 |
| Stickiness (N) | − 1.33 ± 0.40a | − 0.15 ± 0.03b | − 0.15 ± 0.2b | − 0.16 ± 0.03b | 0.000 |
| Cohesiveness | 0.03 ± 0.02a | 0.12 ± 0.04a | 0.23 ± 0.17a | 0.42 ± 0.37a | 0.204 |
| Gumminess (N) | 2.13 ± 2.32a | 0.38 ± 00.22a | 0.82 ± 00.46a | 2.84 ± 3.76a | 0.535 |
| Chewiness (N) | 1.27 ± 1.86a | 0.35 ± 0.22a | 0.62 ± 0.54a | 2.82 ± 3.77a | 0.521 |
Values are expressed as mean and standard deviation (n = 3). Statistically significant at p < 0.05, where a,b indicate significance difference in each row
CB commercial bar
As mentioned earlier, HPB hardens as time passes by. The water–protein interaction plays a modifying role in the textural attributes of the HPB. Studies have suggested that moisture migrates to the protein sources leading to aggregation of whey protein and crystallization of sugar, which may increase the hardness of the bars. Past literature has tried to establish the effect different protein sources have on HPB texture (McMahon et al. 2009; Banach et al 2016; Lu and Zhou 2019). The studies, however, focused on protein powders and hydrosylates, as they’re the principal ingredients in any protein bar. In this study, whey protein concentrate is used as a protein source, which is known to reduce the firmness and stability of the bars (Imtiaz et al. 2012). This may be the reason for the reduced hardness of the PMPB than the commercial bar.
In vitro protein and starch digestibility
In vitro digestibility analysis helps us to understand the percentage of nutrients that are available for absorption. The protein digestibility of the selected variant was 75.65 ± 0.02%. The starch digestibility of the variant revealed that 252.00 ± 10.00 mg of maltose was released per 100 g of the sample. The results imply that while most of the protein is digestible, the maltose release is limited. A comparable study on IVPD of the sorghum-based protein bar reported a maximum digestibility of 68.10% protein and a minimum of 67.90% (Verma et al. 2018). In the same study, the sorghum-based protein bar had the highest starch digestibility value of 45.55 mg maltose released per gram and a lowest of 44.38 mg maltose released per gram. It is observed that in cereal-based protein bars, the digestibility of protein is substantially augmented. In comparison, starch digestibility is considerably lesser.
The processing of cereals or cereal-based food products alters the nutrient profile and the bioavailability of nutrients. The in-vitro protein digestibility of unprocessed pearl millet grain (45.5–49.3%) is substantially lower than dehulled or roasted or germinated grains (80%). The enhancement of protein digestibility is due to the elimination of anti-nutrients, degradation of complex protein to simple and easily digestible protein, which increases the surface area of protein molecules exposed to enzymatic reactions. In contrast, the starch content, as well as starch digestibility of pearl millet, reduces if soaked or dehulled or germinated. But dry-treatment (roasting) doesn’t impact the digestibility of starch though it reduces the amount of starch (Pushparaj and Urooj 2017). The processing methods adopted in the development of pearl millet protein bars certainly influenced its nutrient digestibility. The whey protein concentrates added in the bar were pre-mixed with digestive enzymes that also improve the digestibility of protein in the bar (Mohan 2018). The enhanced protein digestibility of the PMPB signifies that pearl millet incorporated food products can be marketed as a reliable protein source.
Conclusion
Millets are one of the most researched cereals and its importance is well recognized in the field of food and nutrition. Still, there is a decline in the consumption of these nutritionally exceptional crops, due to the challenge in processing the grains, fewer choices of delicious food products, change in lifestyle, and urbanization. The primary objective of this study is to establish and recognize pearl millet as a good source of protein. The bars formulated have a good protein content of 15.74–18.32 g% and an in vitro protein digestibility of 75.65%. The organoleptic and textural analysis reveals that the protein bars formulated have an immense possibility to be accepted by the consumers. Therefore, more ready-to-eat products using pearl millets needs to be developed to increase their consumption.
