Abstract
Wolbachia is one of the most abundant facultative intracellular symbionts in arthropods. It alters host biology in diverse ways, including the induction of reproductive manipulation, association of nutrient supplier and protection against pathogens. Aphids are a group of insects which exhibit interesting biological characteristics such as complex life cycles, alteration of sexual and asexual reproduction and shifts between two different hosts. Wolbachia is widely present in many orders of insects, but so far limited studies on Wolbachia in aphids have been carried out. Galling aphids are a group of aphids that induce galls on their primary host plants at specific life stage. In this study, 15 natural populations representing nine galling aphid species were analyzed for the presence of Wolbachia using species-specific primer pairs. Wolbachia presence in galling aphids was quite low and varied significantly among aphid populations. Only three of the 15 populations we analyzed had detectable Wolbachia and the overall infection rate was 20%. Two Wolbachia strains, O and B, were identified from the galling aphids Kaburagia rhusicola and Schlechtendalia chinensis. Strain O was for the first time to be found in aphids, and it is likely involved with the life stages of galling aphids living in closed microenvironments with specific survival strategies that are different from free-living aphids.
Subject terms: Bacteria, Pathogens
Introduction
Wolbachia is an intracellular facultative symbiont present widely in arthropods. Wolbachia species such as Wolbachia pipientis have many different strains1,2. At present, 16 Wolbachia supergroups have been reported, and named A to F and H to Q from insects3–6. Wolbachia diversity was initially characterized using the genes wsp, 16S rRNA, ftsZ, gltA, and groEL as molecular markers7. One of the consequences of Wolbachia in insects is associated with the induction of different reproductive strategies such as parthenogenesis, feminization, male-killing and cytoplasmic incompatibility (CI)8–11. In addition to reproduction, Wolbachia can also affect the biological characteristics of its hosts, such as providing host nutrients, protecting hosts from RNA viruses, regulating the age structure of the host populations, and improving the proliferation of host stem cells13–16. The presence of Wolbachia in insects may put selective pressure more strongly on a host or create reproductive barriers that can lead to speciation. Wolbachia-based methods have also been developed to control the transmission of insect pests and arboviruses17,18. Aphids are a group of insects, many of which are important pests for agriculture and forestry18,19. However, some species such as galling aphids can manipulate to plant tissues, resulting in the formation of galls, which can provide protection for aphids from predators20–22. Like most aphids, galling aphids exhibit complex biological traits and a complicated life cycle, such as sexual and asexual reproduction, and alternating between two host species23. Among galling aphids, Chinese galling aphids are specific species which induce closed galls rich in tannins on Chinese sumac trees (Anacardiaceae: Rhus) and have been used for medicinal, chemical and other industrial purposes23,24.
Until recently, aphids were thought to be free of Wolbachia26–29. However, data on European aphids indicate that the absence of Wolbachia was an underestimate3. A total of 425 natural samples representing 144 aphid species have been analyzed for the presence of Wolbachia using specific 16S rRNA-based PCR, and 37 (8.7%) samples have been found to have Wolbachia3. Later in China, 109 samples representing 73 aphid species have also been analyzed, and all the samples have been found to have Wolbachia4. Wang et al. (2014)2 suggested that the infection status of Wolbachia in aphid populations from China might differ from other areas and this multiple infection pattern was probably caused by horizontal transmission2,30,31. However, horizontal transmission has not been observed in European aphids.
So far, four Wolbachia supergroups, A, B, M and N have been detected in aphids with supergroup M being the most commonly detected3. The distribution patterns of Wolbachia in Chinese aphids are complex and varied among different species4. Wolbachia in aphids is underestimated primarily because of its low titer and/or high divergence of different strains. Among the 217 aphid species analyzed for Wolbachia before, only 11 were galling aphids (4.1%), and of those, only six galling aphids carried Wolbachia3,4. Therefore, the distribution of Wolbachia in galling aphids and its significance remain to be studied.
The objective of this study is to investigate the presence of Wolbachia in natural populations of galling aphids from China. Specifically, Wolbachia strains were screened and classified based on nine marker genes: 16S rRNA, gltA, groEL, wsp, gatB, fbpA, coxA, hcpA and ftsZ. Phylogenetic analysis was also conducted based on the sequences of the marker genes.
