Abstract
Objective: Women with overweight/obesity have significantly lower rates of exclusive breastfeeding (EBF) at 6 weeks postpartum compared with women of normal weight. We sought to determine whether differences in Baby-Friendly Hospital Initiative (BFHI) adherence, obstetric practices, or social support explain these weight-related EBF disparities.
Methods: One hundred forty-two healthy women who intended EBF (61 normal weight, 50 overweight, and 31 obese by preconception body mass index [BMI]) were enrolled in a cross-sectional study. Obstetric data were collected and participants completed modified Infant Feeding Practices Study II surveys at 6 weeks postpartum.
Results: Women with obesity were significantly less likely to undergo spontaneous labor and more likely to receive synthetic oxytocin and epidural anesthesia compared with women with overweight or normal weight. Women who were overweight were less likely to report extended family support for breastfeeding compared with women with obesity or normal weight; however, BFHI components and composite BFHI score did not differ by maternal BMI. Furthermore, regardless of BMI, women with greater adherence to BFHI practices were more likely to be EBF at 6 weeks postpartum (p-value <0.001). Nonetheless, at 6 weeks postpartum, women with obesity were expressing milk more frequently and less likely to have met their own breastfeeding goals compared with women with overweight and normal weight.
Conclusions: Differences in EBF rates by BMI were not explained by BFHI adherence or obstetric practices. These data suggest physiological differences, rather than intrapartum practices and support services, may explain differences in EBF rates by maternal overweight/obesity.
Keywords: breastfeeding, obesity, Baby-Friendly, social support, obstetric practices, attitude toward breastfeeding
Introduction:
Exclusive breastfeeding (EBF) is recommended for optimal infant nutrition, and is associated with numerous maternal and infant health benefits, including lower rates of obesity, type 2 diabetes, and cardiovascular disease.1 To support establishment of breastfeeding, the Baby-Friendly Hospital Initiative (BFHI) has identified 10 steps to optimize successful breastfeeding. Revised in 2018, these recommendations include immediate skin-to-skin contact, initiation of breastfeeding as soon as possible after birth, support for new mothers during initiation and maintenance of breastfeeding, rooming-in of the newborn with the mother rather than in a nursery, helping mothers to recognize infant feeding cues, and counseling on the recommended use of bottles/teats/pacifiers (dummies).2 Adherence to these steps leads to higher rates of EBF and longer duration of any breastfeeding.3 Health facilities that demonstrate evidence-based maternity practices and policies such as the BFHI have been shown to improve breastfeeding outcomes.4 In addition, other studies have suggested the importance of support for breastfeeding by providers, partners, and family members.5 In contrast, there are several recognized barriers to EBF, including delayed lactogenesis and insufficient milk production, which may be influenced by obstetric complications and maternal factors, including age, obesity, gestational diabetes, pre-eclampsia, cesarean delivery, and separation of mothers and infants after birth.6 Women with obesity have higher rates of obstetric intervention, including cesarean delivery and induction of labor.7,8 There has been limited attention to the impact of obstetric practices on EBF, aside from inconsistent reports on the relationship between mode of delivery (vaginal versus cesarean) and breastfeeding rates,9–11 and, therefore, the impact of obstetric practices on EBF rates by maternal body mass index (BMI) remains unexplored.
Maternal obesity is associated with impaired lactation, including earlier introduction of formula supplementation and earlier breastfeeding cessation.12–14 We have reported significant differences in EBF rates by maternal BMI among women intending EBF, with undesired formula introduction starting within the first 2 weeks after delivery.15 Maternal obesity has previously been associated with decreased exposure to pro-breastfeeding hospital practices according to large retrospective survey data16 as well as psychosocial characteristics that associate with poor breastfeeding outcomes.17 Our objective in this study was to determine whether adherence to BFHI practices in the early postpartum period varied by maternal BMI, and whether there are differences in social support, obstetric practices, and breastfeeding behaviors/breast emptying by maternal BMI that may help to explain the BMI-related disparity in EBF rates.
