Abstract
The present study investigated the nutritional composition of bran from rice (RB) and wheat (WB) and compared the natural virtues of crude extracts based on phenolic composition, antidiabetic and anticancer activities. The profiling of phenolic-rich ethyl acetate extracts (RBE and WBE) confirms that RBE is rich in catechol (0.122 mg/g dw), p-coumaric acid (0.159 mg/g dw), kaempferol (0.374 mg/g dw) and apigenin (0.399 mg/g dw); and WBE is affluent with catechol (0.144 mg/g dw), ferulic acid (0.160 mg/g dw), caffeic acid (0.083 mg/g dw) and ellagic acid (0.074 mg/g dw). RBE exhibited better antioxidant activity, inhibited the activity of α-amylase (IC50-353.41 µg/mL) and α-glucosidase (IC50-314.22 µg/mL), hindered glycation process (IC50-451.11 µg/mL), and enhanced glucose uptake in L6 muscle cells (20.4%) indicating its potential in diabetic management. RBE was toxic to HT29 colon cancer cells and decreased cell membrane integrity. RBE and WBE arrested cell-cycle transition in HT29 cells from G0 to G1 and G2 to M phase respectively and induced apoptosis (27.15% and 5.9%, respectively for RBE and WBE) suggesting anticancer activities of the extract. The study indicates that bran from rice and wheat are a potential source of dietary fibre and phytochemicals with antidiabetic and anticancer properties for developing value-added products with nutraceutical benefits.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04353-1) contains supplementary material, which is available to authorized users.
Keywords: Bran, Dietary fibre, Phenolic compounds, Antioxidant, Antidiabetic, Anticancer
Introduction
Cereals are the essential staple foods for millions of population, among them, wheat and rice are the primary produce globally. The cereal processing generates a considerable amount of bran, the outer layer of the grain kernel, as byproduct where most of the antioxidants, B vitamins and fibre locate. Bran is also an excellent source of soluble and insoluble dietary fibre with promising functional and nutritional properties (Daou and Zhang 2013; Mingyai et al. 2017).
RB and WB are the rich sources of dietary fibre and antioxidants (Daou and Zhang 2013; Mingyai et al. 2017; Arab et al. 2011; Zhou and Yu 2004). The dietary fibre content of WB and RB are reported to vary between 36.5–52.4% (Vitaglione et al. 2008) and 27.04–32.9% (Daou and Zhang 2013) respectively. Consumption of dietary fibre rich food is reported to reduce the hazards of chronic diseases, and the antioxidants play a remarkable role in controlling these diseases. Polyphenols, oryzanols, tocopherols and tocotrienols impart antioxidant potential to RB and WB (Lai et al. 2009; Brewer et al. 2014). Bran is rich in phytosterols which are known to absorb cholesterol and reduces LDL cholesterol in the blood (Ozdestan et al. 2014). The phytochemical constituents of bran significantly rely upon the extraction solvents and the cereal varieties. Abozed et al. (2014) have noted that the acetone extract of durum wheat variety (Egypt) possess high phenolic content and better antioxidant potential. The methanol extract of fajr rice variety (Iran) exhibited a high amount of phenolic compounds and showed remarkable antioxidant action (Arab et al. 2011).
Though RB and WB have been studied for various biological activities attributed by dietary fibre, oryzanols, tocopherols and tocotrienols, comparative studies on detailed phytochemical composition and biological activities are very few. Moreover, there are fewer studies related to the possible mechanism of action underlying the biological activity of crude extracts from these brans. However, there are no detailed reports on the phenolic profiling on the bran from rice and wheat and its associated health benefits. In this context, we have carried out a comparative evaluation of the phytochemical composition and biological activities of ethyl acetate and methanol extracts of the bran for their nutraceutical benefits in terms of antidiabetic and anticancer activity.
Materials and methods
Sample
Rice (Oryza sativa—“Cheradi” variety) and wheat (Triticumaestivum—“HD2967" variety) grains were collected from paddy fields in Kollam district of Kerala and Ambala district of Haryana respectively. The specimen samples of rice (TBGT93995) and wheat (TBGT99996) was deposited in the herbarium of Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Kerala. Dried grains were milled in a local mill (two-stage milling process) to separate husk and bran. The bran was refined through 80 mesh and placed in an airtight container until use.
Chemicals
Fetal bovine serum (FBS), horse serum and Dulbecco's modified eagle media (DMEM) were procured from Life Technologies, USA. Antibiotic–antimycotic solution (with 10,000 units penicillin, 10 mg streptomycin and 25 μg amphotericin B per mL), trypsin-EDTA solution (0.25%), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), polyphenols, trolox, acarbose, 2,2-diphenyl-1-picrylhydrazyl (DPPH), α-amylase, α-glucosidase, 4-nitrophenyl α-d-glucopyranoside and acarbose were supplied by Sigma-Aldrich, USA. Invitrogen, USA supplied 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG). Cupric chloride (CuCl2) and neocuproine were obtained from Alfa Aesar, USA. Sodium nitroprusside, vitamin C and solvents for high-performance liquid chromatography (HPLC) were obtained from Sisco Research Laboratories, India.
Cell lines
L6-rat skeletal myoblast and HT-29 colon cancer cells (NCCS, Pune, India) were grown in DMEM containing FBS (10%) and antibiotic–antimycotic solution (0.5%) and kept in an 37 °C incubator supplied with 5% carbon dioxide (CO2). L6 cells were differentiated with horse serum (5%), trypsinized, diluted and seeded to a density of 104 cells per well in 24 well plates. After the cells attained ≈ 80% confluence, the cells were treated with extracts, and the viable cells were quantified by MTT assay and represented as IC50 values.
Proximate analysis
Moisture (method 930.15), ash (method 923.03), fat (method 983.23), protein (method 984.13), crude fibre (method 962.09), carbohydrate (difference method, [100-{moisture + ash + fat + protein}]), sodium and potassium (method 969.23), were determined by procedure of Association of Official Analytical Chemists (AOAC) protocols (1990).
