Highlights
-
•
Internalizing symptoms in neurotypical youth relate to amygdala connectivity.
-
•
Greater modulation is observed in females than in males.
-
•
Connectivity might be a symptom of or a risk factor for disorders.
Keywords: Resting-state fMRI, Functional connectivity, Amygdala subnuclei, Adolescence, Internalizing symptoms, Sex differences
Abstract
Amygdala resting-state functional connectivity (rsFC) is altered in adolescents with internalizing disorders, though the relationship between rsFC and subclinical symptomatology in neurotypical youth remains unclear. Here we examined whether amygdala rsFC varied across a continuum of internalizing symptoms in 110 typically-developing (TD) youths 8 to 17 years old using functional magnetic resonance imaging (fMRI). We assessed overall internalizing symptoms, as well as anxious-depressed, withdrawn-depressed, and somatic complaints. Given known sex differences in the prevalence of internalizing disorders, we compared connectivity between males and females. As compared to males, females with greater internalizing, anxious-depressed, and somatic symptoms displayed greater connectivity with the cingulate gyrus, insula, and somatosensory cortices. In contrast, males with greater anxious-depressed symptoms demonstrated weaker connectivity with the subcallosal prefrontal cortex. Sex differences in rsFC in relation to symptom severity were evident for the whole amygdala and for two of its subnuclei (centromedial and superficial amygdala). Overall, results suggest that, for females, higher internalizing symptoms are associated with greater rsFC between the amygdala and regions implicated in emotional and somatosensory processing, salience detection, and action selection. Future longitudinal investigations are needed to determine whether this hyperconnectivity may confer resilience to, or pose risk for, the development of internalizing disorders.
1. Introduction
1.1. Overview of Internalizing Disorders
Internalizing disorders constitute a group of related psychiatric conditions including anxiety disorders, somatic disorders, and depression; these disorders display significant overlap of symptom expression and are twice as common in females than in males (Eaton et al., 2012). Such conditions are highly prevalent during adolescence, a time when considerable brain development occurs, specifically in networks relevant for socio-emotional processing (Blakemore and Mills, 2014; Casey, 2015; Casey et al., 2019; Crone and Dahl, 2012; Guyer et al., 2016). Female adolescents in particular exhibit greater subclinical internalizing symptoms than males and are more likely to be diagnosed with an internalizing disorder (Angold et al., 2002; Costello and Angold, 2000; Crick and Zahn-Waxler, 2003; Hankin et al., 1998; Lewinsohn et al., 1995). Nearly 1 in every 3 adolescents will receive a diagnosis of an anxiety disorder before adulthood (Merikangas et al., 2010), and college students are increasingly seeking mental health services due to anxiety (Center for Collegiate Mental Health, 2015). While the DSM-5 (American Psychiatric Association, 2013) conceptualizes these internalizing disorders separately for diagnosis and treatment, anxiety and depression are often sequentially or concurrently comorbid amongst adolescents (Cummings et al., 2014; Garber and Weersing, 2010). Other types of symptoms such as rumination (McLaughlin and Nolen-Hoeksema, 2011) and emotion dysregulation (McLaughlin et al., 2011) are also transdiagnostic features of both anxiety and depression. In the absence of comorbidity, adolescents who meet criteria for one internalizing disorder often endorse subclinical symptoms for another. Such symptom overlap is especially true for adolescents with anxiety who often display high, albeit subthreshold, levels of depression (Garber and Weersing, 2010).
In addition to considerable behavioral evidence suggesting comorbidity and similarities between internalizing disorders, a growing body of neuroimaging research demonstrates shared neural alterations across internalizing disorders. Adolescents with depression and anxiety both demonstrate amygdala hyperactivation to fearful faces relative to neutral faces as compared to neurotypical youth (Beesdo et al., 2009). Relatedly, re-grouping adults with generalized anxiety disorder (GAD) and major depressive disorder (MDD) based on whether they exhibit high vs. low intra-limbic functional connectivity better predicts the extent of their attentional threat bias than when grouping by clinical diagnosis (Bijsterbosch et al., 2018). Notably, another study indicates that clustering MDD patients using limbic and fronto-striatal network connectivity reveals patient subtypes that are predictive of responsivity to repetitive transcranial magnetic stimulation (rTMS) therapy (Drysdale et al., 2017).
