Abstract
Objectives
The accuracy of FRAX® as a screening tool to identify osteoporosis and how it compares with tools such as Osteoporosis Self-Assessment Tool for Asians (OSTA), in Southeast Asian women has so far been unexplored. We aimed to determine the FRAX® thresholds that accurately identify densitometric osteoporosis and to compare its performance with that of OSTA for this purpose.
Methods
Singaporean postmenopausal women (n = 1056) were evaluated. FRAX® Major Osteoporotic Fracture Probability (MOFP), Hip Fracture Probability (HFP) scores, and OSTA indices were calculated. Receiver operating characteristic (ROC) curves were constructed and via the Youden index, the optimal cut-off points of balanced sensitivity and specificity for dual energy X-ray absorptiometry (DXA)-defined osteoporosis were identified and the performance characteristics were compared.
Results
A FRAX® MOFP threshold of ≥3.7% had sensitivity, specificity, positive predictive value and negative predictive value of 0.78 (0.73–0.83), 0.63 (0.59–0.66), 0.4 (0.36–0.44), and 0.9 (0.87–0.92), respectively in identifying osteoporosis. The corresponding values for a HFP threshold of ≥0.6% were 0.85 (0.80–0.89), 0.58 (0.55–0.62), 0.39 (0.35–0.43), and 0.92 (0.9–0.94) and that for an OSTA index cut-off of ≤ −1.2 were 0.76 (0.70–0.81), 0.74 (0.71–0.77), 0.48 (0.43–0.54), and 0.91 (0.88–0.93). The area under the ROC curves were 82.8% (79.9%–85.6%), 77.6% (74.2%–81%), and 79.6% (76.5%–82.8%) for OSTA, MOFP, and HFP thresholds respectively.
Conclusions
FRAX® and OSTA perform comparably in identifying osteoporosis in our population. OSTA has only 2 parameters and may be simpler to use. However, FRAX® may also have a role in primary screening to identify the postmenopausal woman to be referred for DXA scanning and may help facilitate fracture risk reduction discussions with the patient.
Keywords: Asia, Assessment threshold, FRAX, OSTA, Osteoporosis, Screening
1. Introduction
Early identification of patients at risk of osteoporosis is an important step to address the inevitable rise of fragility fractures associated with ageing populations. Though screening for osteoporosis remains a contentious issue, tools that help to assess the likelihood of osteoporosis are many. Currently available screening guidelines recommend either dual energy X-ray absorptiometry (DXA) scanning for at-risk populations below certain age thresholds or universal DXA scanning beyond those. Organizations such as the National Osteoporosis Foundation [1], American Association of Clinical Endocrinologists [2] and the United States Preventive Task Force (USPTF) [3] recommend that all women aged 65 years and above should have a screening DXA. The USPTF also recommends bone mineral density (BMD) testing in women younger than 65 years, if their 10-year predicted risk of major osteoporotic fracture is ≥ to the risk of a 65-year-old woman with no other FRAX® clinical risk factors [3].
Though the availability of DXA is not a significant problem in Singapore with 16.9 DXA machines available per 1 million population (https://iofbonehealth.org/data-publications/regional-audits/asia-pacific-regionalaudit) recommendations for universal DXA screening of women above a certain age cannot be made in most other countries in Asia due the lack of easy access to DXA scanning, nor in Singapore given the associated cost constraints despite easy availability of the resource in the latter country. This approach is thus unlikely to be accepted by physicians and the public alike in this part of the world. Using BMD testing as a modality to screen populations as a whole may also be limited by the fact that this test has a high specificity but low sensitivity, with a large number of osteoporotic fractures occurring in individuals with osteopenic and normal bone mineral densities [4]. Thus, universal testing of BMD is unlikely to have a meaningful impact on the burden of fractures.
In Singapore, guidance for osteoporosis screening is provided through Clinical Practice Guidelines (Appropriate Care Guidance, ACG), published by the Agency for Care Effectiveness of the Ministry of Health (ace-hta.gov.sg/our-guidance/osteoporosis-identification-and-management-in-primary-care.html. Last accessed February 9 2020). The guidance rests on the principle of an opportunistic strategy, wherein it is recommended that BMD screening should be employed selectively through a case-finding approach based on an individual’s risk for low bone mass. The Singapore ACG recommends using the Osteoporosis Self-Assessment Tool for Asians (OSTA); a simple tool based on age and weight that was developed for use in postmenopausal Asian women and recommends those identified as high risk be referred for BMD measurement [5].