Materials and Methods
Participants
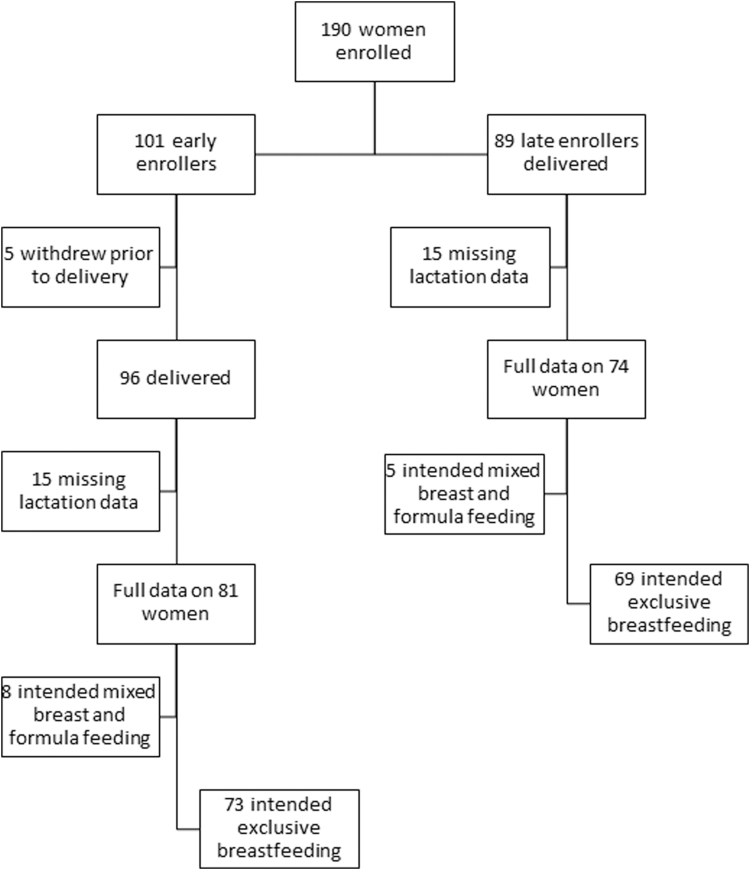
We studied a longitudinal cohort of 190 healthy mother–baby pairs stratified by maternal prepregnancy BMI and enrolled at Oregon Health & Science University from October 2015 to April 2018, as previously described.15 The OHSU Institutional Review Board approved the study protocol, #IRB00011175, and each participant provided signed informed consent before enrollment. One hundred one women were early enrollers (12–16 weeks gestation) and 89 were late enrollers (>37 weeks gestation); both groups were followed through the first year postpartum (Fig. 1). Five early enrollers withdrew before delivery. Women who lacked lactation survey data were excluded (n = 30), leaving 155 participants available for analysis (81 early enrollers, 74 late enrollers). One hundred forty-two women intended EBF and were included in the final sample. Women who did not intend EBF were more likely to have obesity, but otherwise were similar to women who intended EBF. Maternal prepregnancy BMI was determined by measured maternal height and self-reported prepregnancy weight, which strongly correlated with first prenatal visit weight (p < 0.0001, r2 = 0.98). Maternal BMI was categorized using the World Health Organization (WHO) BMI categories: normal weight (BMI 18.5–24.9 kg/m2) (n = 59), overweight (25.0–29.9 kg/m2) (n = 50), and obese (BMI ≥30.0 kg/m2) (n = 31). Two women were underweight (BMI <18.5 kg/m2) and were included in the normal weight category.
FIG. 1.
Study participant flowchart.
Breastfeeding variables
Exclusive breastfeeding of the infant was defined per the WHO criteria as no other food or drink, not even water, except human milk (including milk expressed or from a donor) for 6 months of life; the WHO criteria allows the infant to receive oral rehydration solution, vitamins, and medicines when necessary.
Obstetric data were collected from review of the electronic medical record. Participants completed modified Infant Feeding Practice Study II surveys18 online at 6 weeks postpartum to assess adherence to BFHI steps, obstetric factors, social support, and infant feeding intention (human milk, formula, and both). The survey was completed at 6 weeks postpartum as this is when the routine postpartum obstetric visit occurs for most women, and the majority of women have not yet returned to paid employment. Cessation of EBF was determined by self-report of infant age (in weeks) at first use of formula supplementation.
Analysis
The primary outcome was adherence to BFHI practices while admitted to the hospital, as defined by a composite of (1) skin-to-skin contact immediately after birth, (2) initiation of breastfeeding within 60 minutes after birth, (3) breastfeeding on demand, and (4) no pacifier use in the hospital. Two additional BFHI practices were not included in the composite score: rooming-in and assistance with breastfeeding. All women experienced rooming-in, which is the standard of care in our institution as there is no well-baby nursery available. All women who reported requesting assistance with breastfeeding (58.5%, n = 83) reported receiving assistance (100%, n = 83), and there was no difference by EBF status or BMI category. Secondary outcomes studied included perceived social support, labor practices, frequency of breast emptying, and attitudes about breastfeeding. Each variable was compared by BMI category.