Isolation of dietary fibre
The soluble and insoluble forms of dietary fiber were extracted as per the protocol described by Bureau of Indian Standard Method (IS: 11062 1984) with slight modifications. Defatted, moisture-free bran (3 g) mixed with water (50 mL) and autoclaved. Further, the pH was adjusted to 1.5 with hydrochloric acid (5 M) followed by the addition of 50 mg pepsin (250 units/mg solid) and chloroform (200 mL) and incubated for 20 h with mild stirring at 37 °C. Followed by incubation, the pH was increased to 6 with sodium hydroxide (3 N) and phosphate buffer (25 mL, 0.2 M, pH 7.4), pancreatin (100 mg, P7545 from Sigma), glucoamylase (20 mg, 250 U/mL), amyloglucosidase (20 mg, 260 U/mL) and thymol crystals (2 g) were added. This mixture was incubated for 18 h with mild stirring at 37 °C. The contents were centrifuged (3000g × 30 min) after the incubation; the leftovers were washed with acetone and diethyl ether and lyophilized to obtain the insoluble dietary fiber. The supernatant was precipitated with ethanol [1:4 ratio (v/v)] and centrifuged (3000g × 30 min). The residue was washed with alcohol, acetone and diethyl ether and lyophilized to obtain the soluble dietary fibre.
Preparation of bran extracts
The bran was powdered (Retschultra centrifugal mill ZM200, Germany), refined through vibrating shifter (Vibro Sifter model PVS30, Prism Pharma Machinery, India) coupled with mesh (20 mm), defatted with hexane and dried in an oven at 40 °C. Defatted bran (100 g) was extracted with ethyl acetate followed by methanol with continuous stirring. The crude extracts were filtered and evaporated (Buchi-R215, Switzerland) under reduced pressure at 40 °C to obtain RB ethyl acetate extract (RBE), RB methanol extract (RBM), WB ethyl acetate extract (WBE) and WB methanol extract (WBM). The extracts were dissolved and made up to 100 mL with respective solvents, and kept at 4 °C until further use.
Total phenolic content
The total phenolic content of the extracts was determined using Folin–Ciocalteau reagent Singleton and Rossi (1965). The 1 mL reaction mixture containing 720 μL of test/standard (different concentrations of test samples or gallic acid were made up with methanol), 80 μL of Folin’s reagent and 200 μL of sodium carbonate (7.5%) was incubated for 90 min at room temperature. A blank reaction with methanol was also performed simultaneously. The absorbance was observed at 725 nm using a multiplate reader (Synergy 4 Biotek, USA). The phenolic content of the extracts in total was presented as milligram of gallic acid equivalent per gram dry weight (GAE/g dry weight).
Chromatographic profiling for assessment of polyphenols in extracts
The profiling of polyphenolic compounds was performed using HPLC, as described earlier (Arun et al. 2017). Briefly, 20 µL extract was injected to Shimadzu Prominence UFLC (Japan). The peaks separated [gradient system containing water, methanol and acetic acid water at a ratio of 88:10:2 (solvent A) and 8:90:2 (solvent B)] were tracked using diode array detector at 280 nm and the retention time were correlated with that of the standard polyphenols. For quantification of polyphenols, standard polyphenols at different concentrations (5, 10, 50, 100, 250, 500 and 1000 µg/mL) were injected, and the area under the peak for each concentration was noted. A graph was plotted (concentration versus area under the peak) to obtain a straight line equation. To the equation of specific polyphenols, the value of area under the peak of a particular polyphenol as obtained for the test sample (different extracts of RB and WB) was inserted, and the concentration of that specific polyphenol was quantified.
Antioxidant property
The antioxidant activity of the extracts was assessed in terms of free radical (DPPH, Nitric oxide, superoxide) scavenging and cupric ion reducing activity as described earlier (Venkatachalam and Muthukrishnan 2012; Apak et al. 2008). The assays were performed using different concentrations of extracts (100–600 µg/mL). The IC50 values were obtained by plotting concentration versus absorption values in Origin PRO software, USA (average of three replicates).
Antidiabetic property
The inhibition of α-amylase and α-glucosidase enzymes, antiglycation property and glucose uptake efficacy of rice and wheat bran extract at different concentrations (100–600 µg/mL) was evaluated using the procedure as described earlier (Arun et al. 2017). The extracts at required concentrations were dried and resuspended in DMSO (DMSO final concentration in reaction mixture—0.1%). Appropriate solvent controls were kept while performing the assays. The absorption values were plotted against concentration and IC50 were obtained from the graph.
Inhibition of α-amylase
The assay mixture containing sodium phosphate buffer (0.02 M, pH 6.9 containing 6 mM sodium chloride), α-amylase (0.02 U/mL from porcine pancreas) and extracts at different concentrations were maintained at 37 °C for 10 min, soluble starch (1%, w/v) was added and kept for 15 min incubation. The reaction was ceased by adding 1 M hydrochloric acid and blue colour developed upon addition of iodine reagent (5 mM I2 and 5 mM KI) was read at 620 nm. The relative activity was measured by correlating with the control (without inhibition).
Inhibition of α-glucosidase
The reaction mixture with phosphate buffer (0.1 M, pH 7), 4-nitrophenyl α-d-glucopyranoside (0.5 M), α-glucosidase (0.1 U/mL) enzyme and extract at different concentrations was kept for 30 min incubation at 37 °C. The reaction was ended by adding sodium carbonate (0.2 M) solution. The p-nitrophenol discharged due to the action of the enzyme was measured at 410 nm and percentage inhibition was calculated.
Antiglycation property
To analyze the antiglycation property of the extracts the mixture of bovine serum albumin (1 mg/mL), d-glucose (200 mM), extracts (different concentrations) and phosphate-buffered saline (PBS-0.2 M, pH7.4) were kept for 24 h incubation at 60 °C. To terminate the reaction, trichloroacetic acid was added and maintained at 4 °C for 10 min. The mixture was centrifuged (10,000g) and the residue (glycated end-products) was resuspended in PBS to measure the excitation–emission (370/440 nm) fluorescence.