The inclusion of clinical risk factors enhances the performance of BMD in predicting fractures. Several clinical risk scores have been published in recent years for estimating absolute fracture risk over fixed time periods. The World Health Organization Collaborating Centre for Metabolic Bone Diseases at Sheffield developed FRAX®, an algorithm to compute age specific fracture probabilities according to an individual’s clinical risk factor information with or without BMD measurement [6]. Whether FRAX® which was developed to estimate fracture probabilities and guide treatment decisions can be used as an osteoporosis screening tool remains controversial. It is felt that if the objective of screening is to identify individuals with T scores of −2.5 or lesser, then it is more appropriate to use tools that detect osteoporosis rather than a tool to assess fracture risk. A few studies appear to suggest that FRAX® may perform inferior to screening tools such as the Simple Calculated Osteoporosis Risk Estimate and Osteoporosis Self-Assessment Tool to identify individuals with osteoporotic BMD ranges [[7], [8], [9]]. On the other hand, individuals designated by FRAX® as having high risk for major osteoporotic fracture have been shown to have a T-score in the osteoporotic range at one or more BMD measurement sites [10]. A potential benefit in recommending screening thresholds of FRAX® to health care professionals is that it may help them to discuss the patient’s individual absolute fracture risk at the time of consultation in addition to only just identifying the risk of osteoporosis. It may thus facilitate discussions on fracture prevention also.
Singapore is an island nation in Southeast Asia with a unique multi-ethnic population of Chinese, Malays and Indians (https://singstat.gov.sg/find-data/search-by-theme/population/population-and-population-structure/latest-data-; Accessed February 9, 2020). Local estimates indicate that the overall prevalence of osteoporosis is ∼37% in women over the age of 50 years [11]. A fall in the age-standardized incidence rates of hip fractures have been observed in Singapore in the years 2000–2016 compared to 20 years ago. Hip fracture rates in Singapore are declining in women ≥70 years and in men between the ages of 75–85 [12]. However, despite these reduced incidence rates over time, steep increases in the aging population are driving a rise in the absolute number of hip fractures and the health economic burden of osteoporosis and osteoporotic fractures remain huge and is projected to increase exponentially over the next several years. Recently, an analysis of the health economic burden of osteoporosis in Singapore estimated the total economic burden (including direct and indirect costs to society) associated with these fractures to be S$183.5 million in 2017 [11] This is forecasted to grow to S$289.6 million by 2035. It was also estimated in that study, that increasing the treatment rate for osteoporosis could avert up to 29,096 fractures over the forecast period (2017–2035), generating cumulative total cost savings of up to S$330.6 million [11]. The authors concluded that improving the detection, diagnosis, and treatment of osteoporosis are imperative to reduce the growing clinical, economic, and societal burden of fractures in Singapore.
Chinese, Malay, and Indian ethnic-specific FRAX® models were developed for Singapore and launched in December 2010. FRAX® based intervention thresholds that can be employed in the Singaporean population have also been identified [13]. However, to date, there have been no attempts made to elucidate whether FRAX® based thresholds can potentially be employed to determine who to refer for DXA scanning, neither in Singapore nor in any other country in Southeast Asia. No study has also so far compared the performance characteristics of OSTA and FRAX® to correctly identify osteoporosis on axial DXA scanning. The 2 primary aims of our study were (1) to determine the FRAX® thresholds that could correctly define a densitometric diagnosis of osteoporosis in postmenopausal Singaporean women and, (2) to compare the performance characteristics of FRAX® and OSTA to identify osteoporosis on DXA scanning. The USPTF as mentioned previously has recommended that BMD testing be performed in women 50–64 years old if their FRAX® MOFP is ≥ to that of a 65-year-old woman of average body mass index (BMI), having no clinical risk factors [3]. We wanted to see how well such a threshold would perform in our population and therefore the secondary aim of our study was, to explore the performance characteristics of a FRAX® MOFP threshold of 6.3% and a FRAX® HFP threshold of 1.63% in identifying a densitometric diagnosis of osteoporosis in women below the age of 65 years. 6.3% and 1.63% are the major osteoporotic and hip fracture probabilities respectively of a 65-year-old Singaporean woman of average BMI (23.8 kg/m2) who has no FRAX related clinical risk factors.
2. Methods
The study sample was derived from patients seen at 2 large hospital clusters in Singapore; Singapore General Hospital belonging to the SingHealth Cluster and National University Hospital belonging to the National University Health System Cluster. These 2 clusters essentially cover the health care of most of the Singaporean population. SingHealth Institutional Review Board approval was obtained for this study (CIRB Ref 2018/2574). The dataset consisted of 600 women who had been seen in the outpatient and health screening clinics at Singapore General Hospital; the largest hospital in Singapore and had DXA scans done as a part of their routine work up or had DXA scans done as a part of general health screening and 1201 women, recruited through the gynecology and menopause clinics into the Integrated Women’s Health Programme (IWHP) cohort study at National University Hospital, Singapore [14]. Menopause was defined as at least 6 months of amenorrhoea for women >55 years and at least >12 months of amenorrhoea for women 50–54 years [8]. Women who were pre-menopausal according to these criteria were excluded. BMDs at the hip and lumbar spine (L1–4) of all subjects were measured by DXA technology (Hologic QDR 4500, Hologic, Waltham, MA, USA). Female, ethnic-specific Singaporean reference data base was used to calculate the T-scores [15]. Baseline socio-demographic information, medical, menstrual and fracture history, family history of fractures, smoking, and alcohol history had all been obtained and recorded upon initial visit or at the time of the DXA screening. Subjects who had uninterpretable DXA scans of the hip and lumbar vertebrae were excluded. Subjects who had ever been treated with, or were on current treatment with antiosteoporosis agents were also excluded. These exclusions resulted in a final sample size of 1056 subjects, 50 years old and above.