Results
Screening for Wolbachia in natural populations of galling Aphids
A total of 117 samples of natural galling aphids were collected from various host plants in 7 different locations in China. The 117 samples represented 15 populations of 9 aphid species within 6 genera, 2 tribes of Aphididae. The 15 populations were from genera Kaburagia (1 species, 5 populations), Floraphis (1 species, 1 populations), Schlechtendalia (2 species, 4 populations), Nurudea (1 species, 1 populations), Pemphigus (3 species, 3 populations), and Chaetogeoica (1 species, 1 populations) (Table 1). All samples were screened for the presence of Wolbachia by PCR amplification using 16S rRNA-specific primers 16S-281F/1372R. Our results showed that the presence of Wolbachia in these populations was infrequent and varied significantly among different aphid populations. Among all 15 aphid populations, only 3 populations were detected to harbor Wolbachia. These Wolbachia-carrying aphids were Kaburagia rhusicola and Schlechtendalia chinensis from the tribe Fordini, and Pemphigus yunnanensis from the tribe Pemphigini. No Wolbachia was detected in the remaining populations. Among the Wolbachia-positive species, Wolbachia was detected in four out of six samples in K. rhusicola, and in one out of nine samples in either S. chinensis or P. yunnanensis (Table 1).
Table 1.
Sample for the study of Wolbachia infection in galling aphid.
| Aphid species | Primary host | Collected location | Wolbachia infection prevalence | Number of tested populations | Number of infected populations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16S rRNA | MLST genes | Other genes | Supergroup | |||||||||||
| gatB | fbpA | coxA | hcpA | ftsZ | wsp | gltA | groEL | |||||||
| Kaburagia rhusicola | Rhus potaninii | Yunnan Kunming | + | + | + | − | − | − | − | − | − | O | 6 | 4 |
| Shaanxi Chenggu | − | − | − | − | − | − | − | − | − | − | 6 | 0 | ||
| Shaanxi Ningqiang | − | − | − | − | − | − | − | − | − | − | 6 | 0 | ||
| Yunman Yanjin | − | − | − | − | − | − | − | − | − | − | 6 | 0 | ||
| Sichuan Emei | − | − | − | − | − | − | − | − | − | − | 6 | 0 | ||
| Floraphis meitanensis | Rhus punjabensis var. sinica | Yunnan Kunming | − | − | − | − | − | − | − | − | − | − | 9 | 0 |
| Schlechtendalia peitan | Rhus chinensis | Yunnan Kunming | − | − | − | − | − | − | − | − | − | − | 9 | 0 |
| Nurudea shiraii | Rhus chinensis | Yunnan Kunming | − | − | − | − | − | − | − | − | − | − | 9 | 0 |
| Schlechtendalia chinensis | Rhus chinensis | Yunnan Kunming | − | − | − | − | − | − | − | − | − | − | 9 | 0 |
| Hubei Wufeng | − | − | − | − | − | − | − | − | − | − | 9 | 0 | ||
| Sichuan Emei | + | + | + | − | − | − | − | − | − | O | 9 | 1 | ||
| Pemphigus yangcola | Populus yunnanensis | Yunnan Kunming | − | − | − | − | − | − | − | − | − | − | 6 | 0 |
| Pemphigus yunnanensis | Populus yunnanensis | Yunnan Kunming | + | + | + | + | + | + | − | − | − | B | 9 | 1 |
| Pemphigus populitransversus | Populus yunnanensis | Yunnan Kunming | − | − | − | − | − | − | − | − | − | − | 9 | 0 |
| Chaetogeoica folidentata | Pistacia chinensis | Yunnan Chuxiong | − | − | − | − | − | − | − | − | − | − | 9 | 0 |
+, amplification; −, failed to detect amplification product.
Sequence variation in Wolbachia genes
Nine genes including 16S rRNA, gltA, groEL, wsp, gatB, fbpA, coxA, hcpA and ftsZ of Wolbachia were selected for PCR amplification. However, only six genes were amplified from at least one of the three populations harboring Wolbachia. Three genes including 16S rRNA, gatB and fbpA were amplified from K. rhusicola and S. chinensis. The amplicon lengths were 1,077 bp for 16S rRNA, 497 bp for gatB, and 476 bp for fbpA for samples from K. rhusicola, whereas the amplicons were 1,061 bp for 16S rRNA, 492 bp for gatB, and 479 bp for fbpA for samples from the S. chinensis. Six genes including 16S rRNA, gatB, fbpA, coxA, hcpA and ftsZ were amplified from the P. yunnanensis. The amplicon sizes were 1,054 bp for 16S rRNA, 497 bp for gatB, 480 bp for fbpA, 488 bp for coxA, 509 bp for hcpA, and 518 bp for ftsZ (Table 2).
Table 2.