Descriptive statistics were used to characterize the demographic profile of our cross-sectional sample. In addition to maternal BMI, we described the age, parity, race, gestational age at delivery, mode of delivery, birth weight, prior breastfeeding experience, and return to work timing. We analyzed unadjusted associations between maternal prepregnancy BMI and antepartum and postpartum factors.
Results
Among the 142 women who intended EBF, women who were overweight were slightly but significantly older than women with normal weight or obesity, and women with obesity had the lowest mean gestational weight gain (Table 1). All other demographic variables were matched between participants differing by maternal BMI.
Table 1.
Demographics of Women Intending Exclusive Breastfeeding
| Total (n = 142) | Normal weight (n = 61) | Overweight (n = 50) | Obese (n = 31) | p | |
|---|---|---|---|---|---|
| Maternal age (years) (mean ± SD) | 33.3 (4.7) | 33.0 (4.5) | 34.6 (4.8) | 32.0 (4.6) | 0.039 |
| Race/ethnicity,an (%) | |||||
| White | 121 (85.2) | 54 (88.5) | 41 (82.0) | 26 (83.9) | 0.61 |
| Black | 4 (2.8) | 0 | 3 (6.0) | 1 (3.2) | 0.16 |
| Hispanic | 12 (8.6) | 5 (8.3) | 4 (8.3) | 3 (9.7) | 0.87 |
| Asian | 7 (4.9) | 4 (6.6) | 3 (6.0) | 0 | 0.35 |
| American Indian | 8 (5.6) | 2 93.3) | 3 (6.0) | 3 (9.7) | 0.45 |
| Pacific Islander | 1 (0.7) | 1 (1.6) | 0 | 0 | 0.51 |
| Declined/unknown | 5 (3.5) | 1 (1.6) | 3 (6.0) | 1 (3.2) | 0.46 |
| Nulliparous, n (%) | 82 (58.2) | 36 (59.0) | 26 (52.0) | 20 (66.7) | 0.43 |
| Prenatal smoking, n (%) | 1 (0.7) | 0 | 0 | 1 (3.2) | 0.09 |
| Gestatational Weight Gain (kg) (min–max) | 13.0 | 14.3 (7.7 – 26.0) | 13.1 (−3.6 – 29) | 10.2 (−5.2 – 28.4) | 0.009 |
| GA at delivery (weeks), mean (±SD) | 39.4 (1.3) | 39.5 (1.4) | 39.2 (1.3) | 39.7 (1.1) | 0.20 |
| Birth weight (kg), mean (±SD) | 3.41 (0.43) | 3.38 (0.47) | 3.41 (0.41) | 3.49 (0.39) | 0.48 |
| Fetal sex (male), n (%) | 67 (47.2) | 29 (47.5) | 23 (46.0) | 15 (48.4) | 0.98 |
| Return to work, n (%) | 92 (75.4) | 48 (81.4) | 26 (65.0) | 18 (78.3) | 0.17 |
| Breastfed as infant | 105 (73.9) | 51 (83.6) | 37 (74.0) | 17 (54.8) | 0.039 |
| Partner breastfed as infant | 93 (65.5) | 46 (75.4) | 33 (66.0) | 14 (45.2) | 0.046 |
| Favored breastfeeding | |||||
| Maternal provider | 123 (86.6) | 52 (85.3) | 45 (90.0) | 26 (83.9) | 0.67 |
| Infant provider | 122 (85.9) | 52 (85.3) | 45 (90.0) | 25 (80.7) | 0.49 |
| Partner preference | 119 (85.6) | 53 (88.3) | 39 (81.3) | 27 (87.1) | 0.56 |
| Extended family | 90 (63.4) | 46 (75.4) | 24 (48.0) | 20 (64.5) | 0.012 |
| Partner's extended family | 73 (51.8) | 35 (57.4) | 23 (46.0) | 15 (50.0) | 0.48 |
| BF experience (%) (multiparous only) | 57 (95.0) | 23 (92.0) | 24 (100) | 10 (90.9) | 0.35 |
| Intended BF duration (months), mean (±SD) | 14.0 (6.6) | 15.2 (4.9) (6–24) | 13.6 (8.9) (1–48) | 11.9 (4.7) (6–24) | 0.09 |
| Confident to reach age (1–5 scale), mean (±SD) | 4.03 (0.9) | 4.1 (0.8) | 4.1 (0.9) | 3.8 (1.0) | 0.18 |
| Confident (%) | 100 (73) | 47 (78.3) | 36 (75.0) | 17 (58.6) | 0.14 |
| Lactation class attendance | 39 (27.5) | 16 (26.2) | 11 (22.0) | 12 (38.7) | 0.25 |
Bold represents p-value < 0.05.