FRAX® scores without BMD were calculated separately for women belonging to the 3 races. The mean weighted MOFP and HFP at age 65 of a “Singaporean” woman was calculated using the average BMI at age 65 i.e., 23.8 kg/m2 weighted by the proportion of Chinese, Malays, and Indians in the Singaporean population. The mean weighted MOFP at age 65 was thus calculated using the formula (74.3 × MOFP of 65-year-old Chinese woman without any other CRF +13.4 × MOFP of 65-year-old Malay woman without any other CRF + 9.1 × MOFP of 65-year-old Indian woman without any other CRF) /100 where 74.3, 13.4, and 9.1 are the percentage distribution of Chinese, Malays, and Indians respectively in the Singaporean population (https://www.moh.gov.sg/content/moh_web/home/statistics/Health_Facts_Singapore/Population_And_Vital_Statistics, last accessed February 9, 2020). A similar equation was used to calculate the mean weighted HFP at 65 years old. This same approach of using the weighted proportion of the 3 ethnicities to calculate weighted MOFP and HFP was used in our earlier study to estimate the mean age dependent intervention thresholds in Singaporeans [13].
Measurements by DXA at the neck of femur (NOF) have the highest predictive value for hip fracture and it has been proposed as the reference skeletal site for defining osteoporosis in epidemiologic studies [16]. The proportion of individuals allocated to any diagnostic category as would the risk of fracture vary when T-scores at different sites are used to make a diagnosis of osteoporosis. However, using only the T-score at the NOF to make a diagnosis of osteoporosis might result in an underestimation of osteoporosis and it is recommended that a combination of spinal and femoral densitometry should be used in diagnosing the condition [17]. Therefore, in our study, osteoporosis was diagnosed if the lowest T-score (referenced to local data base) at any of the 3 axial sites on DXA scan viz. NOF, total hip, or lumbar spine was ≤-2.5.
Demographics and other characteristics were summarized using descriptive statistics. The P-values for comparing continuous variables were derived from analysis of variance while Fisher exact test was used for categorical variables. Receiver operating characteristic (ROC) curves that plot the true positive rate (sensitivity) against the false positive rate (1-specificity) for various cut-off values were constructed, and the area under the ROC curve (AUC) and its 95% confidence interval (CI) were estimated. Optimal cut-off points in identifying osteoporosis were obtained using the Youden index (sensitivity + specificity - 1) [18]. The statistical analysis was performed using R (R Core Team [2019]; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; URL https://www.R-project.org/) and package pROC [19].
3. Results
In the study sample, 80% were Chinese, 13% were Indian, and 7% were Malay. The mean age ± standard deviation (SD) was 59.6 ± 7.5 years. 254 out of the 1056 subjects had osteoporosis on DXA scanning. Mean age ±SD of women with normal BMD at all 3 axial sites on DXA was 57.3 ± 6.2 years while those of osteopenic and osteoporotic women were 58.8 ± 6 and 65 ± 8.8 years, respectively. The demographic and baseline characteristics are shown in Table 1.
Table 1.
Demographic and baseline characteristics.