Gene sequence lengths of Wolbachia infected in the galling aphids.
| Aphids and collected location | Gene names | |||||
|---|---|---|---|---|---|---|
| 16S rRNA | MLST genes | |||||
| gatB | fbpA | coxA | hcpA | ftsZ | ||
| Kaburagia rhusicola Kunming | 1,077 | 497 | 476 | – | – | – |
| Schlechtendalia chinensis Emei | 1,061 | 492 | 479 | – | – | – |
| Pemphigus yunnanensis Kunming | 1,054 | 497 | 480 | 488 | 509 | 518 |
Phylogenetic analysis
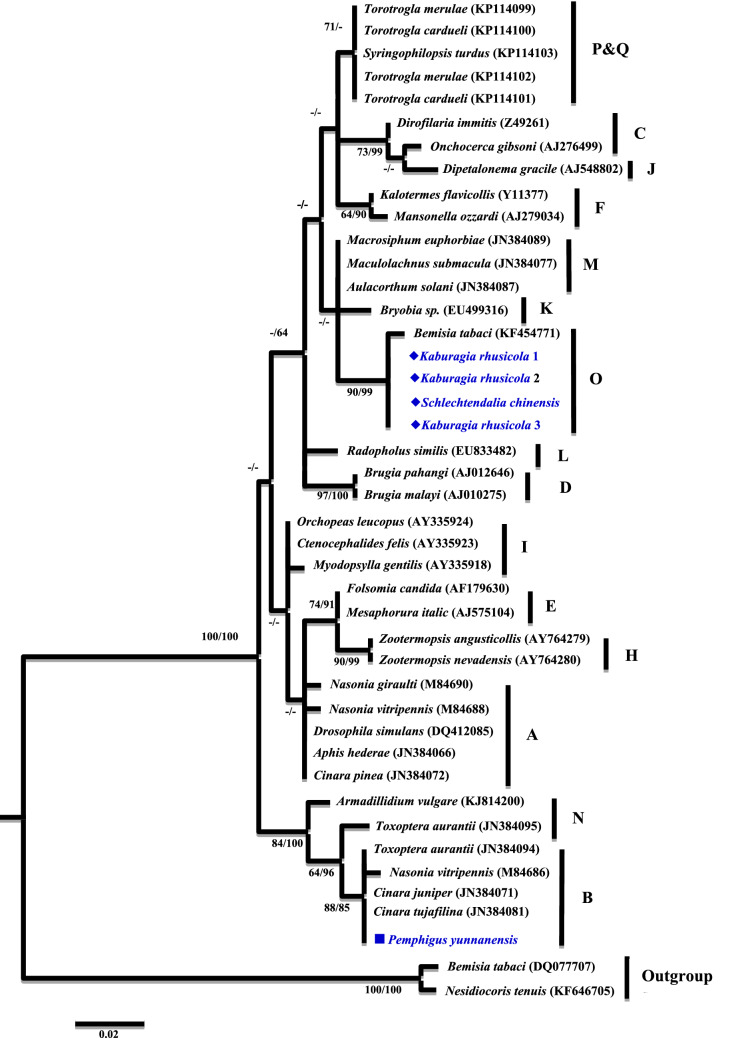
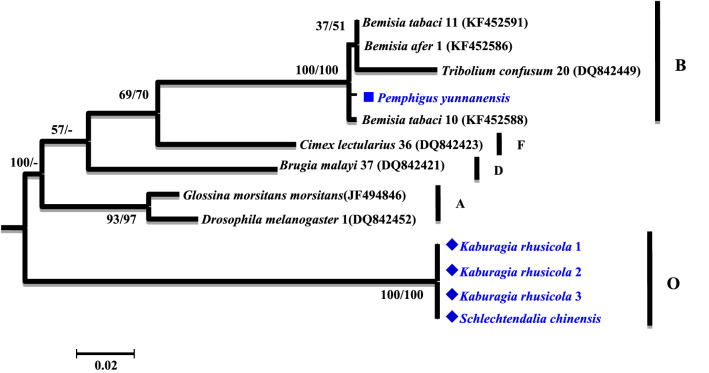
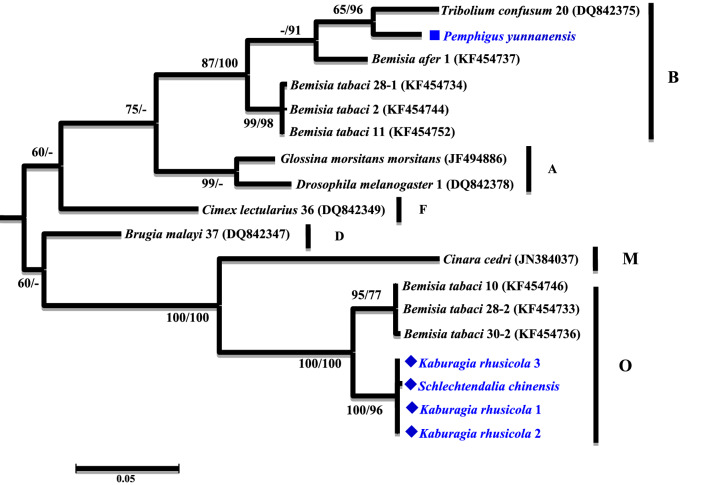
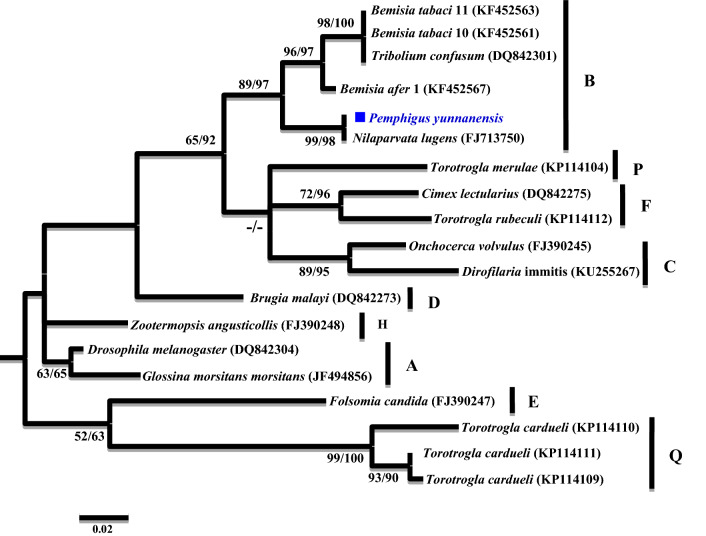
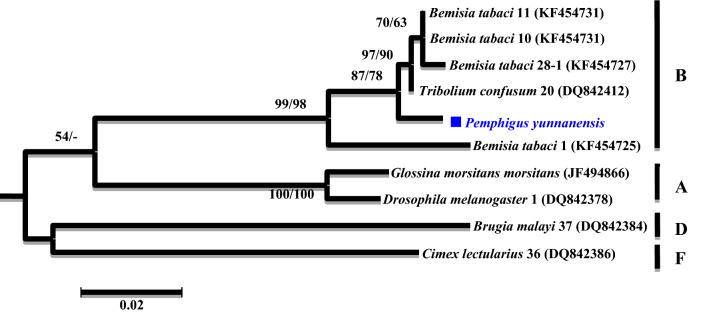
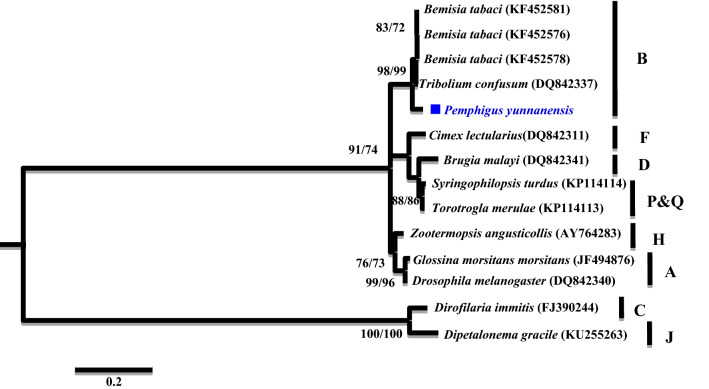
A phylogenetic analysis was conducted based on the partial 16S rRNA gene sequences (about 1,100 bp). Two different groups were revealed. The Wolbachia strains detected from K. rhusicola and S. chinensis clustered within supergroup O with high bootstrap support (90/99), while strains detected from the P. yunnanensis clustered with supergroup B with high bootstrap support values (88/85) (Fig. 1). Moreover, Wolbachia strains detected from K. rhusicola