Reported all that apply.
BF, breastfeeding; GA, gestational age; SD, standard deviation.
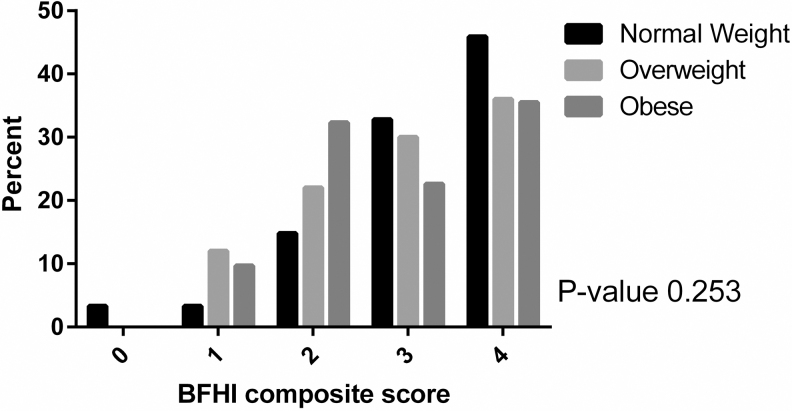
Higher BFHI composite scores were associated with significantly higher rates of EBF at 6 weeks PP compared with lower scores (p-value <0.001) (Supplementary Fig. S1). However, maternal BMI was not significantly associated with BFHI composite score (Fig. 2) or individual BFHI components (Table 2). Similarly, there was no difference by maternal BMI on BFHI composite score when stratified by 6-week EBF status (Supplementary Tables S1 and S2).
FIG. 2.
Adherence to BFHI by maternal body mass index. BFHI, Baby-Friendly Hospital Initiative.
Table 2.
Individual Components of Baby-Friendly Hospital Initiative by Maternal Body Mass Index and Exclusive Breastfeeding Status
| Total (N = 142) |
Normal weight (n = 61) |
Overweight (n = 50) |
Obese (n = 31) |
p | |||||
|---|---|---|---|---|---|---|---|---|---|
| EBF (n = 109) | Non-EBF (n = 32) | EBF (n = 55) | Non-EBF (n = 5) | EBF (n = 36) | Non-EBF (n = 14) | EBF (n = 18) | Non-EBF (n = 13) | ||
| Skin-to-skin | 98 (89.9) | 25 (78.1) | 51 (92.7) | 2 (40.0) | 32 (88.9) | 12 (85.7) | 15 (83.3) | 11 (84.6) | 0.81 |
| Breastfed in <60 minutes | 82 (75.9) | 14 (43.8) | 43 (79.6) | 2 (40.0) | 26 (72.2) | 7 (50.0) | 13 (72.2) | 5 (38.5) | 0.21 |
| Pacifier in hospital | 22 (20.2) | 16 (50) | 9 (16.4) | 2 (40.0) | 9 (25.0) | 8 (57.1) | 4 (22.2) | 6 (46.2) | 0.46 |
| Feeding on demand | 82 (75.2) | 18 (56.3) | 40 (72.7) | 2 (40.0) | 26 (72.2) | 9 (64.3) | 16 (88.9) | 7 (53.9) | 0.23 |
| Needed help nursing while in hospitala | 60 (55.6) | 23 (71.9) | 28 (51.9) | 4 (80.0) | 22 (61.1) | 10 (71.4) | 10 (55.6) | 9 (69.2) | 0.57 |
| Received help in hospitala | 60 (100) | 23 (100) | 28 (100) | 4 (100) | 22 (100) | 10 (100) | 10 (100) | 9 (100) | 1.0 |
n (%).
Not included in composite score.
Women with obesity experienced significantly different obstetric practices compared with women with overweight and normal weight, including lower rates of spontaneous labor (p = 0.018), higher likelihood of receiving synthetic oxytocin in labor (p = 0.043), and higher rates of epidural usage (p = 0.03) (Table 3). There was no difference by maternal BMI on labor support persons at the birth (family, friends, and/or doula) or mode of delivery (Table 3).
Table 3.