| Variable | Total (N = 1056) | Normal (N = 494) | Osteopenia (N = 308) | Osteoporosis (N = 254) | P-value |
|---|---|---|---|---|---|
| Age, yr | 59.6 ± 7.5 | 57.3 ± 6.2 | 58.8 ± 6 | 65 ± 8.8 | <0.001 |
| Race | |||||
| Chinese | 841 (80) | 371 (75.1) | 240 (77.9) | 230 (90.6) | <0.001 |
| Indian | 137 (13) | 77 (15.6) | 47 (15.3) | 13 (5.1) | <0.001 |
| Malay | 77 (7) | 46 (9.3) | 20 (6.5) | 11 (4.3) | <0.001 |
| Others | 1 (0) | 0 (0) | 1 (0.3) | 0 (0) | |
| Weight, kg | 57.5 ± 11.5 | 62.4 ± 10.7 | 56 ± 11 | 49.8 ± 8.4 | <0.001 |
| Height, cm | 155.6 ± 6.7 | 157.3 ± 5.7 | 155.2 ± 7.5 | 152.9 ± 6.4 | <0.001 |
| BMI, kg/m2 | 23.6 ± 4.3 | 25.2 ± 4.4 | 23 ± 3.8 | 21.3 ± 3.4 | <0.001 |
| FRAX® MOFP score, % | 5.7 ± 6.2 | 3.8 ± 3.5 | 4.5 ± 3.5 | 10.8 ± 9.3 | <0.001 |
| FRAX® HFP score, % | 2 ± 3.8 | 1 ± 2 | 1.3 ± 1.5 | 4.9 ± 6.3 | <0.001 |
| OSTA index ([weight-age] × 0.2) | −0.4 ± 3.1 | 1 ± 2.6 | −0.6 ± 2.5 | −3.1 ± 2.6 | <0.001 |
Values are presented as mean ± standard deviation or number (%).
BMI, body mass index; MOFP, Major Osteoporotic Fracture Probability; HFP, Hip Fracture Probability; OSTA, Osteoporosis Self-Assessment Tool for Asians.
3.1. Screening thresholds of FRAX® and OSTA index to identify osteoporosis
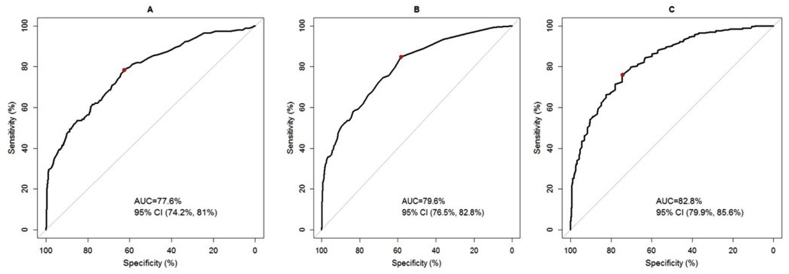
The sensitivities, specificities, positive predictive value (PPV) and negative predictive value (NPV) of the optimal cut-off point for FRAX® MOFP threshold, FRAX® HFP threshold and OSTA index are shown in Table 2. An MOFP Youden index cut-off of ≥3.7%, had a sensitivity of 78%, specificity of 63%, a PPV of 40%, and an NPV of 90% to identify a densitometric diagnosis of osteoporosis in Singaporean postmenopausal women. An HFP Youden index cut-off ≥0.6% had a sensitivity, specificity, PPV, and NPV of 85%, 58%, 39%, and 92% respectively. An OSTA index Youden cut-off of ≤ -1.2 had a sensitivity of 76%, specificity of 74%, a PPV of 48%, and an NPV of 91% to accurately define a densitometric diagnosis of Osteoporosis The AUC for FRAX® MOFP was 77.6% (95% CI, 74.2%–81%), that for FRAX® HFP was 79.6% (95% CI, 76.5%–82.8%) and that for OSTA index was 82.8% (95% CI, 79.9%–85.6%) (Fig. 1).
Table 2.
Sensitivity, specificity, positive predictive value, and negative predictive values of the optimal cutoff by Youden index for FRAX® MOFP score, FRAX® HFP score, and OSTA index to identify Osteoporosis on DXA.
| Threshold | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| FRAX® MOFP score ≥ 3.7% | 0.78 (0.73–0.83) | 0.63 (0.59,0.66) | 0.4 (0.36–0.44) | 0.9 (0.87–0.92) |
| FRAX® HFP score ≥ 0.6% | 0.85 (0.80–0.89) | 0.58 (0.55–0.62) | 0.39 (0.35–0.43) | 0.92 (0.9–0.94) |
| OSTA Index ≤ −1.2 | 0.76 (0.70–0.81) | 0.74 (0.71–0.77) | 0.48 (0.43–0.54) | 0.91 (0.88–0.93) |
MOFP, Major Osteoporotic Fracture Probability; HFP, Hip Fracture Probability; OSTA, Osteoporosis Self-Assessment Tool for Asians; DXA, dual energy X-ray absorptiometry; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Fig. 1.
Receiver operating characteristic (ROC) curves and area under the ROC curve (AUCs) for FRAX® MOFP score (plot A), FRAX® HFP score (plot B), and OSTA index (plot C) to identify osteoporosis on DXA. MOFP, Major Osteoporotic Fracture Probability; HFP, Hip Fracture Probability; OSTA, Osteoporosis Self-Assessment Tool for Asians; DXA, dual energy X-ray absorptiometry; CI, confidence interval.
When we explored various arbitrarily chosen MOFP thresholds varying from ≥3% to ≥15% and HFP thresholds varying from ≥0.5% to ≥3%, it was found that increasing the cut-off values decreased sensitivity, but increased the specificity and PPVs of the thresholds (Table 3).