Glucose uptake assay
After preexposing with the extracts (100 µg/mL), the differentiated L6 myoblast cells were washed with PBS followed by adding 2-NBDG, the fluorescent glucose analogue, for 30 min. The cells were rewashed, trypsinized and reconstituted in 1 mL buffer to carry out flow cytometry (BD FACS Aria II, USA).
Anticancer property
The anticancer effect of various extracts was analyzed by different in vitro assays. The extracts were dried and resuspended in DMSO, and the final concentration of DMSO in the wells of cell culture plates was maintained at 0.1% by proper dilution with PBS.
Cell membrane integrity (lactate dehydrogenase release assay)
The potential of the extract on cell membrane integrity of HT29 cells was analyzed by lactate dehydrogenase release assay kit (Cayman Chemical Company, USA). The HT29 cells were exposed to extracts in varying concentrations (1–500 µg/mL) and incubated for 24 h. Following the incubation time, 100 µL of media from each well was transferred to 96 well plates. To that 100 µL of the reaction mixture was added (prepared as per manufacturer’s indications) and the absorbance was measured at 490 nm.
Cell cycle arrest
The propidium iodide staining was followed to analyze the cell cycle. Concisely, HT29 cells pre-exposed with extracts (75 µg/mL) were washed and fixed with 70% ethanol. The cells in each well were stained with a solution containing 200 µL of propidium iodide (50 µg/mL) and 50 µL of RNase (100 µg/mL) and quantified by flow cytometry (BD FACS Aria II, USA).
Analysis of apoptosis
Annexin V-FITC apoptosis detection kit (Cayman Chemical Company, USA) was utilized to depict the early and late-stage apoptosis in HT29 cells exposed with extracts (75 µg/mL). The annexin V forms a strong bond with the exposed phosphatidylserine on the membranes of apoptotic cells. This affinity differs in early and late stages and is quantified using flow cytometry.
Statistical data analysis
The average of triplicate observations with standard deviation was evaluated. The information obtained from various experiments was scrutinized by one-way ANOVA and Duncan’s multiple range test using SPSS version 7.5.1 (Chicago SPSS Inc. USA) with p value ≤ 0.05.
Results and discussion
The proximate contents of rice and wheat bran
The proximate composition of RB and WB was investigated in the present study (Table 1). The moisture, fat, protein and ash content of RB (10 ± 0.3%, 5.1 ± 0.074%, 12.54 ± 0.28% and 11.42 ± 0.04% respectively) was significantly higher than WB (8 ± 0.48%, 1.42 ± 0.053%, 9.85 ± 0.14% and 6.34 ± 0.17%. respectively). On the other hand, the carbohydrate content was higher in WB (74.39 ± 1.19%) as compared to RB (60.94 ± 0.98%), and values fall within the reported range (Sairam et al. 2011; Lin et al. 2019).
Table 1.
Proximate contents, minerals, soluble and insoluble fibre from rice bran and wheat bran
| Proximate | Rice bran | Wheat bran |
|---|---|---|
| Moisture (%) | 10.0 ± 0.30* | 8.0 ± 0.48 |
| Protein (%) | 12.54 ± 0.28* | 9.85 ± 0.14 |
| Fat (%) | 5.1 ± 0.074* | 1.42 ± 0.053 |
| Ash (%) | 11.42 ± 0.04* | 6.34 ± 0.17 |
| Carbohydrate (%) | 60.94 ± 0.98* | 74.39 ± 1.19 |
| Sodium (mg/100 g) | 4.6 ± 0.40* | 2.1 ± 0.09 |
| Potassium (mg/100 g) | 1164 ± 2.48* | 1498 ± 3.27 |
| Soluble dietary fibre (%) | 0.92 ± 0.097 | 2.22 ± 1.02# |
| Insoluble dietary fibre (%) | 32.17 ± 2.45 | 40.94 ± 3.40# |
| Total dietary fibre (%) | 33.09 ± 2.55 | 43.16 ± 4.42# |
Each value represents mean ± SD (standard deviation) from triplicate measurements.
*RB significantly different from WBE; #WB significantly different from RB (p value ≤ 0.05)
The enzymatic hydrolysis of bran yields soluble and insoluble dietary fibre. The total dietary fibre content of RB and WB were 33.09 ± 2.55% and 43.16 ± 4.42% respectively. The insoluble (40.94 ± 3.40%) and soluble (2.22 ± 1.02%) dietary fibre content were more in WB, and the results are comparable to that of data compiled in the review by Chawla and Patil (2010). However, the soluble dietary fibre content of RB (0.92 ± 0.097%) is significantly less when compared to published reports (Chawla and Patil 2010), which may be due to the difference in factors like extraction protocol, plant variety, geographical area etc.
The study indicated that RB and WB are better sources of dietary fibre as compared to many other agro-industrial byproducts (Arun et al. 2017; Sagar et al. 2018). Consumption of dietary fibre is reported to lower the risk of developing lifestyle associated diseases. The soluble dietary fibre is associated with the maintenance of gut homeostasis by improving gut microbiota growth. Experimental studies have shown that the prebiotic nature of soluble dietary fibre boost the growth of probiotic gut bacteria (Poeker et al. 2018) and thus maintains gut health. Therefore, there is an excellent scope for the value addition of RB and WB for developing functional foods and nutraceutical products. It is reported that the preeminent part of bioactive phytochemicals is destined to fibre. Since RB and WB were rich in fibre, we further investigated the phytochemical composition and its biological activity.
Total Phenolic Content and its HPLC profiling
The yield and TPC of extracts of RB and WB are shown in Table 2. Methanol extraction showed better yield for both RB and WB. However, this has not reflected in the phenolic content, as TPC is less in methanol extracts compared to that of ethyl acetate extracts. Sugars that usually get eluted with methanol might be the reason for increased yield of methanol extracts. The RB extracts possessed higher phenolic content than WB, and these TPC values fall within the range of reported values of various rice and wheat varieties (Arab et al. 2011; Brewer et al. 2014; Lai et al. 2009; Zhou and Yu 2004; Abozed et al. 2014; Wang et al. 2013).