Intrapartum and Postpartum Practices
| Total (N = 142) | Normal weight (n = 61) | Overweight (n = 50) | Obese (n = 31) | p | |
|---|---|---|---|---|---|
| Labor support | |||||
| Partner/family friends | 141 (99.3) | 61 (100) | 50 (100) | 30 (96.8) | 0.17 |
| Doula | 29 (20.4) | 13 (21.3) | 12 (24.0) | 4 (12.9) | 0.47 |
| Spontaneous labor (not induced)a | 62 (43.7) | 35 (57.4) | 22 (44.0) | 5 (16.1) | 0.018 |
| Pain medication in labor | |||||
| Epidural | 96 (67.6) | 37 (60.7) | 32 (64.0) | 27 (87.1) | 0.03 |
| IV narcotics | 46 (32.4) | 15 (24.6) | 17 (34.0) | 14 (45.2) | 0.13 |
| Nitrous oxide | 30 (21.1) | 17 (27.9) | 6 (12.0) | 7 (22.6) | 0.12 |
| Unmedicated | 32 (22.5) | 17 (27.9) | 13 (26.0) | 2 (6.5) | 0.052 |
| Pitocin in labora | 79 (59.9) | 31 (52.5) | 24 (54.6) | 24 (82.8) | 0.017 |
| Mode of delivery (%) | 0.21 | ||||
| Vaginal | 103 (72.5) | 47 (77.1) | 37 (74.0) | 19 (61.3) | |
| Assisted vaginal | 1 (0.7) | 1 (1.6) | 0 | 0 | |
| Scheduled cesarean | 10 (7.0) | 2 (3.3) | 6 (12.0) | 2 (6.5) | |
| Unscheduled cesarean | 28 (19.7) | 11 (18.0) | 7 (14.0) | 10 (32.3) | |
| Separated from infant | 33 (23.2) | 13 (21.3) | 11 (22.0) | 9 (29.0) | 0.69 |
| Another woman's milk | 28 (19.9) | 11 (18.3) | 7 (14.0) | 10 (32.3) | 0.13 |
| Milk bank | 17 (13.8) | 9 (14.8) | 5 (12.5) | 5 (20.8) | 0.54 |
| Family/friend | 4 (3.3) | 2 (3.4) | 1 (2.5) | 1 (4.2) | 0.93 |
| Started formula before discharge | 8 (5.6) | 2 (3.3) | 3 (6.0) | 3 (9.7) | |
| 6 Weeks postpartum | |||||
| Currently breastfeeding | 137 (96.5) | 60 (98.4) | 48 (96.0) | 29 (93.6) | 0.48 |
| How many times daily, mean (±SD) | 9.15 (3.0) | 9.6 (2.9) | 9.25 (2.5) | 8.0 (3.5) | 0.06 |
| Currently expressing milk | 98 (69.0) | 39 (63.9) | 36 (72.0) | 23 (74.2) | 0.51 |
| How many times daily, mean (±SD) | 2.39 (2.2) | 1.84 (1.8) | 2.25 (1.9) | 3.54 (3.0) | 0.012 |
| How much total milk (oz), mean (±SD) | 5.16 (0.98) | 5.31 (0.92) | 5.09 (0.98) | 5.0 (1.07) | 0.44 |
| How use expressed milk | |||||
| Give to infant now | 29 (20.4) | 9 (14.8) | 10 (20) | 10 (32.3) | 0.14 |
| Some infant, freeze some | 50 (35.2) | 24 (39.3) | 16 (32.0) | 10 (32.3) | 0.67 |
| Freeze it all | 25 (17.6) | 8 (13.1) | 11 (22.0) | 6 (19.4) | 0.45 |
| Give away/donate | 6 (4.2) | 3 (4.9) | 2 (4.0) | 1 (3.2) | 0.93 |
| Longest stretch without breastfeeding (hours), mean (±SD) | 4.35 (1.4) | 4.46 (1.6) | 4.25 (1.1) | 4.28 (1.5) | 0.72 |
| Galactagogue use | 64 (45.1) | 28 (45.9) | 24 (48.0) | 12 (38.7) | 0.71 |
| How well infant gaining weight | 0.41 | ||||
| Not gaining enough | 4 (2.8) | 3 94.9) | 0 | 1 (3.2) | |
| Gaining well | 136 (95.8) | 57 (93.4) | 50 (100) | 29 (93.6) | |
| Gaining too much | 0 | 0 | 0 | 0 | |
| Not sure | 2 (1.4) | 1 91.6) | 0 | 1 (3.2) | |
| Satisfied with current feeding method | 123 (86.6) | 56 (91.8) | 46 (92.0) | 21 (67.7) | 0.002 |
| Like breastfeeding | 101 (74.3) | 51 (85.0) | 31 (64.6) | 19 (67.9) | 0.037 |
| Met goals for breastfeeding | 120 (84.5) | 55 (90.2) | 44 (88.0) | 21 (67.7) | 0.013 |
Bold represents p-value < 0.05.
n (%).
Excludes scheduled cesarean.