and S. chinensis formed distinct Wolbachia lineage analogous to Wolbachia supergroup O based on gatB and fbpA sequences with high bootstrap (100/100, 100/96) (Figs. 2, 3). Wolbachia strains detected from P. yunnanensis clustered within supergroup B with moderate bootstrap support (88/85) based on partial 16S rRNA gene sequences, and on coxA, hcpA and ftsZ gene sequences with high or moderate bootstrap (99/98, 87/78, 98/99) (Figs. 4, 5, 6).
Figure 1.
Phylogenetic analysis inferred from Wolbachia 16S rRNA gene sequences using Maximum Likelihood (ML) and Bayesian Inference (BI). Scale bar indicates substitutions per site. Aphid K. rhusicola and S. chinensis indicated by ‘filled diamonds’, and P. yunnanensis indicated by ‘filled squares’. ‘–’ indicated support rate less than 50%.
Figure 2.
Phylogenetic analysis inferred from Wolbachia gatB gene sequences using Maximum Likelihood (ML) and Bayesian Inference (BI). Scale bar indicates substitutions per site. Aphid K. rhusicola and S. chinensis indicated by ‘filled diamonds’, and P. yunnanensis indicated by ‘filled squares’. ‘–’ indicated support rate less than 50%.
Figure 3.