Table 2.
Yield, TPC and CUPRAC values for different extracts of rice and wheat bran
| Yield% (g/g dry weight) | TPC (mg GAE/g dry weight) | CUPRAC (µM TR/g dry weight) | |
|---|---|---|---|
| RBE | 3.74 ± 0.245* | 6.50 ± 0.89* | 6.28 ± 1.23* |
| RBM | 6.58 ± 0.112# | 4.11 ± 1.10# | 10.51 ± 1.45# |
| WBE | 3.20 ± 0.117 | 4.70 ± 1.23 | 3.34 ± 1.50 |
| WBM | 5.70 ± 0.184 | 2.31 ± 0.92 | 5.91 ± 1.27 |
Each value represents mean ± SD (standard deviation) from triplicate measurements
*RBE significantly different from WBE; #RBM significantly different from WBM (p value ≤ 0.05)
Taken in the account of the fact that the medicinal properties of plants are generally associated with constituent phenolic compounds, the RB and WB extracts were analyzed qualitatively and quantitatively by HPLC (Table 3). Thirteen polyphenolic compounds were initially inspected with their corresponding retention times are as follows: (1) Gallic acid, (2) Catechol, (3) Chlorogenic acid, (4) Caffeic acid, (5) Syringic acid, (6) p-coumaric acid, (7) Ferulic acid, (8) Ellagic acid, (9) Myricetin, (10) Cinnamic acid, (11) Quercetin, (12) Kaempferol and (13) Apigenin.
Table 3.
Polyphenol quantification in ethyl acetate and methanol extracts of RB and WB
| Polyphenols | Yield (mg/g dry weight) | |||
|---|---|---|---|---|
| RBE | RBM | WBE | WBM | |
| Gallic acid | – | 0.004 ± 0.001b | – | – |
| Catechol | 0.122 ± 0.002 | – | 0.144 ± 0.004c | – |
| Chlorogenic acid | 0.017 ± 0.008a | – | – | – |
| Caffeic acid | 0.053 ± 0.001 | 0.071 ± 0.005 | 0.083 ± 0.005c | 0.064 ± 0.006 |
| Syringic acid | 0.041 ± 0.005 | 0.062 ± 0.008b | 0.035 ± 0.004 | – |
| p-Coumaric acid | 0.159 ± 0.021a | 0.040 ± 0.007 | 0.052 ± 0.007 | 0.115 ± 0.003d |
| Ferulic acid | 0.049 ± 0.022 | 0.030 ± 0.010 | 0.160 ± 0.002c | 0.044 ± 0.007 |
| Ellagic acid | 0.063 ± 0.008 | 0.033 ± 0.004b | 0.074 ± 0.001c | 0.023 ± 0.004 |
| Myercetin | 0.021 ± 0.002a | 0.014 ± 0.008b | 0.008 ± 0.001 | – |
| Cinnamic acid | 0.024 ± 0.004a | 0.013 ± 0.004 | 0.012 ± 0.007 | 0.010 ± 0.005 |
| Quercetin | 0.013 ± 0.001a | 0.011 ± 0.002b | – | – |
| Kaempferol | 0.374 ± 0.088a | 0.018 ± 0.002b | – | – |
| Apigenin | 0.399 ± 0.075a | 0.246 ± 0.054b | 0.053 ± 0.014 | 0.027 ± 0.001 |
Each value represents mean ± SD (standard deviation) from triplicate measurements
aValues of RBE significantly different from WBE
bValues of WBE significantly different from RBE
cValues of RBM significantly different from WBM
dValues of WBM significantly different from RBM
Twelve polyphenols were identified in RBE, whereas eleven polyphenols were identified in RBM (Fig. 1). RBE is rich in catechol, p-coumaric acid, kaempferol and apigenin (0.122, 0.159, 0.374 and 0.399 mg/g dry extract respectively). Nine polyphenols were identified in WBE, whereas six polyphenols were identified in WBM (Fig. 1). WBE is rich in catechol, ferulic acid, caffeic acid and ellagic acid (0.144, 0.160, 0.083 and 0.074 mg/g dry extract respectively). From the chromatograms, it is evident that RB extracts are endowed with more polyphenols than the WB. This may be correlated with the higher value of TPC for RB extracts as compared to WB. It may also be noted that the phytochemicals are distributed based on their solubility and polarity and flavonoids like myricetin, quercetin, kaempferol and apigenin which are less polar are more abundant in ethyl acetate extract as compared to methanol extract, as reported earlier (Brglez Mojzer et al. 2016).
Fig. 1.
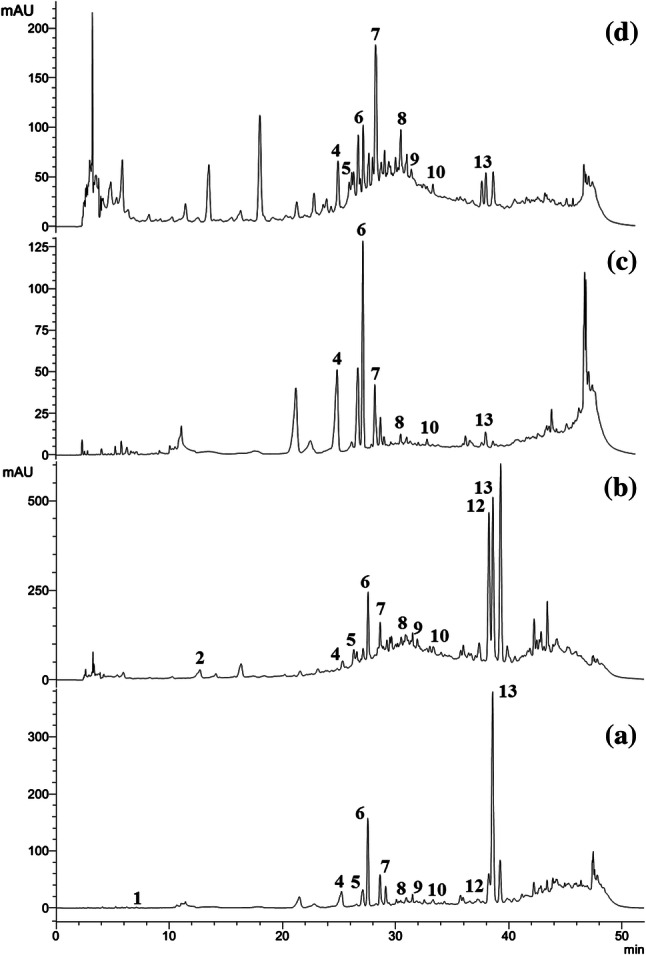
Chromatogramsdepicting HPLC profiling of polyphenols in extracts of RB and WB. (a) RBE, (b) RBM, (c) WBE and (d) WBM. The numbers assigned to corresponding peaks are referred to in the text
Antioxidant property
The outsets of lifestyle-associated diseases are characterized by excessive production of ROS combined with oxidative stress, and there is a continuous quest for new antioxidant sources which can play a significant role in avoiding the threat of acquiring lifestyle-associated diseases. The antioxidant efficacy is a multifactorial phenomenon that strongly relies on phytochemical nature, oxidative situations, and mechanisms of action. Hence, the antioxidant activity could not be assessed based on a single antioxidant assay (Silva et al. 2006). To address the antioxidant potential of bran extracts, we have performed Cu2+ reducing potential and free radical scavenging (DPPH, NO, superoxide radical) activities.