1.2. Roles of the Amygdala Subnuclei
Neural network alterations observed in children and adolescents with internalizing disorders are broadly evident in brain circuits relevant for emotional processing and salience detection, particularly involving the amygdala (for review, see Blackford and Pine, 2012). Alterations in these networks can manifest as atypical resting-state functional connectivity (rsFC), a metric indexing co-activation history between regions supporting similar functions which allows for the identification of functionally related-brain networks (Raichle, 2010). The basolateral amygdala (BLA), centromedial amygdala (CMA), and superficial amygdala (SFA) demonstrate separable rsFC networks that relate to distinct roles in both the processing of sensory stimuli and the expression of different behaviors (Amunts et al., 2005; Bzdok et al., 2013; Roy et al., 2009). The BLA receives sensory and threat-related information and is thought to process emotions via communication between regions such as visual association cortices, medial prefrontal cortex, and the cingulate gyrus (Dolan and Vuilleumier, 2006; LeDoux, 2000; Pessoa and Adolphs, 2010). The CMA contributes to the expression of emotions via its communication with sensorimotor regions, brainstem, and cerebellum (LeDoux, 2007, 2000; Roy et al., 2009). The SFA plays a role in emotion processing, displaying connectivity with the ventral striatum, orbitofrontal cortex, cingulate, insula, and hippocampus (Koelsch et al., 2013). Given their distinct connectivity patterns and their relation to different processes, assessing connectivity for each amygdala subnuclei could better inform our understanding of neural circuit alterations in relation to internalizing symptoms.
1.3. Prior Research and Present Study
Research in neurotypical youth has also characterized normative developmental trajectories of functional connectivity with the amygdala and its subnuclei (Alarcón et al., 2015; Gabard-Durnam et al., 2014; Jalbrzikowski et al., 2017; Qin et al., 2014); however, less is known about how subclinical symptomatology amongst typically-developing (TD) youth may relate to amygdala rsFC. Within this existing literature, reports are mixed with regard to the directionality of the relationship between amygdalar connectivity and internalizing symptoms, as well as with regard to the brain regions showing such relationships. These disparities may reflect the specificity or severity of the symptoms examined, the developmental period under investigation (i.e., childhood versus late adolescence), whether whole amygdala rsFC or amygdala subnuclei rsFC is considered, and other sample characteristics. A longitudinal study of TD youth (average age ∼13) with no psychopathology at baseline compared amygdala rsFC, on average 2.5 years later, between youth who remained free of symptoms and those who showed greater depressive symptomatology (referred to as “escalators”). Relative to this group, neurotypical youth showed greater baseline connectivity between the right amygdala and left IFG, supramarginal gyrus, and right mid-cingulate cortex, as well as less connectivity between left amygdala and cerebellum (Scheuer et al., 2017). In contrast, a cross-sectional study investigating amygdala subnuclei rsFC in a younger sample of TD children (average age ∼8) showed that greater symptoms of anxiety related to greater connectivity between the left amygdala, particularly the BLA, and several regions implicated in sensory processing, higher-order frontal regions, and subcortical regions (Qin et al., 2014). In a sample of youth exposed to early-life stress, greater adolescent amygdala-vmPFC connectivity related to less symptoms of anxiety, but more symptoms of depression, particularly for females (Burghy et al., 2012). Assessments of TD adolescents who exhibit subclinical internalizing symptoms can help inform whether known circuit-level alterations in functional connectivity seen in adolescents with a clinical diagnosis arise prior to disorder onset and could thus be used as biomarkers, or instead are merely symptomatic of a clinical diagnosis. Further, investigating the association between amygdala functional connectivity and distinct internalizing symptoms affords the ability to detect similarities and differences across disorders with shared symptom expression.
Here, we used a dimensional approach to understand how subclinical internalizing symptomatology might modulate rsFC of the amygdala and its subnuclei in a sample of 110 TD children and adolescents. Given differential rates of internalizing symptoms and disorders in males and females, we particularly focused on sex differences in rsFC. To our knowledge, this is the first study to specifically examine the role of sex when relating subclinical internalizing symptoms to amygdala rsFC in a large sample of otherwise typically-developing youth. The entire bilateral amygdala, as well as the BLA, CMA, and SFA subnuclei in exploratory analyses, were used as seed regions of interest in independent analyses. The internalizing scale of the Child Behavior Checklist (CBCL), a parent-report of child and adolescent symptoms, was used to assess symptom severity; furthermore, its three constituent subscales – anxious-depressed, somatic complaints, and withdrawn-depressed – were used to examine how specific types of symptoms might relate to distinct patterns of amygdala functional connectivity. Supplemental analyses were also conducted to explore how age might affect any observed relationships between amygdala connectivity and internalizing symptomatology. Given the limited relevant literature and lack of consistent results, we refrained from formulating specific hypotheses as per the relationship between internalizing symptoms and functional connectivity of the amygdala or its subnuclei. However, prior work does suggest the presence of sex differences in whole amygdala and amygdala subnuclei functional connectivity (Engman et al., 2016), and that atypicalities in amygdala task-based functional connectivity may vary as a function of mood in females but not in males (Mareckova et al., 2016). Thus, we predicted that internalizing symptomatology would modulate amygdala connectivity, and that this relationship would differ between females and males.