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive values of various FRAX(R) MOFP and HFP thresholds to identify osteoporosis on DXA.
| Variable | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| FRAX® MOFP threshold | ||||
| ≥3.7% (optimal cutoff by Youden index) | 0.78 (0.73–0.83) | 0.63 (0.59–0.66) | 0.4 (0.36–0.44) | 0.9 (0.87–0.92) |
| ≥3% | 0.84 (0.79–0.89) | 0.51 (0.47–0.54) | 0.35 (0.32–0.39) | 0.91 (0.88–0.94) |
| ≥8% | 0.48 (0.42–0.54) | 0.89 (0.86–0.91) | 0.58 (0.51–0.64) | 0.84 (0.82–0.87) |
| ≥10% | 0.39 (0.33–0.46) | 0.94 (0.92–0.95) | 0.67 (0.59–0.74) | 0.83 (0.8–0.85) |
| ≥15% | 0.3 (0.24–0.36) | 0.99 (0.98–0.99) | 0.87 (0.78–0.93) | 0.81 (0.79–0.84) |
| FRAX® HFP threshold | ||||
| ≥0.6% (optimal cutoff by Youden index) | 0.85 (0.8–0.89) | 0.58 (0.55–0.62) | 0.39 (0.35–0.43) | 0.92 (0.9–0.94) |
| ≥0.5% | 0.89 (0.84–0.93) | 0.46 (0.43–0.5) | 0.34 (0.31–0.38) | 0.93 (0.9–0.95) |
| ≥1% | 0.75 (0.69–0.8) | 0.68 (0.64–0.71) | 0.42 (0.38–0.47) | 0.89 (0.87–0.92) |
| ≥2% | 0.54 (0.47–0.6) | 0.85 (0.83–0.88) | 0.54 (0.48–0.6) | 0.85 (0.83–0.88) |
| ≥3% | 0.45 (0.39–0.51) | 0.92 (0.9–0.94) | 0.63 (0.56–0.7) | 0.84 (0.81–0.86) |
MOFP, Major Osteoporotic Fracture Probability; HFP, Hip Fracture Probability; DXA, dual energy X-ray absorptiometry; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
The sensitivity, specificity, PPV, and NPV of FRAX MOFP threshold of 6.3% in the women 50–64 years old to identify a densitometric diagnosis of osteoporosis were 0.17 (0.11–0.24), 0.95 (0.93–0.96), 0.39 (0.26–0.52), and 0.85 (0.83–0.88) respectively and those of FRAX® HFP threshold of 1.63% to do this were 0.19 (0.12–0.28), 0.95 (0.93–0.96), 0.33 (0.21–0.47), and 0.89 (0.87–0.91), respectively (Table 4).
Table 4.
Sensitivity, specificity, positive predictive value, and negative predictive value for FRAX® MOFP and FRAX® HFP thresholds of 6.3% and 1.63% (i.e., FRAX® probabilities equivalent to that of a 65-year-old Singaporean woman of average BMI and no clinical risk factors) to identify osteoporosis on DXA scan in women <65 years old.
| Threshold | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| FRAX® MOFP score ≥ 6.3% | 0.17 (0.11–0.24) | 0.95 (0.93,0.96) | 0.39 (0.26–0.52) | 0.85 (0.83–0.88) |
| FRAX® HFP score ≥ 1.63% | 0.19 (0.12–0.28) | 0.95 (0.93–0.96) | 0.33 (0.21–0.47) | 0.89 (0.87–0.91) |
MOFP, Major Osteoporotic Fracture Probability; HFP, Hip Fracture Probability; BMI, body mass index; DXA, dual energy X-ray absorptiometry; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
4. Discussion
Antifracture efficacy of antiosteoporosis agents has been shown to be related to changes in bone density [20,21] and hence identification of densitometric osteoporosis and early initiation of antiosteoporosis treatment might be helpful to prevent fractures in women. Our primary aims were to compare the performance of a commonly used screening tool viz. the OSTA against another such as the FRAX®; the latter a tool primarily designed to evaluate fracture risk, to identify a densitometric diagnosis of Osteoporosis. We aimed to provide threshold values for these screening indices to help health care providers refer patients for DXA scans so that osteoporosis can be detected efficiently and early.