The Cu2+ reducing assay gives an idea about the total antioxidant capacity of the sample which is otherwise difficult to quantify by measuring the activity of specific antioxidants. The RBM and WBM depicted higher Cu2+ reducing activity than its corresponding ethyl acetate extracts. Among the methanol extract, the RBM was more active (10.51 µM TR/g dry weight). The results are given in Table 2.
The DPPH, NO and superoxide radical scavenging assays are generally used for the measurement of the antioxidant potential of compounds present in plant extracts. The NO and superoxide radicals when generated uncontrollably in biological systems results in increased inflammation, cellular components got damaged, and leads to chronic diseases. The IC50 values for DPPH, NO and Superoxide radical scavenging assays are given in Table S1. RBE and WBE exhibited better radical scavenging activity than the corresponding methanol extracts. Among the four extracts, RBE showed potent DPPH scavenging activity (IC50—250.14 µg/mL). Arab et al. (2011) reported that the methanol extract of RB scavenge DPPH free radical better.
In contrast, Zhou and Yu (2004) indicated that 70% methanol extract owns the highest DPPH scavenging activity among various extracts of WB. The results from the present study are comparable to these reports. The variation on the IC50 values may be attributed to the varietal difference of sources and solvents selected for the study. The NO radical scavenging activity revealed that the ethyl acetate extracts displayed high scavenging activity compared to corresponding methanol extracts of both RB and WB. RBE demonstrated the highest activity among the four extracts (IC50—346.81 µg/mL). The data from superoxide radical scavenging by the RB and WB extracts indicated that RBE (IC50—290.09 µg/mL) possesses the highest activity which may be correlated with the higher total phenolic content. As per our knowledge, to date, there are no studies that report the NO and superoxide radical scavenging activity of both RB and WB. Since RBE is affluent with polyphenols such as apigenin, kaempferol, p-coumaric acid and catechol, the better antioxidant potential of RBE can be attributed to the synergistic effect of these polyphenols. These specific polyphenols are reported to scavenge free radicals very efficiently (Hirano et al. 2001; Shen et al. 2019).
Though the activity of the extracts was lower than the respective standards, the extracts exhibited antioxidant activities, which may be of interest. The presence of phenolic compounds such as catechol, p-coumaric acid, kaempferol and apigenin in RBE and catechol, ferulic acid, caffeic acid and ellagic acid in WBE indicated promising biological activity of the extracts against degenerative diseases. Antidiabetic and anticancer activities of these particular polyphenols independently have been reported earlier (Proestos et al. 2005; Na et al. 2016; Li et al. 2018; Fang et al. 2008; Rajeswari and Sridevi 2014; Wang et al. 2000; Nair et al. 2009; Jaganathan et al. 2013; Song et al. 2014). Therefore, preliminary studies were carried out to evaluate the efficacy of the extracts in modulating diabetes and cancer using in vitro biochemical assays.
Antidiabetic and anticancer property
RB and WB extracts inhibit α-amylase and α-glucosidase action
Postprandial hyperglycemia is one of the key features of type 2 diabetes. Inhibiting primary enzymes (α-amylase and α-glucosidase) of carbohydrate metabolism is one of the methods adopted to reduce postprandial hyperglycemia. The potential of the bran extracts to reduce hyperglycemia was analyzed by its efficacy to suppress α-amylase and α-glucosidase, which release glucose from carbohydrate. The enzymes inhibitory activity was represented as IC50 values (Table S1). The RBE exhibited better α-amylase and α-glucosidase inhibiting activity (353.41 and 314.22 µg/mL). However, the activity of the extracts was less compared to acarbose. This is the first time report as far as to our knowledge about the potential of RB against α-amylase and α-glucosidase. The abundance of kaempferol and apigeninin RBE would have played a role in enzyme inhibition as these polyphenols are reported to interfere α-glucosidase and α-amylase action (Na et al. 2016; Li et al. 2018).
RB and WB extracts reduce the synthesis of advanced glycated end products
The antiglycation study demonstrated that RB is more active than WB (Table S1). RBE and WBE found to possess better activity when compared to the corresponding methanol extracts (451.11 and 515.39 µg/mL). Two standard molecules—ascorbic acid and aminoguanidine were used as positive controls for antiglycation assay, which exhibited an activity of 28.11 and 60.33 µg/mL respectively. It is well known that there exists a definite correlation between the inhibition of advanced glycated end-products synthesis with the phenolic content of the extracts from plant sources. p-Coumaric acid has been proved to associate with antiglycation property (Proestos et al. 2005) which might have contributed to the antiglycation property of RBE. So far, there is no study reporting the antiglycation potential of RB and WB.