2. Methods
2.1. Study Participants
Participants included 124 typically developing children and adolescents who were recruited as part of the Gender Exploration of Neurogenetics and Development to Advance Autism Research (GENDAAR) multisite consortium. Participants were recruited for this study through traditional recruitment strategies (e.g., flyers distributed at community events, youth organizations, school events, etc.); the fact that these youth were enrolled as neurotypical controls in a study focused on Autism Spectrum Disorder (ASD) had no influence on the choice of strategy. Data collection occurred at 4 institutions: Harvard Medical School, Seattle Children’s Research Institute, University of California Los Angeles (UCLA), and Yale University (scanner and site were both included as covariates in all analyses). Fourteen subjects were excluded from the analyses due to excessive motion during the MRI scan (see Methods 2.5), yielding a total sample of 110 participants (57 females: Mage = 13.1, SD = 2.9, range = 8.28 – 17.9 years; 53 males: Mage = 13.7, SD = 2.5, range = 8.26-17.8 years; see Table 1). All participants were right-handed, had no contraindications for MRI, had no previous or current history of neurological, psychiatric, or neurodevelopmental disorders, and no history of ASD in any first-degree relatives. Further, the Social Responsiveness Scale (Constantino and Gruber, 2012) was used to exclude participants with elevated ASD symptomatology. 75% of participants identified as White, 12% as more than one race, 8% as Asian, and 5% as Black or African American (Table 1). Family income was collected as a proxy for socioeconomic status; however, this information was missing for 36 participants. Additionally, all participants self-reported about their pubertal status using the Pubertal Development Scale (Table 1; PDS, Petersen et al., 1988). Written informed consent and assent were obtained from all legal guardians and study participants in accordance with all sites’ Institutional Review Boards. All participants were compensated for their participation in this study.
Table 1.
Sample Descriptives
| Females (n = 57) |
Males (n = 53) |
P-value | |
|---|---|---|---|
| Age | 13.1 (2.9) | 13.7 (2.5) | 0.22 |
| Mean Relative Motion (mm) | 0.14 (0.12) | 0.11 (0.08) | 0.21 |
| Framewise Displacement (mm) | 0.23 (0.20) | 0.19 (0.13) | 0.20 |
| Percentage ICA Components Kept | 49.3% (11.5) | 51.2% (10.9) | 0.38 |
| Pubertal Development Status (PDS) | 12.6 (4.2) | 11.2 (3.8) | 0.08 |
| Self-Reported Ethnicity | |||
| Asian | 4 | 5 | - |
| Black/African American | 4 | 1 | - |
| More than one race | 7 | 6 | - |
| White | 42 | 41 | - |
| Internalizing Scale | 4.10 (4.22) | 2.73 (3.80) | 0.07 |
| Anxious-Depressed Subscale | 2.03 (2.38) | 1.35 (1.94) | 0.10 |
| Somatic Complaints Subscale | 1.10 (1.65) | 0.60 (1.47) | 0.10 |
| Withdrawn-Depressed Subscale | 0.96 (1.25) | 0.77 (1.46) | 0.46 |
Mean values with standard deviations in parentheses. P-values were derived using two sample t-tests.
2.2. Child Behavior Checklist (CBCL)
Internalizing symptoms were assessed via parental reports on the Child Behavior Checklist (CBCL) internalizing scale (Achenbach, 1991) which provides a global categorization of all internalizing problems. We also examined the scores on its 3 subscales (anxious-depressed, somatic complaints, and withdrawn-depressed) to explore how specific symptomatology might differentially modulate amygdala connectivity. Appendix A lists the specific items parents were asked, grouped by each subscale. Each CBCL subscale score is generated by summing values indicating how often a parent perceives their child experiencing a symptom on a scale from 0-2, where 0 indicates that this symptom is not endorsed by their child (“Not True (as far as you know)”), 1 indicates that parents believe this symptom is sometimes expressed by their child (“Somewhat or Sometimes True”), and 2 indicates that parents believe this symptom is often expressed by their child (“Very True or Often True”). There are 32 total items on the Internalizing scale (scores range from 0-64), which is composed of 13 items on the anxious-depressed subscale (scores range from 0-26), 11 items on the somatic complaints subscale (scores range from 0-22), and 8 items on the withdrawn-depressed subscale (scores range from 0-16).