Prenatal factors
There were no significant differences by maternal BMI on intended duration of breastfeeding, confidence to reach desired length, lactation class attendance, or prior breastfeeding experience (Table 1). Women with obesity were significantly less likely to have been breastfed themselves compared with women with normal weight and overweight (54.8% versus 83.6% versus 74.0%, p = 0.039). The partners of women with obesity (BMI not collected) were also less likely to have been breastfed compared with partners of women with normal weight and overweight (45.2% versus 75.4% versus 66.0%, p = 0.046).
Social support
When asked about their perception of the attitude of others toward favoring EBF as the best way to feed their infant, there was no difference by maternal BMI on maternal provider, infant provider, partner, or partner's extended family (e.g., in-laws) support for EBF (Table 1). However, maternal BMI was associated with a difference in perceived support for breastfeeding by their own extended family members (e.g., parents, siblings) among women with overweight compared with women with normal weight or obesity (48.0% versus 75.4% versus 64.5%, p = 0.012). Also, independent of maternal BMI, there was a significant difference in perceived familial support for breastfeeding depending on whether the woman and/or her partner had been breastfed. More than 70% of the families of women who were breastfed as infants supported breastfeeding, compared with only 41.9% of families of women who had not been breastfed as infants (p-value 0.012). Similarly, when a partner had been breastfed, 59.1% of partner's families supported breastfeeding compared with 37% of families of partners who had not been breastfed (p-value 0.051).
Postpartum breastfeeding practices and attitudes
At 6 weeks postpartum, women with obesity were expressing milk more frequently than women with overweight and normal weight (3.54 × daily versus 2.25 × versus 1.84 × , p = 0.012, Table 3). There was no difference by maternal BMI on frequency of breastfeeding sessions, whether currently expressing milk, how expressed milk was used, use of galactagogues, or longest gap between breast emptying. The majority of women felt like their babies were gaining weight well. However, women with overweight and obesity were significantly less likely to report that they enjoyed breastfeeding (liked or liked very much) compared with women with normal weight (64.6% versus 67.9% versus 85.0%, p = 0.037). In addition, women with obesity were significantly less likely to be satisfied or very satisfied with their current feeding method compared with women with normal weight and overweight (67.7% versus 91.8% versus 92.0%, p = 0.002) and less likely to feel like they had met their own goals for breastfeeding (67.7% versus 90.2% versus 88.0%, p = 0.013).
Discussion
In this study, we did not find significant differences in BFHI practices immediately after birth by maternal BMI that would explain discrepancies in EBF rates. We also did not find significant differences in reported social support for breastfeeding by maternal BMI aside from decreased extended family support for EBF among women with overweight, but not obesity. These findings could help to explain why current efforts to address maternal weight-related EBF disparities that have focused primarily on peer support and immediate postpartum practices have met with limited improvement in EBF rates.19
Our study did find significant differences in intrapartum obstetrical events and practices among women with obesity compared with women with normal weight and overweight, including lower rates of spontaneous labor, increased use of epidural anesthesia, and increased synthetic oxytocin administration. Although differences in these obstetrical events might be anticipated to account for differences in EBF rates, these obstetric differences do not explain the reduced EBF rates in women with overweight. Specifically, intrapartum obstetrical events and experiences were not different between women with normal weight and overweight, yet we have previously reported that women with overweight experienced EBF rates at 6 weeks postpartum similar to women with obesity rather than women with normal weight (69.6% versus 64.0% versus 91.8%, for overweight, obese, and normal weight, respectively, p-value 0.003).15 This suggests that factors other than intrapartum obstetrical practices may alter EBF ability, or that intrapartum obstetrical factors have an impact that varies by maternal BMI.
Consistent expression of milk on an ongoing basis is required to establish and maintain milk supply, and women seeking lactation assistance are commonly told to increase their frequency of breastfeeding and expressing milk. In this study, we found that women with obesity were emptying their breasts more frequently than women with normal weight and overweight, and this was due to greater frequency of milk expression with similar breastfeeding frequency. This is in agreement with Leonard et al. who used the Infant Feeding Practices II survey and found that at 2 months postpartum, women with obesity were more likely to be expressing milk and report that they did so to maintain adequate milk production.20,21 Insufficient milk supply in the early postpartum period has been postulated as one of the drivers in the association between maternal obesity and decreased EBF rates.6 One of the commonly cited causes of insufficient milk supply is delayed lactogenesis, as defined by onset of stage II lactogenesis beyond 72 hours postpartum, which has been reported to occur in up to 44% of primiparous women and to be more likely among women with maternal age ≥30 years and BMI ≥25.22
The additional maternal work related to infant feeding as demonstrated by increased milk expression in addition to breastfeeding may contributed to the difference in attitude toward breastfeeding at 6 weeks postpartum by maternal BMI. Women with overweight and obesity were less likely to report that they enjoyed breastfeeding compared with women with normal weight, and women with obesity were less likely to report feeling satisfied with their infant feeding method and to have met their breastfeeding goals compared with women with normal weight and overweight.