The thresholds for FRAX® and OSTA that were identified using the Youden index in our study had acceptable but not very high sensitivity. This does mean that a fraction of patients who are truly osteoporotic would be missed (false negatives) if these thresholds were used. However, if thresholds with a higher sensitivity were chosen, this would compromise specificity further. The latter would mean that many people who really have normal BMD, may be classified as high risk of having osteoporosis (false positives). These people may thus undergo unnecessary DXA scanning resulting in higher costs and unnecessary worry in them till their DXA results are available and they are shown not to have osteoporosis. The thresholds that we obtained, viz. 3.7% or more for FRAX® MOFP and 0.6% or more for FRAX® HFP would offer a reasonable trade-off between sensitivity and specificity to identify osteoporosis in Singaporean postmenopausal women. An OSTA index threshold of ≤ -1.2 has a sensitivity of 76% and a specificity of 74%. Although lower than the sensitivities, the relatively acceptable specificities of both tools at the given cut-off values (albeit higher for OSTA than for FRAX®) also provides opportunities for cost savings by excluding patients who do not need a BMD assessment. By setting thresholds that should trigger referrals for DXA scanning, a balance between identifying an opportunity for fracture prevention through early detection of osteoporosis and avoidance of driving up costs which would have been the case if universal DXA screening was recommended can be achieved. In our population of Singaporean women, OSTA and FRAX® performed similarly without any significant differences in AUCs.
Since both OSTA index and FRAX® perform quite similarly in identifying osteoporosis in our multi-ethnic population, it can be argued that OSTA which has only 2 parameters i.e., age and weight and therefore is much simpler to use should preferably be the screening tool of choice. However, employing a fracture risk assessment tool such as FRAX® that can be added on to the patient’s risk profile and provide fracture probabilities in addition to just identifying the risk of osteoporosis may provide an extra aid to the physician to discuss options for fracture prevention at the time of initial consultation. A 7-centre randomized controlled trial on the clinical efficacy and cost-effectiveness of screening older women in primary care for the prevention of fractures, based on the FRAX® risk assessment tool, demonstrated encouraging findings with regard to a reduction in hip fracture [22].
It has to be noted that the OSTA cut-off point identified by Koh et al. [5] in their original Asian 8-country study, was different from the threshold we identified in that low risk of osteoporosis was seen at an OSTA index cut-off of > -1 by the former. However, in that study, the performance of OSTA within the individual countries was not reported and it is very likely that the cut-off would have been different if it had been reported thus. The former study was also limited by the small sample size of only 100 Singaporean women whereas ours had a number of more than 1000.
In addition, multiple studies have established that modifications to the originally proposed index cut-off value may be necessary to ensure the optimal performance of OSTA. For instance, the OSTA index at the standard cut-point of < -1 had only sensitivities of 36.2% and 40.6% respectively for the lumbar spine and the neck of the femur in predicting osteopenia to osteoporosis status in Thai postmenopausal women [23]. The OSTA indices that have been identified in various studies vary from ≤-3.5 to ≤6 [24,25]. This could be because of differences in ethnicity, body weight and other characteristics of the study populations.
The very poor sensitivities we found when the discriminatory value analysis was done with a FRAX® MOFP threshold of 6.3% (equal to that of a 65-year-old woman of average BMI and no clinical risk factors) in the 50- to 64-year-old Singapore women suggests that this particular cut-off value cannot be used as a screening threshold, since it would mean that a large majority of women with BMD in the range of osteoporosis would be missed. Employing the same strategy that the USPTF employed, of recommending universal DXA scanning to more than 65-year-old women may also not be a feasible approach given the constraints in the Asian context mentioned previously.
In order to derive screening thresholds for a given population, multiple factors should be taken into consideration including costs of DXA scanning, that of consultations with the health care provider and of medications, the efficacy of these medications to reduce fractures, and the threshold of cost effectiveness at which intervention is considered appropriate or willingness to pay [26]. This of course has to be balanced with the potential of rare but significant side effects from long-term use of potent antiosteoporosis medications [27] especially in younger women. Cost effective analysis of screening and intervention thresholds for osteoporosis in Singapore is in planning and will be available soon.
It could be argued that using uniform thresholds be it OSTA or FRAX®, for all 3 races is inappropriate given that the risk of osteoporosis and osteoporotic fractures is likely to be different in Chinese, Malays, and Indians [12]. Indeed, when we analysed the Chinese, Malay, and Indian women in our study sample separately, different OSTA and FRAX® score thresholds with varying sensitivities and specificities were obtained (data not shown). However, implementing different screening (and intervention) thresholds for different races in the same country though not difficult theoretically or technically, carries with it grave societal, philosophic and health care policy implications. The decision to use ethnic-specific thresholds would also have been reasonable if there is good evidence that clinical efficacy and cost effectiveness of diagnosis and intervention at a certain level of risk differ between ethnicities. In the absence of such evidence, it would thus be more practical to consider the same thresholds for all ethnicities in a particular country and this is the same philosophy adopted by other countries with a multi-ethnic population such as the United Kingdom, the United States, etcetera.