The anxious-depressed subscale primarily measures various types of fears (e.g., fear of being perfect, fear of social situations) as well as some symptoms of depression (e.g., cries a lot). The somatic complaints subscale addresses physical manifestations of internalizing problems, including nausea, tiredness, and digestive problems. The withdrawn-depressed subscale primarily assesses symptoms of depression, particularly related to social situations (e.g., would rather be alone than with others). These subscales were chosen for their ability to capture dimensional symptom severity in youth with and without a clinical diagnosis. Internalizing symptom severity was compared between males and females using two-tailed t-tests. All analyses were conducted using CBCL raw scores. As CBCL T-scores are normed separately for males and females, using T-scores would have precluded meaningful sex comparisons.
2.3. MRI Data Acquisition
Resting-state functional magnetic resonance imaging (rs-fMRI) data were obtained for all participants using either a Siemens 3 T Trio (12-channel head coil) or a Prisma 3 T whole-body scanner (20-channel head coil). For registration, each participant also received a matched-bandwidth echo-planar image (TR = 5000 ms, TE = 34 ms for Trio or 35 ms for Prisma, FOV = 192 mm, 34 slices, slice thickness 4 mm, in-plane voxel size 1.5 x 1.5 mm). The T2*-weighted rs-fMRI sequence (TR = 2000 ms, TE = 30 ms, FOV = 192 mm, 34 slices, slice thickness 4 mm, in-plane voxel size 3 x 3 mm on both platforms) was acquired while participants were instructed to view a white crosshair on a black background, and at least 5.5 minutes of resting state data were acquired for each participant.
2.4. MRI Preprocessing
MRI data were analyzed using FSL and AFNI (Analysis of Functional NeuroImages; Cox, 1996). The following pre-processing steps were implemented prior to analyzing amygdala functional connectivity. Images were skull-stripped using AFNI, then realigned using the mean functional volume via FSL’s Motion Correction Linear Registration Tool (MCFLIRT; Jenkinson et al., 2002). Registration of rs-fMRI data to structural images was conducted via a 2-step process whereby functional data were linearly registered to the matched-bandwidth EPI volume (6 degrees of freedom), and then registered to the MNI 152 2 mm standard brain (12 degrees of freedom). Images were smoothed using a 6 mm FWHM Gaussian kernel. To remove potential confounds resulting from head motion, smoothed data were denoised using Independent Component Analysis (ICA)-based Automatic Removal of Motion Artifacts (ICA-AROMA; Pruim et al., 2015) to regress out single-subject components labeled as motion or noise. ICA-AROMA has been shown to be one of the very best approaches for addressing head motion when compared to 18 other commonly employed denoising pipelines (Parkes et al., 2018). We chose to use ICA-AROMA, as opposed to motion scrubbing, as scrubbing results in both data loss and alterations in subjects’ time series (Parkes et al., 2018). As compared with other pipelines, ICA-AROMA also does not alter estimates of long-range connectivity, which is a concern especially for pediatric samples (Van Dijk et al., 2012). Eight subjects were removed for having fewer than 10 resting-state components remaining after implementing ICA-AROMA; an additional six subjects were removed due to high maximum absolute motion (greater than 8 mm). Data from the remaining 110 participants were bandpass filtered (0.1 Hz > t > 0.01 Hz). FSL’s Automatic Segmentation Tool (FAST) was then used to create white matter, cerebrospinal fluid, and global signal masks from high-resolution anatomical scans, and signal from these masks and their derivatives were regressed out from functional data using FSL’s FEAT. Resulting subject-level residuals were normalized and registered to standard space. As global signal regression (GSR) may introduce spurious anti-correlations (Murphy & Fox, 2017), only positive connectivity findings are reported and discussed here. We opted to apply GSR as it is effective at removing motion and respiratory related global artifacts, as well as increasing the relationship between functional connectivity and neuroanatomical structures; when used in conjunction with ICA-AROMA, GSR can be especially effective at denoising data (Parkes et al., 2018).