Cell viability assay
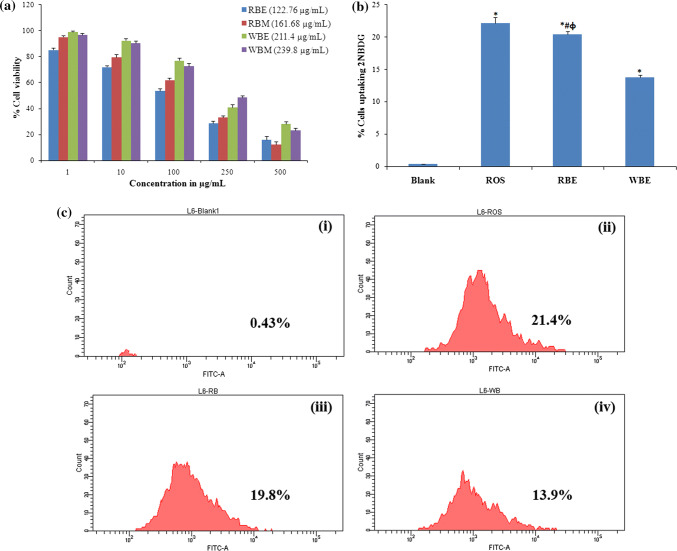
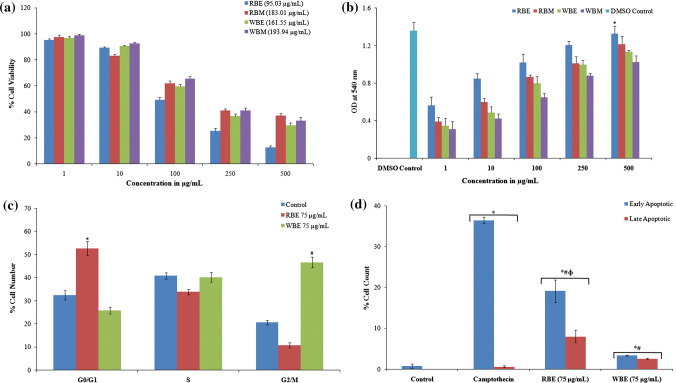
The cytotoxic effect of the extracts against L6 myoblast (Fig. 2a) and HT29 colon cancer cells (Fig. 3a) was assessed by MTT assay. The RBE and WBE were more toxic to L6 (IC50—122.76 µg/mL and 211.4 µg/mL) and HT29 (IC50—95.03 µg/mL and 161.55 µg/mL) cell lines. Concentrations below IC50 were used for further cell culture studies.
Fig. 2.
The effect of extracts on cell viability and glucose uptake efficacy of L6 muscle cells. a MTT assay showing cytotoxicity of ethyl acetate extracts of rice (RBE) and wheat (WBE) bran against L6 (IC50 values are shown in brackets). b Graphical representation of glucose uptake assay using 2-NBDG [*significance of difference from untreated, #significance of difference from Rosiglitazone (ROS positive control) and Φsignificance of difference between RBE and WBE] and c representative flow cytometry data of glucose uptake, i untreated control, ii rosiglitazone, iii ethyl acetate extract of rice bran-RBE (100 μg/mL) and iv ethyl acetate extract of wheat bran-WBE (100 μg/mL)
Fig. 3.
Analysis of cytotoxicity and anticancer action of extracts against HT-29 cancer cells. a MTT assay showing cytotoxicity of RBE and WBE against HT29 cells (IC50 values are shown in brackets); b LDH release assay showing extracts effect on cell membrane integrity [*RBE-500 μg/mL significantly different from other extracts]; c cell cycle arrest. Histogram illustrating the cells (%) arrested in various stages of the cell division cycle as obtained from flow cytometry analysis. Treatment–untreated control, camptothecin (50 μM), RBE (75 μg/mL) and WBE (75 μg/mL) (*#significance of difference of RBE and WBE with the untreated sample respectively); and d graphical representation showing cells in early and late phase apoptosis (*significance of difference from untreated, #significance of difference from positive control and Φsignificance of difference between RBE and WBE)
RB and WB extracts enhance uptake of Glucose in L6 cells
RBE and WBE were further studied for their activity in enhancing glucose uptake in L6 cells. RBE and WBE (100 µg/mL, concentration below IC50) were taken for the study. The results demonstrated that the pre-incubation of L6 cells with RBE and WBE effectively induced glucose uptake (Fig. 2b, c). RBE and WBE enhanced the cellular uptake of glucose by 20.4 ± 0.5% and 13.8 ± 0.361% respectively, compared to the control cells. It was exciting to note that the RBE activity was similar to that of rosiglitazone 100 nM (22.15 ± 0.87%). As muscle and adipose cells fail to respond to the impaired insulin in Type 2 diabetes, researchers are in search of natural constituents that enhance the response of adipose and muscle cells to insulin, which in turn leads to increased glucose uptake in the adipocytes or myotubes. The study suggested that bran is an excellent source of phytochemicals with antidiabetic potential. The better activity of RBE can be linked to the higher content of kaempferol and apigenin (Fang et al. 2008; Rajeswari and Sridevi 2014), along with the synergistic effect of other polyphenol compounds present in the extract.
RB and WB extracts impair cell membrane integrity and release LDH
To understand the effects of RB and WB on the prevention and management of cancer, its effect on cell membrane integrity by LDH release, cell cycle arrest and induction of apoptosis were studied. During cell death, lactate dehydrogenase (LDH) released as the cells losses its membrane integrity and is, therefore, is considered as a marker of cell death. Therefore, leakage of the membrane-bound LDH was studied to assess the effect of the extracts on membrane integrity of HT29 colon cancer cell. The results are illustrated in Fig. 3b. The cells treated with 100% DMSO exhibited maximum LDH release. In the case of extracts, the LDH release increased with increase in the concentration of the extracts. The maximum LDH release was exhibited when treated with RBE. Thus, the assay established that pretreatment with extract significantly affects the integrity of the membrane of HT29 cells.