Acknowledgements
The principal author expresses her sincere gratitude to the University Grants Commission for NET-Senior Research Fellowship (UGC- Ref. No.: 1460/(NET-DEC. 2013)).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kerenhappuch Susan Samuel, Email: happuch.avril@gmail.com.
Nazni Peerkhan, Email: naznip@gmail.com.
References
- AACC (2010) Approved methods of analysis, 11th edn. Method 46-10.01. Method 32-10.01, Method 40-20.01. Method 40-70.01 Cereals and Grains Association, St. Paul, MN, USA
- AOAC Authors (2005) Official methods of analysis, 18th edn. Association of Analytical Communities, Gaithersburg, MD. Reference data: Method 934.01, Method 923.03, Method 920.39, Method 995.11
- Banach JC, Clark S, Lamsal BP. Instrumental and sensory texture attributes of high-protein nutrition bars formulated with extruded milk protein concentrate. J Food Sci. 2016;81(5):S1254–S1262. doi: 10.1111/1750-3841.13270. [DOI] [PubMed] [Google Scholar]
- Bukya A, Ankush SA, Nagesh GD. Development, characterization and optimization of cereal, legume and millet incorporated nutri-bar and its storage studies. Int J Sci Res. 2018;7(3):81–83. [Google Scholar]
- Childs JL, Yates MD, Drake MA. Sensory properties of meal replacement bars and beverages made from whey and soy proteins. J Food Sci. 2007;72(6):S425–S434. doi: 10.1111/j.1750-3841.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- Chima CE, Igyor MA. Micronutrients and anti-nutritional contents of selected tropical vegetables grown in SouthEast, Nigeria. Niger Food J. 2007 doi: 10.4314/nifoj.v25i1.33659. [DOI] [Google Scholar]
- Dhami P, Devi SS. Estimation of in vitro starch digestibility (IVSD) in the fermented rice and ragi based products. Int J Pure Appl Biosci. 2017;5(4):1406–1411. doi: 10.18782/2320-7051.2855. [DOI] [Google Scholar]
- FAO (2016) https://www.fao.org/faostat/en/#data/QC. Accessed 21 Aug 2019
- Izzo M, Niness K. Formulating nutrition bars with inulin and oligofructose. Cereal Foods World. 2001;46(3):102–106. [Google Scholar]
- Kumar A, Tomer V, Kaur A, Kumar V, Gupta K. Millets: a solution to agrarian and nutritional challenges. Agric Food Secur. 2018 doi: 10.1186/s40066-018-0183-3. [DOI] [Google Scholar]
- Loveday SM, Hindmarsh JP, Creamer LK, Singh H. Physicochemical changes in a model protein bar during storage. Food Res Int. 2009;42(7):798–806. doi: 10.1016/j.foodres.2009.03.002. [DOI] [Google Scholar]
- Lu R, Tipper NC. A portable device for the bioyield detection to measure apple firmness. Appl Eng Agric. 2009;25(4):517–523. doi: 10.13031/2013.27455. [DOI] [Google Scholar]
- Lu N, Zhou P (2019) Whey protein-based nutrition bars. In: Whey protein, pp 495–517. 10.1016/b978-0-12-812124-5.00014-x [DOI]
- Marques TR, Corrêa AD, de Carvalho-Alves AP, Simão AA, Pinheiro ACM, de Oliveira RV. Cereal bars enriched with antioxidant substances and rich in fiber, prepared with flours of acerola residues. J Food Sci Technol. 2014;52(8):5084–5092. doi: 10.1007/s13197-014-1585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DJ, Adams SL, McManus WR. Hardening of high-protein nutrition bars and sugar/polyol-protein phase separation. J Food Sci. 2009;74(6):E312–E321. doi: 10.1111/j.1750-3841.2009.01225.x. [DOI] [PubMed] [Google Scholar]
- Mohan M (2018) Do we need to supplement with digestive enzymes for digesting whey? https://asitisnutrition.com/blogs/health/do-we-need-to-supplement-with-digestive-enzymes-for-digesting-whey. Accessed 3 Mar 2020