Phylogenetic analysis inferred from Wolbachia fbpA gene sequences using Maximum Likelihood (ML) and Bayesian Inference (BI). Scale bar indicates substitutions per site. Aphid K. rhusicola and S. chinensis indicated by ‘filled diamonds’, and P. yunnanensis indicated by ‘filled squares’. ‘–’ indicated support rate less than 50%.
Figure 4.
Phylogenetic analysis inferred from Wolbachia coxA gene sequences using Maximum Likelihood (ML) and Bayesian Inference (BI). Scale bar indicates substitutions per site. Aphid P. yunnanensis indicated by ‘filled squares’. ‘–’ indicated support rate less than 50%.
Figure 5.
Phylogenetic analysis inferred from Wolbachia hcpA gene sequences using Maximum Likelihood (ML) and Bayesian Inference (BI). Scale bar indicates substitutions per site. Aphid P. yunnanensis indicated by ‘filled squares’. ‘–’ indicated support rate less than 50%.
Figure 6.
Phylogenetic analysis inferred from Wolbachia ftsZ gene sequences using Maximum Likelihood (ML) and Bayesian Inference (BI). Scale bar indicates substitutions per site. Aphid P. yunnanensis indicated by ‘filled squares’. ‘–’ indicated support rate less than 50%.
Discussion
There are 16 supergroups of Wolbachia that have been identified in insects at present3–5. Four of them, named supergroup A, B, M and N, have been detected in aphids. So far Wolbachia was detected mostly in free-living aphids3,4. In our study, supergroup O and B were detected from three galling aphid species, including K. rhusicola, S. chinensis and P. yunnanensis. Supergroup O has not been reported from aphids before our study. Supergroup O from these two galling aphids was clustered with the supergroup O from a whitefly, forming a distinct Wolbachia lineage, which is analogous to the linkage formed based on gatB and fbpA gene sequences with robust bootstrap values (Figs. 1, 2, 3).
The relation between aphids and their facultative symbiont Wolbachia can be affected by different factors such as the ability of symbionts to spread from aphids to aphids within or across populations, the cost of infection for hosts, and aphid living environments (host plant, natural enemy pressure, or temperature)32,33. Compared to non-galling aphids, most life stages of galling aphids are in closed microenvironments21,23. Generally, hundreds to thousands of aphid individuals living in a gall are produced parthenogenetically by a single fundatrix19,21,34. If a fundatrix did not carry Wolbachia before she induces a gall, its offspring have basically no chance to be infected by Wolbachia in an enclosed environment. This means that Wolbachia can hardly spread across galling aphid populations. Moreover, galling aphids receive less pressure from natural enemies than non-galling aphids since they are protected by the gall wall20,35. Although vertical transfer is the predominant mode for Wolbachia, horizontal transfer also appeared in nature by infrequently. Horizontal transfer of Wolbachia was observed when infected and uninfected larvae of Trichogramma wasps shared the same hosts31. Also, Hymenopteran parasitoids of frugivorous Drosophila acquired Wolbachia through horizontal transmission with high frequency36. The three galling aphids in our study are host alternation, so they may readily acquire Wolbachia when they free-living on the secondary hosts by shared the same host mosses or attacked by parasitoid wasps which carried Wolbachia. All aphid samples of our study were collected from closed galls, so they are quite different in their living environments, natural enemy pressures and host plants compared to non-galling aphids20,21,34. This is probably the reason why they harbor Wolbachia strains or supergroups different to those in free-living aphids3,4.
Many marker genes have been used to detect Wolbachia in insects, but the consistency of these markers varies among different insect species37–39. The most conserved marker gene among different Wolbachia strains is the 16S rRNA gene, which also provides more consistent PCR amplification. However, because the 16S rRNA gene is highly conserved, non-target amplification occurs during PCR amplification5. The wsp gene evolves faster among different Wolbachia strains. Therefore, PCR amplification is not highly consistent with current primer pairs7. In this study, the primers for the 16S rRNA, gatB and fbpA genes could detect Wolbachia in some galling aphid populations. However, primers for the coxA, hcpA and ftsZ genes detected Wolbachia only in P. yunnanensis (Table 1). Primers for the wsp, groEL and gltA genes did not detect Wolbachia in any samples, indicating that the primer regions in these genes varied and the primers could not achieve specific PCR amplifications. Our results also revealed that Wolbachia in the galling aphids was more difficult to detect using the existing primers possibly because of the variation in the sequences of target genes in the Wolbachia strains. Thus, the selection of more efficient and specific Wolbachia gene primers is needed to advance future research on Wolbachia analyses in aphids, especially in galling aphids.
Materials and methods
Aphid sample collection
Aphids of each population were collected from a mature gall. Fresh aphid galls were collected from host plants manually and dissected in the lab. Aphids were transferred to an Eppendorf tube from a gall, placed in 100% ethanol and then stored at − 20 °C until DNA extraction. Galls collected from each location within ten meters were treated as the same population. Since three generations of aphids are produced parthenogenetically by a mating female in a gall to achieve hundreds to thousands of offspring, aphids from a gall were treated as a clone. Galls collected from a location outside 10 m were treated as different populations.