The extracts of RB and WB arrest cell cycle
The fact that defects in cell cycle checkpoints will lead to the development of cancer is well established (Barnum and O'Connell 2014). The cytotoxicity and LDH release assay showed that both RBE and WBE inhibit the growth of HT29 cells. Based on these data, we further analyzed the effect of the extracts on cell cycle progression. Following the treatment with extracts at 75 μg/mL (concentration below IC50), the dissemination of cells in diverse stages of the cell division cycle was interpreted employing flow cytometry. The experimental outcome indicated that pre-incubation with RBE resulted in the arrest of 52.7 ± 3.1% cells at G0/G1 phase, whereas WBE arrest 46.6 ± 2.3% cells at the G2/M phase. The results are presented in Fig. 3c. The results from the present study indicated that the extracts were able to interfere with the cell. However, further experiments need to be performed to analyze the role of extracts in modulating checkpoint proteins to confirm the exact mechanism by which the extracts demonstrate this activity.
RB and WB extracts activate apoptosis
The effect of extracts to induce apoptosis in HT29 cells was determined via a combination of Annexin V-FITC and propidium iodide. The mean values of cells in early and late-stage apoptosis are depicted in Fig. 3d, and the representative flow cytometry data are shown in Figure S1. The data depicted that RBE was more active in inducing apoptosis when compared to WBE. The percentage of cells that were in the early stage apoptosis after exposing to RBE was 21.1 ± 2.75% whereas 8.0 ± 1.55% of cells were found to be in the late-stage apoptosis. At the same time, only 3.35 ± 0.07% and 2.55 ± 0.21% of cells exhibited early and late stage of apoptosis, respectively, after treatment with WBE.
It was observed that RBE exhibited better activity than WBE. The anticancer effect of RBE can be attributed to its higher phytochemicals that include apigenin, kaempferol, p-coumaric acid and catechol; as these compounds were proved to have anticancer potential against diverse group of cancer cell lines (Wang et al. 2000; Nair et al. 2009; Jaganathan et al. 2013; Song et al. 2014).
Conclusion
Bran is the most nutritious and versatile part of the cereal kernels which has been underutilized as a food source. The bran from rice and wheat as investigated under the present work has been found to be a good source of dietary fibre and phytochemicals such as kaempferol, apigenin, catechol, p-coumaric acid, ferulic acid etc. The extracts demonstrated suitable antioxidant activities. The antidiabetic properties that were evaluated in terms of inhibition of α-amylase and α-glucosidase, antiglycation and glucose uptake assays showed that RBE demonstrated better activity in enhancing glucose uptake efficacy and inhibiting the key enzymes involved in carbohydrate metabolism. The antiglycation property suggests the potential of the bran extracts against diabetes-associated complications. Abnormal cell cycle progression and inhibition of apoptosis are the signature hallmark of cancer development and the bran extracts, especially RBE, demonstrated promising activity by hindering the cell cycle and activating apoptosis. The data obtained from the present investigation confirms the biological properties of rice bran and wheat bran in terms of antidiabetic and anticancer potential. The study emphasizes the potential of value addition of bran in developing functional foods and nutraceutical products for preventive management of lifestyle-associated diseases and these less studied cereal varieties may be a good source of dietary fibre and phytochemicals with enormous applications.
Electronic supplementary material
Figure S1. Flow cytometry determination of apoptosis. The quadrant Q1, Q2, Q3 and Q4 correspondingly portrays cells at necrotic, late apoptotic, live and early apoptotic stage. (i) Untreated control, (ii) Positive control – camptothecin (50 μM), (iii) RBE (75 μg/mL) and (iv) WBE (75 μg/mL); (D) LDH release assay showing extracts effect on cell membrane integrity [*RBE-500 μg/mL significantly different from other extracts] (JPG 287 kb)
Table S1. IC50 values of various assays for different extracts of RB and WB (DOCX 14 kb)
Acknowledgements
The authors appreciate the research amenities provided by CSIR India. The first author is grateful to ICMR India for the junior research fellowship.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abozed SS, El-kalyoubi M, Abdelrashid A, Salama MF. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann Agric Sci. 2014;59(1):63–67. doi: 10.1016/j.aoas.2014.06.009. [DOI] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) In: Official methods of analysis. 15. Helrich K, editor. Arlington, VA: AOAC Inc; 1990. [Google Scholar]
- Apak R, Guclu K, Ozyurek M, Celik SE. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim Acta. 2008;160(4):413–419. doi: 10.1007/s00604-007-0777-0. [DOI] [Google Scholar]
- Arab F, Alemzadeh I, Maghsoudi V. Determination of antioxidant component and activity of rice bran extract. Sci Iran. 2011;18(6):1402–1406. doi: 10.1016/j.scient.2011.09.014. [DOI] [Google Scholar]
- Arun KB, Sithara T, Reshmitha TR, Akhil GC, Nisha P. Dietary fibre and phenolic-rich extracts from Musa paradisiaca inflorescence ameliorates type 2 diabetes and associated cardiovascular risks. J Funct Foods. 2017;31:198–207. doi: 10.1016/j.jff.2017.02.001. [DOI] [Google Scholar]
- Barnum KJ, O'Connell MJ. Cell cycle regulation by checkpoints. Methods Mol Biol. 2014;1170:29–40. doi: 10.1007/978-1-4939-0888-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer LR, Kubola J, Siriamornpun S, Herald TJ, Shi YC. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014;152(1):483–490. doi: 10.1016/j.foodchem.2013.11.128. [DOI] [PubMed] [Google Scholar]
- Brglez Mojzer E, Knez Hrnčič M, Škerget M, Knez Ž, Bren U. Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21(7):901. doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Indian Standards (1984) Method for estimation of total dietary fiber in food stuffs. IS, 11062-1984