- Padmashree A, Sharma GK, Srihari KA, Bawa AS. Development of shelf stable protein rich composite cereal bar. J Food Sci Technol. 2011;49(3):335–341. doi: 10.1007/s13197-011-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo G. Cereal bars: they're not just for breakfast anymore. Cereal Foods World. 2003;48(2):70. [Google Scholar]
- Prakash VHP, Prakash J. In vitro protein digestibility of legumes cooked with spices. Nahrung. 1999;43(1):19–21. doi: 10.1002/(SICI)1521-3803(19990101)43:1<19::AID-FOOD19>3.0.CO;2-R. [DOI] [Google Scholar]
- Pushparaj FS, Urooj A. Impact of household processing methods on the nutritional characteristics of pearl millet (Pennisetum typhoideum): a review. MOJ Food Process Technol. 2017 doi: 10.15406/mojfpt.2017.04.00082. [DOI] [Google Scholar]
- Radhakrishan R. Food and nutrition security of the poor. Econ Political Wkly. 2005;18:1817–1821. [Google Scholar]
- Ragaee S, Abdelaal E, Noaman M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006;98(1):32–38. doi: 10.1016/j.foodchem.2005.04.039. [DOI] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods . 2. New Delhi: New Age Int. Publishers; 2005. [Google Scholar]
- Sangeetha N, Devi M. Effect of dehydration on the quality characteristics of extruded pasta using millet milk powder. J Nutr Food Sci. 2012 doi: 10.4172/2155-9600.1000175. [DOI] [Google Scholar]
- Saxena R, Vanga S, Wang J, Orsat V, Raghavan V. Millets for food security in the context of climate change: a review. Sustainability. 2018;10(7):2228. doi: 10.3390/su10072228. [DOI] [Google Scholar]
- Sharma C, Kaur A, Aggarwal P, Singh B. Cereal bars—a healthful choice a review. Carpathian J Food Sci Technol. 2014;6(2):29–36. [Google Scholar]
- Sobana RM. Quality evaluation of millet based composite sports bar. Int J Food Sci Nutr. 2017;2(4):65–68. [Google Scholar]
- Sr I, Kuhn-Sherlock B, Campbell M. Effect of dairy protein blends on texture of high protein bars. J Texture Stud. 2012;43(4):275–286. doi: 10.1111/j.1745-4603.2011.00337.x. [DOI] [Google Scholar]
- Stefan J (2003) Made to last. Prepared Foods. https://www.preparedfoods.com/articles/103794-made-to-last. Accessed 29 Feb 2020
- TechSci Research (2019) https://www.techsciresearch.com/report/india-nutritional-bars-market/2095.html. Accessed 2 Aug 2019
- Vahini V. Nutritional and microbiological assessment of nutrient enriched millet bar for adolescent female athletes. Int J Eng Sci Res. 2018;8(1, Special Issue ICON17-MCC):204–208. [Google Scholar]
- Verma S, Khetrapaul N, Verma V. Development and standardisation of protein rich sorghum based cereal bars. Int J Curr Microbiol Appl Sci. 2018;7(05):2842–2849. doi: 10.20546/ijcmas.2018.705.330. [DOI] [Google Scholar]
- Weber SA, Fuller DQ. Millets and their role in early agriculture. Pragdhara. 2008;18(69):e90. [Google Scholar]
- Yadav OP (2011) Review of pearl millet research. In: Proceedings of 46th annual pearl millet workshop of all India coordinated pearl millet improvement project (AICPMIP) on 12–14 March 2011, Hisar, Jodhpur, Rajasthan, India
- Zhu D, Labuza TP. Effect of cysteine on lowering protein aggregation and subsequent hardening of whey protein isolate (WPI) protein bars in WPI/buffer model systems. J Agric Food Chem. 2010;58(13):7970–7979. doi: 10.1021/jf100743z. [DOI] [PubMed] [Google Scholar]