Nine galling aphid species (Aphididae: Eriosomatinae: Fordini and Pemphigini) were collected from five host trees at eight locations in Yunnan, Sichuan, Hubei and Shaanxi provinces of China. K. rhusicola was the most common species and five populations were obtained from this species. S. chinensis was the second common species with three populations collected. The other six galling aphid species included Floraphis meitanensis, Schlechtendalia peitan, Pemphigus yangcola, Pemphigus yunnanensis, Pemphigus populitransversus and Chaetogeoica folidentata, each with only one population collected (Table 1).
DNA extraction
Ten to twenty aphid individuals of a population were selected for DNA extraction. Genomic DNA was extracted using Dzup Genomic DNA Isolation Reagent (Sangon Biotech, China) from pooled aphids of the same clone following the manufacturer's protocol and was then stored at − 20 °C until detected.
Wolbachia detection
DNA samples of 117 natural galling aphid populations were screened for the presence of Wolbachia strains. Detection was based on the amplification of the 16S rRNA gene fragment (about 1,100 bp) with the Wolbachia-specific primers 16S 281F 5′-CTATAGCTGATCTGAGAGGAT-3′ and 16S 1372R 5′-YGCTTCGAGTGAAACCAATTC-3′ (Table 3)4. PCR procedures were an initial step of 94 °C for 3 min, followed by 35 cycles of 94 °C for 45 s, 55 °C for 60 s, and 72 °C for 90 s and a final step of 72 °C for 10 min. Amplified DNA products were electrophoresed on agarose gels and stained. PCR products were sequenced from both directions in an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA). Sequencing results were then checked by Blast in NCBI nr database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Only those samples, which were blasted to expected products of the specific primers, were selected for further analysis. These selected samples were further examined by PCR analyses of the genes gltA, groEL, wsp, gatB, fbpA, coxA, hcpA and ftsZ using specific primers based on previous reports (Table 3)40–43. PCR amplifications were carried out in 25 μl reaction volume, consisting of 1 μl DNA, 1 μl of forward and reverse primers (10 μmol/l), 12.5 μl Taq Master Mix (Sangon Biotech, China), and 9.5 μl ddH2O. PCR products (4 μl) were electrophoresed on a 1.5% agarose gel. Positive samples were further analyzed.
Table 3.
Primer list used for Wolbachia detection.
| Gene | Hypothetical product | Primer name and sequences (5′–3′) | Product size | Tm (°C) | References |
|---|---|---|---|---|---|
| 16S rRNA | Ribosomal RNA 16S |
16S 281F: CTATAGCTGATCTGAGAGGAT 16S 1372R: YGCTTCGAGTGAAACCAATTC |
1,100 | 55 | Wang et al.4 |
| 16S rRNA | Ribosomal RNA 16S |
WspecF: CAT ACC TAT TCG AAG GGA TAG WspecR: AGC TTC GAG TGA AAC CAA TTC |
440 | 52 | Werren and Windsor43 |
| wsp | Outer surface protein |
Wsp81F: TGGTCCAATAAGTGATGAAGAAAC Wsp691R: AAAAATTAA ACGCTACTCCA |
546 | 52 | Zhou and Rousset40 |
| groEL | Chaperonin GroEL |
GroELF: GGTGAGCAGTTRCARSAAGC GroELR: TARCCRCGRTCAAAYTGCATRCCA |
491 | 55 | Wang et al.4 |
| ftsZ | Cell division protein |
FtsZF: GTTGGYAAAGGTGCAGCAGAAGA FtsZR: CGYACYCATTTKGCTGCAGMATCAA |
524 | 53 | Wang et al.4 |
| groEL | Chaperonin GroEL |
groEL F1: GGTGAGCAGTTGCAAGAAGC groEL R1: AGRTCTTCCATYTTRATTCC |
491 | 55 | Casiraghi et al.41 |
| gltA | Citrate synthase |
gltAF1: TACGATCCAGGGTTTGTTTCTAC gltAR1: CTCATTAGCTCCACCGTGTG |
659 | 56 | Casiraghi et al.41 |
| gatB | Glutamyl-tRNA(Gln) amidotransferase, subunit B |
GatB F1: GAKTTAAAYCGYGCAGGBGTT GatB R1: TGGYAAYTCRGGYAAAGATGA |
497 | 54 | Paraskevopoulos et al.42 |
| coxA | Cytochrome coxidase, subunit I |
CoxA-F1: TTGGRGCRATYAACTTTATAG CoxA-R1: CTAAAGACTTTKACRCCAGT |
488 | 55 | Paraskevopoulos et al.42 |
| hcpA | Conserved hypothetical protein |
HcpA-F1: GAAATARCAGTTGCTGCAAA HcpA-R1: GAAAGTYRAGCAAGYTCTG |
515 | 53 | Paraskevopoulos et al.42 |
| fbpA | Fructose-bisphosphate aldolase |
FbpA-F1: GCTGCTCCRCTTGGYWTGAT FbpA-R1: CCRCCAGARAAAAYYACTATTC |
509 | 59 | Paraskevopoulos et al.42 |
Sequencing
Nine genes 16S rRNA, gltA, groEL, wsp, gatB, fbpA, coxA, hcpA and ftsZ of Wolbachia were amplified and sequenced from the populations harboring Wolbachia. PCR products were sequenced from both directions in an ABI 3730 DNA analyzer. Original sequences were manually processed and then assembled by DNA Star Lasergene v7.1-SeqMan.