2.5. MRI Data Analysis
All statistical analyses were performed using the general linear model in FSL’s FEAT. Time series were extracted from the whole bilateral amygdala at a probabilistic threshold of 25% in the Harvard-Oxford atlas, consistent with previous amygdala rsFC studies (e.g., Bickart et al., 2012). Separate exploratory analyses were also conducted for the bilateral centromedial (CMA), basolateral (BLA), and superficial (SFA) nuclei. These bilateral amygdala subnuclei ROIs were generated using the Jülich histological atlas available in FSL (FMRIB’s Software Library, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki; Smith et al., 2004). Voxels were assigned to the subnuclei with the highest probability of containing them only if they had at least a 40% probability of belonging to that subnuclei and not to any other nearby structures. Then, each thresholded subnuclei was subtracted from the other two and binarized to generate final non-overlapping ROIs of each subnuclei. These time series were then independently correlated with every other voxel in the brain to generate distinct functional connectivity maps for each participant. Finally, all subject-level correlation maps were transformed to z-statistic maps using Fisher’s r to z transform to allow for between-subject comparisons. Group analyses were conducted in FSL’s FEAT using FLAME 1 + 2, a mixed effects model. All regression analyses described below were focused on (i.e., masked by) brain regions where either males or females showed significant whole amygdala functional connectivity (thresholded at z > 2.3, p < 0.05), in keeping with our main aim of characterizing how amygdala connectivity might be modulated by internalizing symptoms in males and females. Restricting our search space to the networks of regions showing significant connectivity with the amygdala (in either group) does not allow for identification of regions which may show amygdala connectivity only as a function of symptoms; however, given that our sample involved neurotypical youth experiencing a limited range of symptoms, we opted to assess the modulatory role of internalizing symptomatology only in brain regions that showed significant amygdala connectivity in either group.
Raw, demeaned scores for the CBCL internalizing scale, as well as for the anxious-depressed, somatic complaints, and withdrawn-depressed subscales, were entered as covariates of interest in separate higher-level FEAT regression analyses to assess whole-group (males + females), within-group (males and females separately), and between-group (males vs. females) effects. Scanner and data collection site were also entered as regressors of no interest. Analyses were first conducted for the internalizing scale to identify general patterns of altered connectivity; these were followed by separate analyses for each subscale to assess the specific relationship between amygdala functional connectivity and distinct symptom profiles. All bottom-up analyses were thresholded at z > 3.1 (p < 0.001) and corrected for multiple comparisons at p < 0.05 in accordance with current recommendations in the field for more stringent statistical thresholding (Kessler et al., 2017). Correction for multiple tests (i.e., across multiple seeds) is not feasible using FEAT; however, false discovery rate (FDR) correction was applied whereby the p-values averaged across each significant cluster resulting from 16 independent tests (i.e., 4 amygdala ROIs x 4 internalizing symptoms scales) were entered into the p.adjust function of the R stats package (R Core Team, 2019) to correct for multiple tests. All analyses were additionally conducted including age as a covariate, given that amygdala functional connectivity might change over the course of development. Results that differed when including age in the models are described in the relevant sections below; all results from these additional analyses with age as a covariate are included in supplementary tables (Tables S1–4). Finally, to examine the extent to which any observed relationship between internalizing symptoms and amygdala connectivity might have been moderated by age, we extracted parameter estimates of connectivity from the regions where internalizing symptoms significantly modulated amygdala connectivity and conducted moderation analyses in R; only one significant moderating effect of age was observed as detailed in Section 3.3.2 below.
3. Results
3.1. Demographics and Head Motion
Males and females did not significantly differ in age (p = 0.22, Table 1) or in pubertal status as measured by the PDS (p = 0.08, Table 1). Males and females also did not differ in mean relative motion, the percentage of ICA components retained following ICA-AROMA, or framewise displacement (Table 1).
3.2. Internalizing Symptoms
There were no significant differences between males and females on any of CBCL measures of internalizing (internalizing scale: t(108) = 1.78, p = 0.08; anxious-depressed subscale: t(108) = 1.63, p = 0.10; somatic complaints subscale: t(108) = 1.68, p = 0.10; withdrawn-depressed subscale: t(108 = 0.73, p = 0.46; see Table 1)). None of the CBCL measures correlated with age or with the PDS. 84 participants (76% of the sample) were reported by parents as exhibiting some internalizing symptoms.