Despite its few limitations including its non-randomized nature, and despite it not being population based, our study has several advantages. It is the first such study aimed at determining FRAX® based screening thresholds for osteoporosis management in Singapore and to compare their performance characteristics with that of an earlier established screening methodology such as OSTA. Though the study population was not randomly recruited from the community, we had the advantage of having very large cohorts from the two largest hospitals from the two health care clusters in Singapore that cover most of the population of Singapore. This makes it more plausible that the results can be extrapolated to the larger population of Singapore though the thresholds we identified will have to be validated before being recommended for clinical practice. There were more than adequate numbers of normal women who had presented for simple health screening as well as women with densitometrically diagnosed osteoporosis. Unlike as with large anonymized data sets or claims databases, our study had the unique advantage in that individual data and history could be checked because the study subjects were individually identified. All subjects had a complete history and laboratory work done either as part of routine clinical care or if they were part of the IWHP cohort, as part of the study protocol. The distribution of ethnicities in our study sample did not exactly mirror the ethnic distribution in Singapore with the percentage of Indians being more than the Malays in our study population. However, this is likely a representation of patient numbers seen in actual clinical practice in Singapore where certain ethnicities are more likely to seek medical attention than others.
5. Conclusions
Early identification is key to appropriate management of osteoporosis and to prevent its devastating complication of fragility fractures. FRAX® was originally developed to identify women at a high fracture risk and not to identify a densitometric diagnosis of osteoporosis. However, it may still have a role in primary screening to identify the postmenopausal woman to be referred for DXA scanning. Employing the FRAX® tool for this purpose may also aid the primary care physician to discuss fracture probabilities with the patient who may not relate being sent for osteoporosis screening with the eventual benefit of fracture reduction with appropriate management. The fact that FRAX® and OSTA had almost similar performance characteristics as screening modalities in our population is reassuring. Future research focusing on randomized controlled trials that test whether the use of these risk assessment tools to select individuals for BMD testing will decrease fracture incidence have to be conducted in our population.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
CRediT author statement
Manju Chandran: Conceptualization, Methodology, Data curation, Writing - original draft, Writing - review & editing. Yun Ann Chin: Data curation. Kuan Swen Choo: Data curation. Wan Chen Ang: Data curation. Xiao Feng Huang: Data curation. Xiao Ming Liu: Data curation. Donovan Tay: Data curation. Tin Kyaw Kyaw Aung: Data curation. Amin Ali: Data curation. Win Pa Pa Thu: Data curation. Susan Logan: Data curation, Writing - review & editing. Sean Xuexian Yan: Data curation. Sarath Lekamwasam: Methodology, Writing - review & editing. Ying Hao: Methodology, Formal analysis.
Acknowledgments
ORCID Manju Chandran: 0000-0001-9119-8443. Yun Ann Chin: 0000-0002-4311-2104. Kuan Swen Choo: 0000-0002-9275-228X. Wan Chen Ang: 0000-0002-8891-4055. Xiao Feng Huang: 0000-0001-6550-999X. Xiao Ming Liu: 0000-0002-3263-082X. Donovan Tay: 0000-0002-2192-2245. Tin Kyaw Kyaw Aung: 0000-0002-4017-0317. Amin Ali: 0000-0003-1201-5202. Win Pa Pa Thu: 0000-0002-5793-2488. Susan Logan: 0000-0002-2667-7146. Sean Xuexian Yan: 0000-0001-6178-7386. Sarath Lekamwasam: 0000-0002-3541-9982. Ying Hao: 0000-0002-1785-2467.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Cosman F., de Beur S.J., LeBoff M.S., Lewiecki E.M., Tanner B., Randall S. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho P.M., Petak S.M., Binkley N., Clarke B.L., Harris S.T., Hurley D.L. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(Suppl 4):1–42. doi: 10.4158/EP161435.GL. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force, Curry S.J., Krist A.H., Owens D.K., Barry M.J., Caughey A.B. Screening for osteoporosis to prevent fractures: US preventive services Task Force recommendation statement. J Am Med Assoc. 2018;319:2521–2531. doi: 10.1001/jama.2018.7498. [DOI] [PubMed] [Google Scholar]
- 4.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 5.Koh L.K., Sedrine W.B., Torralba T.P., Kung A., Fujiwara S., Chan S.P. A simple tool to identify asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12:699–705. doi: 10.1007/s001980170070. [DOI] [PubMed] [Google Scholar]