Nucleotide sequence accession numbers
Twelve gene sequences of 16S rRNA, gltB, fbpA, coxA, hcpA and ftsZ generated in this study have been deposited in the GenBank database under accession numbers MT554837-MT554842 and MT634226-MT634228.
Sequence alignment and phylogenetic analysis
Genes representative of Wolbachia strains from different supergroups were selected from the NCBI database (https://www.ncbi.nlm.nih.gov), and used to classify Wolbachia strains detected in our aphid samples. Sequence alignments were carried out using ClustalX 1.8344. Maximum likelihood (ML) and Bayesian inference (BI) were used for phylogenetic analysis. The ML analysis was carried out with MEGA 6.0 and node support rates were evaluated with 1,000 bootstrap replicates. The Bayesian inference (BI) method was performed in MrBayes 3.1.245. In MrBayes, four chains (one cold and three heated chains) which were run for 4 million generations. Trees were sampled every 100 generations, and the first 25% of samples were discarded as burn-in. From the remaining trees, 50% majority-rule consensus trees were generated. Posterior probabilities were computed from the remaining trees. To obtain the appropriate evolution model, the parameters were evaluated using the Akaike Information Standard (AIC) in MrModeltest 2.346. Using this method, the following gene models were obtained: the HKY + G model of 16S rRNA gene, the GTR + G model of ftsZ gene, the HKY + G model of coxA gene, the GTR + I + G model of gatB gene and the HKY + G model of fbpA gene.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (31872305; U1402263), the National Key R & D Program of China (2018YFD0600403), and the grant for Innovative Team of ‘Insect Molecular Ecology and Evolution’ of Yunnan Province. We thank to Prof. Ming-Shun Chen, Kansas State University, and Prof. Kirst King-Jones, University of Alberta, for their helpful comments.
Author contributions
Z.Y and X.C designed and led the project. W.R collected samples, carried out experiments. W.R and Z.Y analyzed the data and wrote the manuscript. W.R, H.W, Y.Y, S.S and H.W designed Figures. All authors reviewed the manuscript. The manuscript is approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Werren JH. Biology of Wolbachia. Annu. Rev. Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 2.Stouthamer R, Breeuwer JA, Hurst GD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 3.Augustinos AA, et al. Detection and characterization of Wolbachia infections in natural populations of aphids is the hidden diversity fully unraveled. PLoS ONE. 2011;6:e28695. doi: 10.1371/journal.pone.0028695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Su XM, Wen J, Jiang LY, Qiao GX. Widespread infection and diverse infection patterns of Wolbachia in Chinese aphids. Insect Sci. 2014;21:313–325. doi: 10.1111/1744-7917.12102. [DOI] [PubMed] [Google Scholar]
- 5.Bing XL, et al. Diversity and evolution of the Wolbachia endosymbionts of Bemisia (Hemiptera: Aleyrodidae) whiteflies. Ecol. Evol. 2014;13:2714–2737. doi: 10.1002/ece3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glowska E, Dragun-Damian A, Dabert M, Gerth M. New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae) Infect. Genet. Evol. 2015;30:140–146. doi: 10.1016/j.meegid.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Baldo L, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rigaud T, et al. Feminizing endocytobiosis in the terrestrial crustacean Armadillidiumvulgare Latr. (Isopoda): recent acquisitions. Endocytobiosis Cell Res. 1991;7:259–273. [Google Scholar]
- 9.Gdd H, Men M, Walker LE. The importance of cytoplasmic male killing elements in natural populations of the two spot ladybird, Adalia bipunctata (Linnaeus) (Coleoptera: Coccinellidae) Biol. J. Linn. Soc. 1993;49:195–202. [Google Scholar]
- 10.Stouthamer R, Breeuwer JAJ, Luck RF, Werren JH. Molecular identification of microorganisms associated with parthenogenesis. Nature. 1993;361:66–68. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- 11.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 12.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702–702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 13.Moreira LA, et al. Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;7:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Mcmeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fast EM, et al. Wolbachia enhances Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourtzis K. Wolbachia-based technologies for insect pest population control. Adv. Exp. Med. Biol. 2008;627:104–113. doi: 10.1007/978-0-387-78225-6_9. [DOI] [PubMed] [Google Scholar]