We also evaluated whether any participant was an outlier on the CBCL measures, as defined as at least two standard deviations above the group average. Six participants were considered outliers across one or more scale (i.e., outliers on the anxious-depressed and somatic complaints subscales), 1 on only the somatic subscale, 1 on only the anxious-depressed subscale, and 4 on only the withdrawn-depressed subscale. All of these participants were included in our sample as our main study question of interest was to understand how a continuous range of symptoms would impact connectivity patterns. However, we ensured that all reported connectivity findings remained significant when behavioral outliers were removed.
Lastly, to ensure that observed sex differences in connectivity did not merely reflect sex differences in internalizing symptoms, we more closely matched the groups (all ps > 0.3) by removing females with the highest reported symptoms and males with the lowest reported symptoms (2 males and 2 females for the anxious-depressed and somatic complaints subscales, 3 males and 3 females for the internalizing scale). All reported between-group effects remained significant after excluding these participants.
3.3. Amygdala Functional Connectivity
While our primary analyses focused on examining amygdala rsFC in relation to internalizing symptoms, the patterns of whole amygdala rsFC (not accounting for internalizing symptoms) for both males and females are presented in Supplementary Fig. 1 (displayed at z > 3.1, p < 0.05).
3.3.1. Amygdala Connectivity as a Function of Overall Internalizing Symptoms
As a function of increasing internalizing symptoms, females displayed increased whole amygdala connectivity with the posterior mid-cingulate cortex (pMCC); this pMCC cluster extended into the supplementary motor area (SMA) and precentral gyri (Table 2). A similar pattern of connectivity was also evident for the SFA and CMA (Table 2). In separate analyses conducted with age as a covariate of no interest, females also displayed greater connectivity between the pMCC and the BLA as a function of increased internalizing symptoms (Table S1). The CMA also uniquely displayed stronger connectivity with the left somatosensory cortex as a function of increased internalizing symptoms (Table 2). Overall internalizing symptoms did not significantly modulate whole amygdala connectivity in males. Direct between-group comparisons showed that, as a function of increased internalizing symptoms, females displayed significantly greater whole amygdala connectivity than males with pMCC, SMA, precentral gyri, and right superior frontal gyrus (SFG). Again, these results held for the SFA and CMA subnuclei (Fig. 1, Table 2, Supplementary Fig. 2A, B), except for the SFG for which significant sex differences were only observed for the whole amygdala. At the whole-group level (across male and female youth), greater internalizing symptoms were associated with stronger connectivity between the whole amygdala and the pMCC (Table 2).
Table 2.
Peak coordinates of brain regions where amygdala connectivity varied as a function of internalizing symptoms (Int); z < 3.1, corrected for multiple comparisons at p < 0.05.
| Internalizing Modulation – Whole Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Male and Female Int + | Posterior Mid-Cingulate Cortex | −2 | −4 | 40 | 4.65 |
| Female Int + | Posterior Mid-Cingulate Cortex | 2 | −10 | 48 | 4.99 |
| Female > Male Int + | Supplementary Motor Area | 8 | −12 | 70 | 5.06 |
| Internalizing Modulation – Centromedial Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Int + | Posterior Mid-Cingulate Cortex | 2 | −10 | 54 | 4.38 |
| Left Somatosensory Cortex | −38 | −22 | 34 | 4.42 | |
| Female > Male Int + | Supplementary Motor Area | 10 | −6 | 50 | 4.77 |
| Internalizing Modulation – Superficial Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Int + | Precentral Gyrus | 0 | −16 | 64 | 4.78 |
| Female > Male Int + | Posterior Mid-Cingulate Cortex | 8 | −6 | 44 | 4.81 |
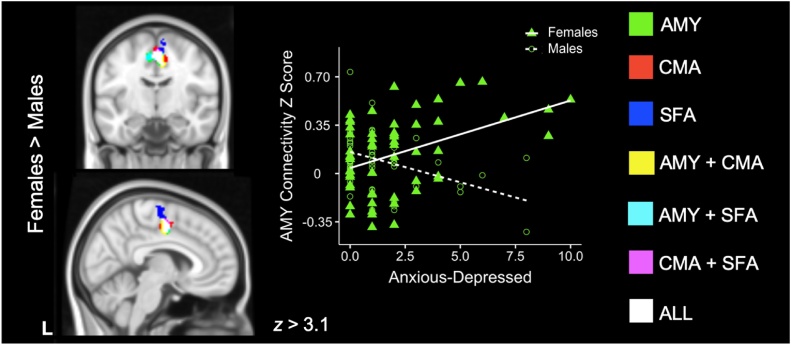
Fig. 1.