- 6.Kanis J.A., Oden A., Johnell O., Johansson H., De Laet C., Brown J. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 7.Cherian K.E., Kapoor N., Shetty S., Naik D., Thomas N., Paul T.V. Evaluation of different screening tools for predicting femoral neck osteoporosis in rural South Indian postmenopausal women. J Clin Densitom. 2018;21:119–124. doi: 10.1016/j.jocd.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Crandall C.J., Larson J., Gourlay M.L., Donaldson M.G., LaCroix A., Cauley J.A. Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US Preventive Services Task Force strategy and two traditional strategies in the Women’s Health Initiative. J Bone Miner Res. 2014;29:1661–1666. doi: 10.1002/jbmr.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecina J.L., Romanovsky L., Merry S.P., Kennel K.A., Thacher T.D. Comparison of clinical risk tools for predicting osteoporosis in women ages 50-64. J Am Board Fam Med. 2016;29:233–239. doi: 10.3122/jabfm.2016.02.150237. [DOI] [PubMed] [Google Scholar]
- 10.Leslie W.D., Majumdar S.R., Lix L.M., Johansson H., Oden A., McCloskey E. High fracture probability with FRAX usually indicates densitometric osteoporosis: implications for clinical practice. Osteoporos Int. 2012;23:391–397. doi: 10.1007/s00198-011-1592-3. [DOI] [PubMed] [Google Scholar]
- 11.Chandran M., Lau T.C., Gagnon-Arpin I., Dobrescu A., Li W., Leung M.Y.M. The health and economic burden of osteoporotic fractures in Singapore and the potential impact of increasing treatment rates through more pharmacological options. Arch Osteoporos. 2019;14:114. doi: 10.1007/s11657-019-0664-4. [DOI] [PubMed] [Google Scholar]
- 12.Yong E.L., Ganesan G., Kramer M.S., Logan S., Lau T.C., Cauley J.A. Hip fractures in Singapore: ethnic differences and temporal trends in the new millennium. Osteoporos Int. 2019;30:879–886. doi: 10.1007/s00198-019-04839-5. [DOI] [PubMed] [Google Scholar]
- 13.Chandran M., McCloskey E.V., Thu W.P.P., Logan S., Hao Y., Tay D. FRAX® based intervention thresholds for management of osteoporosis in Singaporean women. Arch Osteoporos. 2018;13:130. doi: 10.1007/s11657-018-0542-5. [DOI] [PubMed] [Google Scholar]
- 14.Thu W.P.P., Logan S.J.S., Lim C.W., Wang Y.L., Cauley J.A., Yong E.L. Cohort profile: the Integrated Women’s Health Programme (IWHP): a study of key health issues of midlife Singaporean women. Int J Epidemiol. 2018;47:389–390. doi: 10.1093/ije/dyx278. [DOI] [PubMed] [Google Scholar]
- 15.Leong K.H., Feng P.H. Bone mineral density measurements using the Hologic QD2000 in 175 Singaporean women aged 20-80. Singap Med J. 1997;38:25–26. [PubMed] [Google Scholar]
- 16.Kanis J.A., Glüer C.C. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11:192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamsen B., Hansen T.B., Jensen L.B., Hermann A.P., Eiken P. Site of osteodensitometry in perimenopausal women: correlation and limits of agreement between anatomic regions. J Bone Miner Res. 1997;12:1471–1479. doi: 10.1359/jbmr.1997.12.9.1471. [DOI] [PubMed] [Google Scholar]
- 18.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.C. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasnich R.D., Miller P.D. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85:231–236. doi: 10.1210/jcem.85.1.6267. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg M.C., Greenspan S., Wasnich R.D., Miller P., Thompson D.E., Ross P.D. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87:1586–1592. doi: 10.1210/jcem.87.4.8415. [DOI] [PubMed] [Google Scholar]
- 22.Shepstone L., Lenaghan E., Cooper C., Clarke S., Fong-Soe-Khioe R., Fordham R. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2018;391:741–747. doi: 10.1016/S0140-6736(17)32640-5. [DOI] [PubMed] [Google Scholar]
- 23.Lerttrakul S., Soontrapa S. Modified OSTA index for referring women for DEXA measurement. J Med Assoc Thai. 2005;88(Suppl 5):S80–S83. [PubMed] [Google Scholar]
- 24.Chaovisitsaree S., Namwongprom S.N., Morakote N., Suntornlimsiri N., Piyamongkol W. Comparison of osteoporosis self assessment tool for Asian (OSTA) and standard assessment in Menopause Clinic. Chiang Mai J Med Assoc Thai. 2007;90:420–425. [PubMed] [Google Scholar]
- 25.Steuart Richards J., Lazzari A.A., Teves Qualler D.A., Desale S., Howard R., Kerr G.S. Validation of the osteoporosis self-assessment tool in US male veterans. J Clin Densitom. 2014;17:32–37. doi: 10.1016/j.jocd.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Eichler H.G., Kong S.X., Gerth W.C., Mavros P., Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–528. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 27.Harvey N.C., McCloskey E., Kanis J.A., Compston J., Cooper C. Cost-effective but clinically inappropriate: new NICE intervention thresholds in osteoporosis (Technology Appraisal 464) Osteoporos Int. 2018;29:1511–1513. doi: 10.1007/s00198-018-4505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]