Importantly, in our study, women with obesity did not perceive a difference in support for breastfeeding by their health care providers or their infant's providers. Providers own biases can impact breastfeeding counseling, and Garner et al. completed a qualitative study of health care providers who reported increased challenges with providing breastfeeding-related care for women with obesity.23 Although women reported similar breastfeeding support from providers and partners by maternal BMI, EBF ultimately is entirely dependent on the individual women's ability (perceived and or physiological) to produce enough milk to meet all of her infant's needs, which requires time and effort.
Other factors that have been postulated to decrease the duration of breastfeeding for women with obesity include concerns about body image and embarrassment about nursing in front of other people,24 although not all studies are in agreement.25 Our findings align with the systematic review by Negin et al. that showed increased rates of breastfeeding and support for breastfeeding by families with breastfeeding experience, specifically grandmothers who breastfed their own children.26 In addition, breastfeeding is associated with decreased rates of childhood obesity,27–29 and our observation that women with obesity were less likely to have been breastfed themselves compared with women with normal weight and overweight may lend additional support to the developmental origins of health and disease hypothesis that supports breastfeeding as a tool to decrease obesity.30 The potential transgenerational impact of breastfeeding to mitigate risk of obesity adds additional urgency to understanding the factors that influence EBF ability.
Strengths of this study include survey completion at 6 weeks postpartum to minimize the potential impact of return to paid employment, and limiting the population to women intending EBF to eliminate maternal intention as a confounding variable. This is particularly important when studying the impact of obesity on EBF as prior studies have found no difference by maternal BMI on breastfeeding outcomes after adjusting for confounding variables, including intended breastfeeding duration and self-report of the importance of breastfeeding.31 In addition, by focusing on data at 6 weeks postpartum, the majority of women who eventually worked for pay (91/142) had not returned to work (87.9%, including 88.9% of women with obesity and 84.5% of women with overweight), thus decreasing the likelihood of employment barriers as a primary etiology for BMI-related disparities in EBF.
Limitations include the relatively homogenous population with limited racial/ethnic diversity, as our population was 85% white. A prior systematic review found a relationship between higher BMI and lower BF initiation only among certain racial/ethnic groups,21 specifically among Hispanic but not African American women,32 and among white women but not African American women.33 We were unable to evaluate the impact of underweight on EBF due to the small sample (n = 2).
Conclusion
Despite similar adherence to BFHI objectives in the immediate postpartum period and similar perceived support for EBF by providers and family members, women with overweight and obesity were significantly less likely to achieve desired EBF at 6 weeks postpartum. This suggests that attention to factors other than BFHI practices and postnatal support may be critical for establishing EBF equity by maternal BMI.
Supplementary Material
Disclosure Statement
No competing financial interests exist.
Funding Information
N.E.M. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Grant No. K23HD069520-01A1).
Supplementary Material
References
- 1. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841 [DOI] [PubMed] [Google Scholar]
- 2. Implementation Guidance: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services—The Revised Baby-Friendly Hospital Initiative. Geneva: World Health Organization, 2018 [PubMed] [Google Scholar]
- 3. Pérez-Escamilla R, Martinez JL, Segura-Pérez S. Impact of the Baby-friendly Hospital Initiative on breastfeeding and child health outcomes: A systematic review. Matern Child Nutr 2016;12:402–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson JM, Perrine CG, Freedman DS, et al. . Infant feeding-related maternity care practices and maternal report of breastfeeding outcomes. Birth 2018;45:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. wallenborn jt, wheeler dc, lu j, et al. importance of familial opinions on breastfeeding practices: Differences between father, mother, and mother-in-law. Breastfeed Med 2019;14:560–567 [DOI] [PubMed] [Google Scholar]
- 6. O'Sullivan EJ, Perrine CG, Rasmussen KM. Early breastfeeding problems mediate the Negative Association between maternal obesity and exclusive breastfeeding at 1 and 2 months postpartum. J Nutr 2015;145:2369–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marchi J, Berg M, Dencker A, et al. . Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes Rev 2015;16:621–638 [DOI] [PubMed] [Google Scholar]