Amygdala rsFC and Overall Internalizing Symptoms.
Brain regions displaying connectivity modulated by internalizing symptoms with the whole amygdala (green); the centromedial amygdala (CMA, red); the superficial amygdala (SFA, blue); both the whole amygdala and CMA (yellow); both the whole amygdala and SFA (cyan); both the CMA and SFA (pink); or the whole amygdala, CMA, and SFA (white). Scatterplots are included for illustrative purposes. Females displayed greater connectivity than males as a function of higher internalizing symptoms; scatterplot shows whole amygdala connectivity as related to internalizing symptoms in females (green triangles) and males (open green circles).
3.3.2. Amygdala Connectivity as a Function of Anxious-Depressed Symptoms
With increasing anxious-depressed symptoms, females displayed stronger connectivity of the whole amygdala, CMA, and SFA with midline precentral gyri and SMA (Table 3). In contrast, males demonstrated weaker connectivity between the SFA and subcallosal cortex as a function of anxious-depressed symptoms (Table 3). This last effect was not significant when age was included as a covariate in separate bottom-up analyses; a moderation analysis further revealed a significant interaction (p < .01) between age and anxious-depressed symptoms in males whereby weaker connectivity between the SFA and subcallosal cortex as a function of greater anxious-depressed symptoms was more pronounced for younger than older males.
Table 3.
Peak coordinates of brain regions where amygdala connectivity varied as a function of anxious-depressed symptoms (Anx-Dep); z < 3.1, corrected for multiple comparisons at p < 0.05.
| Anxious-Depressed Modulation – Whole Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Anx-Dep + | Precentral Gyrus | 2 | −16 | 48 | 4.37 |
| Female > Male Anx-Dep + | Posterior Mid-Cingulate Cortex | 6 | −8 | 44 | 4.56 |
| Anxious-Depressed Modulation – Basolateral Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Male and Female Anx-Dep + | Left Thalamus | −2 | −26 | 2 | 3.97 |
| Anxious-Depressed Modulation – Centromedial Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Anx-Dep + | Precentral Gyrus | −2 | −16 | 56 | 4.1 |
| Female > Male Anx-Dep + | Posterior Mid-Cingulate Cortex | 8 | −8 | 44 | 4.33 |
| Anxious-Depressed Modulation – Superficial Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Anx-Dep + | Supplementary Motor Area | 2 | −16 | 64 | 4.42 |
| Male Anx-Dep - | Subcallosal Cortex | 2 | 24 | −18 | 3.67 |
| Female > Male Anx-Dep + | Posterior Mid-Cingulate Cortex | 8 | −6 | 44 | 4.28 |
When directly comparing male and female youth, females displayed greater connectivity between the whole amygdala and the pMCC, a pattern also seen for the SFA and CMA (Fig. 2, Table 3, Supplementary Fig. 3A, B). At the whole-group level (across male and female youth combined), greater anxious-depressed symptomatology was associated with stronger connectivity between the BLA and the left thalamus (Table 3). Whole-group connectivity between the BLA and left thalamus was no longer significant when including age as a covariate.
Fig. 2.
Amygdala rsFC and Anxious-Depressed Symptoms.
Brain regions displaying connectivity modulated by anxious-depressed symptoms with the whole amygdala (green); centromedial amygdala (CMA, red); superficial amygdala (SFA, blue); both the whole amygdala and CMA (yellow); both the whole amygdala and SFA (cyan); both the CMA and SFA (pink); and the whole amygdala, CMA, and SFA (white). Scatterplots are included for illustrative purposes. Females displayed greater connectivity than males as a function of higher anxious-depressed symptoms; scatterplot shows that higher anxious-depressed symptoms were associated with greater whole amygdala connectivity in females (green triangles) as compared to males (open green circles).
3.3.3. Amygdala Connectivity as a Function of Somatic complaints
Females displayed greater connectivity between the whole amygdala and the pMCC as a function of increased somatic complaints (Table 4). This pattern was mirrored for the SFA (Table 4); stronger SFA connectivity with increasing somatic complaints was also observed in the right anterior superior temporal gyrus (STG), left central operculum, and left insula (Table 4). CMA connectivity with the bilateral postcentral gyri was stronger in females with more somatic complaints (Table 4). In males, amygdala connectivity did not vary as a function of somatic symptoms. In direct between-group comparison with males, females showed greater connectivity between the SFA and the precentral gyrus, SMA, and pMCC (Fig. 3, Table 4) as a function of increased somatic symptoms. Additionally, females showed greater connectivity than males between the SFA and the left anterior STG with increasing somatic complaints (Fig. 3, Table 4), as well as increased connectivity between the CMA and bilateral pre and postcentral gyri (Fig. 3, Table 4).