- 18.Blackman RL, Eastop VF. Aphids on the World’s herbaceous plants and shrubs. West Sussex: Wiley; 2006. [Google Scholar]
- 19.Zhang GX, Qiao GX, Zhong TS, Zhang WY. Fauna Sinica Insecta 14, Homoptera, Mindaridae and Pemphigidae. Beijing: Science Press ; 1999. [Google Scholar]
- 20.Wool D. Galling aphids: specialization, biological complexity, and variation. Annu. Rev. Entomol. 2004;49:175–192. doi: 10.1146/annurev.ento.49.061802.123236. [DOI] [PubMed] [Google Scholar]
- 21.Stone GN, Schonrogge K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003;18:512–522. [Google Scholar]
- 22.Schultz JC, Edger PP, Body MJA, Appel HM. A galling insect activates plant reproductive programs during gall development. Sci. Rep. 2019;9:1833. doi: 10.1038/s41598-018-38475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran NA. The evolution of aphid life cycles. Annu. Rev. Entomol. 1992;37:321–348. [Google Scholar]
- 24.Zhang GX, Zhong TS. Economic insect fauna of China. 25 Homoptera: Aphidinea. Beijing: Science Press; 1983. [Google Scholar]
- 25.Yang ZX. High yield cultivation techniques of Chinese Gallnut. Beijing: China Forestry Publishing House; 2011. [Google Scholar]
- 26.Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002;11:2123–2135. doi: 10.1046/j.1365-294x.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Valero L, et al. Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. J. Bacteriol. 2004;86:6626–6633. doi: 10.1128/JB.186.19.6626-6633.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Shen ZR, Song Y, Liu HY, Li ZX. Distribution and diversity of Wolbachia in different populations of the wheat aphid Sitobion miscanthi (Hemiptera: Aphididae) in China. Eur. J. Entomol. 2009;106:49–55. [Google Scholar]
- 30.Reuter M, Keller L. High levels of multiple Wolbachia infection and recombination in the ant Formica exsecta. Mol. Biol. Evol. 2003;20:748–753. doi: 10.1093/molbev/msg082. [DOI] [PubMed] [Google Scholar]
- 31.Huigens ME, et al. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. Lond. B. 2004;271:509–515. doi: 10.1098/rspb.2003.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo J, et al. Nine facultative endosymbionts in aphids. A review. J. Asia-Pac. Entomol. 2017;21:794–801. [Google Scholar]
- 33.De Clerck C, et al. A metagenomic approach from aphid’s hemolymph sheds light on the potential roles of co-existing endosymbionts. Microbiome. 2015;3:1–11. doi: 10.1186/s40168-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao SX, Yang ZX, Chen XM. Gall development and clone dynamics of the galling aphid Schlechtendalia chinensis (Hemiptera: Pemphigidae) J. Econ. Entomol. 2012;106:1628–1637. doi: 10.1603/ec13114. [DOI] [PubMed] [Google Scholar]
- 35.Oliver KM, et al. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 36.Vavre F, Fleury F, Lepetit D, Fouillet P, Boulétreau M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 1999;16:1711–1723. doi: 10.1093/oxfordjournals.molbev.a026084. [DOI] [PubMed] [Google Scholar]
- 37.Gerth M. Classification of Wolbachia (Alphaproteobacteria, Rickettsiales): No evidence for a distinct supergroup in cave spiders. Infect. Genet. Evol. 2016;43:378–380. doi: 10.1016/j.meegid.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Lefoulon E, et al. Breakdown of coevolution between symbiotic bacteria Wolbachia and their filarial hosts. PeerJ. 2016;4:e1840. doi: 10.7717/peerj.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bleidorn C, Gerth M. A critical re-evaluation of multilocus sequence typing (MLST) efforts in Wolbachia. FEMS Microbiol. Ecol. 2018 doi: 10.1093/femsec/fix163. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Rousset F, O'Neill S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casiraghi M, et al. Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology. 2005;151:4015–4022. doi: 10.1099/mic.0.28313-0. [DOI] [PubMed] [Google Scholar]
- 42.Paraskevopoulos C, Bordenstein SR, Wernegreen JJ, Werren JH, Bourtzis K. Toward a Wolbachia multilocus sequence typing system: discrimination of Wolbachia strains present in Drosophila species. Curr. Microbiol. 2006;53:388–395. doi: 10.1007/s00284-006-0054-1. [DOI] [PubMed] [Google Scholar]
- 43.Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. Biol. Sci. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 46.Nylander JAA. MrModeltest v2.2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University; 2004. [Google Scholar]