- Chawla R, Patil GR. Soluble dietary fibre. Compr Rev Food Sci Food Saf. 2010;9:178–196. doi: 10.1111/j.1541-4337.2009.00099.x. [DOI] [Google Scholar]
- Daou C, Zhang H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J Food Sci Technol. 2013;51(12):3878–3885. doi: 10.1007/s13197-013-0925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X-K, Gao J, Zhu D-N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82(11–12):615–622. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Hirano R, Sasamoto W, Matsumoto A, Itakura H, Igarashi O, Kondo K. Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J Nutr Sci Vitaminol. 2001;47(5):357–362. doi: 10.3177/jnsv.47.357. [DOI] [PubMed] [Google Scholar]
- Jaganathan SK, Supriyanto E, Mandal M. Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World J Gastroenterol. 2013;19(43):7726–7734. doi: 10.3748/wjg.v19.i43.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai P, Li KY, Lu S, Chen HH. Phytochemicals and antioxidant properties of solvent extracts from Japonica rice bran. Food Chem. 2009;117(3):538–544. doi: 10.1016/j.foodchem.2009.04.031. [DOI] [Google Scholar]
- Li K, Yao F, Xue Q, Fan H, Yang L, Li X, Sun L, Liu Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem Cent J. 2018;12(1):82. doi: 10.1186/s13065-018-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Chen K, Tu D, Yu X, Dai Z, Shen Q. Characterization of dietary fiber from wheat bran (Triticum aestivum L.) and its effect on the digestion. LWT Food Sci Technol. 2019;102:106–112. doi: 10.1016/j.lwt.2018.12.024. [DOI] [Google Scholar]
- Mingyai S, Kettawan A, Srikaeo K, Singanusong R. Physicochemical and antioxidant properties of rice bran oils produced from colored rice using different extraction methods. J Oleo Sci. 2017;66(6):565–572. doi: 10.5650/jos.ess17014. [DOI] [PubMed] [Google Scholar]
- Na B, Nguyen PH, Zhao BT, Vo QH, Min BS, Woo MH. Protein tyrosine phosphatase 1B (PTP1B) inhibitory activity and glucosidase inhibitory activity of compounds isolated from Agrimonia pilosa. Pharm Biol. 2016;54(3):474–480. doi: 10.3109/13880209.2015.1048372. [DOI] [PubMed] [Google Scholar]
- Nair PK, Melnick SJ, Wnuk SF, Rapp M, Escalon E, Ramachandran C. Isolation and characterization of an anticancer catechol compound from Semecarpus anacardium. J Ethnopharmacol. 2009;21(3):450–456. doi: 10.1016/j.jep.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Ozdestan O, Erol T, Acar B (2014) Phytosterols in rice bran and usage of rice bran in food industry. In: Straumite E, Jelgava LLU (eds) 9th Baltic conference on food science and technology “food for consumer well-being” FOODBALT 2014 conference proceedings, pp 24–27
- Poeker SA, Geirnaert A, Berchtold L, Greppi A, Krych L, Steinert RE, de Wouters T, Lacroix C. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS) Sci Rep. 2018;8:4318. doi: 10.1038/s41598-018-22438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proestos C, Chorianopoulos N, Nychas GJE, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts: investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem. 2005;53:1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- Rajeswari R, Sridevi M. Study of in vitro glucose uptake activity of isolated compounds from hydro alcoholic leaf extract of Cardiospermum halicacabum Linn. Int J Pharm Pharm Sci. 2014;6(11):181–185. [Google Scholar]
- Sagar AN, Pareek S, Sharma S, Yahia EM, Lobo MG. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf. 2018;17(3):512–531. doi: 10.1111/1541-4337.12330. [DOI] [PubMed] [Google Scholar]
- Sairam S, Gopala Krishna AG, Urooj A. Physico-chemical characteristics of defatted rice bran and its utilization in a bakery product. J Food Sci Technol. 2011;48(4):478–483. doi: 10.1007/s13197-011-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Song X, Li L, Sun J, Jaiswal Y, Huang J, Liu C, Yang W, Williams L, Zhang H, Guan Y. Protective effects of p-coumaric acid against oxidant and hyperlipidemia—an in vitro and in vivo evaluation. Biomed Pharmacother. 2019;111:57–587. doi: 10.1016/j.biopha.2018.12.074. [DOI] [PubMed] [Google Scholar]
- Silva EM, Souza JNS, Rogez H, Rees JF, Larondella Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2006;10:1012–1018. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Song W, Dang Q, Xu D, Chen Y, Zhu G, Wu K, Zeng J, Long Q, Wang X, He D, Li L. Kaempferol induces cell cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38 signalling. Oncol Rep. 2014;31(3):1350–1356. doi: 10.3892/or.2014.2965. [DOI] [PubMed] [Google Scholar]
- Venkatachalam U, Muthukrishnan S. Free radical scavenging activity of ethanolic extract of Desmodium gangeticum. J Acute Med. 2012;2(2):36–42. doi: 10.1016/j.jacme.2012.04.002. [DOI] [Google Scholar]
- Vitaglione P, Napolitano A, Fogliano V. Cereal dietary fibre: a natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci Technol. 2008;19:451–463. doi: 10.1016/j.tifs.2008.02.005. [DOI] [Google Scholar]
- Wang W, Heidman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28(2):102–110. doi: 10.1002/1098-2744(200006)28:2<102::AID-MC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wang T, Raddatz J, Chen G. Effects of microfluidization on antioxidant properties of wheat bran. J Cereal Sci. 2013;58(3):380–386. doi: 10.1016/j.jcs.2013.07.010. [DOI] [Google Scholar]
- Zhou K, Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT Food Sci Technol. 2004;37:717–721. doi: 10.1016/j.lwt.2004.02.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow cytometry determination of apoptosis. The quadrant Q1, Q2, Q3 and Q4 correspondingly portrays cells at necrotic, late apoptotic, live and early apoptotic stage. (i) Untreated control, (ii) Positive control – camptothecin (50 μM), (iii) RBE (75 μg/mL) and (iv) WBE (75 μg/mL); (D) LDH release assay showing extracts effect on cell membrane integrity [*RBE-500 μg/mL significantly different from other extracts] (JPG 287 kb)
Table S1. IC50 values of various assays for different extracts of RB and WB (DOCX 14 kb)