- 8. Ellis JA, Brown CM, Barger B, et al. . Influence of maternal obesity on labor induction: A systematic review and meta-analysis. J Midwif Womens Health 2019;64:55–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watt S, Sword W, Sheehan D, et al. . The effect of delivery method on breastfeeding initiation from The Ontario Mother and Infant Study (TOMIS) III. J Obstet Gynecol Neonatal Nurs 2012;41:728–737 [DOI] [PubMed] [Google Scholar]
- 10. Prior E, Santhakumaran S, Gale C, et al. . Breastfeeding after cesarean delivery: A systematic review and meta-analysis of world literature. Am J Clin Nutr 2012;95:1113–1135 [DOI] [PubMed] [Google Scholar]
- 11. Kling DOI, Haile ZT, Francescon JOI, et al. . Association between method of delivery and exclusive breastfeeding at hospital discharge. J Am Osteopath Assoc 2016;116:430–439 [DOI] [PubMed] [Google Scholar]
- 12. Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr 2007;27:103–121 [DOI] [PubMed] [Google Scholar]
- 13. Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donath SM, Amir LH. Maternal obesity and initiation and duration of breastfeeding: Data from the longitudinal study of Australian children. Matern Child Nutr 2008;4:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marshall NE, Lau B, Purnell JQ, et al. . Impact of maternal obesity and breastfeeding intention on lactation intensity and duration. Matern Child Nutr 2018:e12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kair LR, Nickel NC, Jones K, et al. . Hospital breastfeeding support and exclusive breastfeeding by maternal prepregnancy body mass index. Matern Child Nutr 2019:e12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hauff LE, Leonard SA, Rasmussen KM. Associations of maternal obesity and psychosocial factors with breastfeeding intention, initiation, and duration. Am J Clin Nutr 2014;99:524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fein SB, Labiner-Wolfe J, Shealy KR, et al. . Infant Feeding Practices Study II: Study Methods. Pediatrics 2008;122(Supplement 2):S28–S35 [DOI] [PubMed] [Google Scholar]
- 19. Fair FJ, Ford GL, Soltani H. Interventions for supporting the initiation and continuation of breastfeeding among women who are overweight or obese. Cochrane Database Syst Rev 2019;9:Cd012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leonard SA, Labiner-Wolfe J, Geraghty SR, Rasmussen KM. Associations between high prepregnancy body mass index, breast-milk expression, and breast-milk production and feeding. Am J Clin Nutr 2011;93:556–563 [DOI] [PubMed] [Google Scholar]
- 21. Wojcicki JM. Maternal prepregnancy body mass index and initiation and duration of breastfeeding: A review of the literature. J Womens Health (Larchmt) 2011;20:341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nommsen-Rivers LA, Chantry CJ, Peerson JM, et al. . Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr 2010;92:574–584 [DOI] [PubMed] [Google Scholar]
- 23. Garner CD, Ratcliff SL, Devine CM, et al. . Health professionals' experiences providing breastfeeding-related care for obese women. Breastfeed Med 2014;9:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newby RM, Davies PS. Antenatal breastfeeding intention, confidence and comfort in obese and non-obese primiparous Australian women: Associations with breastfeeding duration. Eur J Clin Nutr 2016;70:935–940 [DOI] [PubMed] [Google Scholar]
- 25. Han SY, Brewis AA. Influence of weight concerns on breastfeeding: Evidence from the Norwegian mother and child cohort study. Am J Hum Biol 2018;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Negin J, Coffman J, Vizintin P, et al. . The influence of grandmothers on breastfeeding rates: A systematic review. BMC Pregnancy Childbirth 2016;16:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bider-Canfield Z, Martinez MP, Wang X, et al. . Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes 2017;12:171–178 [DOI] [PubMed] [Google Scholar]
- 28. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr 2015;104:30–37 [DOI] [PubMed] [Google Scholar]
- 29. Marseglia L, Manti S, D'Angelo G, et al. . Obesity and breastfeeding: The strength of association. Women Birth 2015;28:81–86 [DOI] [PubMed] [Google Scholar]
- 30. Friedman JE. Developmental programming of obesity and diabetes in mouse, monkey, and man in 2018: Where are we headed? Diabetes 2018;67:2137–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartok CJ, Schaefer EW, Beiler JS, et al. . Role of body mass index and gestational weight gain in breastfeeding outcomes. Breastfeed Med 2012;7:448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kugyelka JG, Rasmussen KM, Frongillo EA. Maternal obesity is negatively associated with breastfeeding success among Hispanic but not Black women. J Nutr 2004;134:1746–1753 [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Smith MG, Dobre MA, et al. . Maternal obesity and breast-feeding practices among white and black women. Obesity 2010;18:175–182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.