Table 4.
Peak coordinates of brain regions where amygdala connectivity varied as a function of somatic complaints (Somatic); z > 3.1, corrected for multiple comparisons at p < 0.05.
| Somatic Complaints Modulation – Whole Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Somatic + | Posterior Mid-Cingulate Cortex | −8 | −6 | 42 | 4.09 |
| Somatic Complaints Modulation – Centromedial Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Somatic + | Left Somatosensory Cortex | −40 | −24 | 34 | 4.36 |
| Right Somatosensory Cortex | 58 | 0 | 26 | 3.93 | |
| Female > Male Somatic + | Left Precentral Gyrus | −50 | −6 | 26 | 4.57 |
| Right Precentral Gyrus | 58 | 0 | 28 | 4.50 | |
| Somatic Complaints Modulation – Superficial Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female Somatic + | Posterior Mid-Cingulate Cortex | −6 | −2 | 42 | 4.71 |
| Right Anterior Superior Temporal Gyrus | 60 | 0 | −4 | 4.17 | |
| Left Central Operculum | −46 | −2 | 14 | 4.18 | |
| Female > Male Somatic + | Precentral Gyrus | −10 | −14 | 66 | 4.68 |
| Left Anterior Superior Temporal Gyrus | −42 | −2 | −18 | 4.05 | |
Fig. 3.
Amygdala rsFC Relationship and Somatic Complaints.
Brain regions displaying connectivity modulated by somatic complaints with the centromedial amygdala (CMA, red) and the superficial amygdala (SFA, blue). Scatterplots are included for illustrative purposes. Females displayed greater connectivity than males as a function of higher somatic complaints; scatterplots show that CMA connectivity (red) and SFA connectivity (blue) was stronger as a function of greater somatic complaints in females (triangles) as compared to males (open circles).
3.3.4. Amygdala Connectivity as a Function of Withdrawn-Depressed Symptoms
Females showed greater connectivity between both the whole amygdala and the SFA with the pMCC and SMA cluster (Table 5) with increasing withdrawn-depressed symptoms. This was the only observed modulation of amygdala connectivity with regards to withdrawn-depressed symptomatology.
Table 5.
Peak coordinates of brain regions where amygdala connectivity varied as a function of withdrawn-depressed symptoms (W-D); z > 3.1, corrected for multiple comparisons at p < 0.05.
| Withdrawn-Depressed Modulation – Whole Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female W-D+ | Posterior Mid-Cingulate Cortex | 2 | −10 | 48 | 4.48 |
| Withdrawn-Depressed Modulation – Superficial Amygdala | MNI Peak (mm) |
Max | |||
|---|---|---|---|---|---|
| X | Y | Z | Z | ||
| Female W-D+ | Precentral Gyrus | −6 | −16 | 66 | 4.5 |
4. Discussion
The goals of the present study were to examine how subclinical internalizing symptoms in typically-developing youth might relate to functional connectivity of the amygdala, and whether distinct patterns might be observed between males and females in light of known sex differences in the rate of internalizing problems (Eaton et al., 2012). While not a significant difference, females in our sample were rated by their parents as showing slightly more internalizing symptomatology overall than males, specifically more anxious-depressed and somatic symptoms. Significant sex differences in amygdala functional connectivity, as related to internalizing symptoms, were also observed. As a function of increasing overall internalizing symptoms and as compared to males, females displayed hyperconnectivity between the whole amygdala and several regions associated with emotional and sensory processing, salience detection, and action selection, including the posterior mid-cingulate cortex (pMCC), insula, and somatosensory cortices. As detailed below, although internalizing symptoms modulated amygdala connectivity similarly across its three subnuclei, some specific relationships were also observed between different types of internalizing symptoms and functional connectivity of distinct amygdala subnuclei in males and females.
At the whole-group level (i.e., in both males and females), increased anxious-depressed symptomatology was associated with stronger connectivity between the basolateral amygdala (BLA) and the left thalamus. Some afferent thalamic relays of sensory information converge in the BLA (Amaral et al., 1992), and this sensory input, in conjunction with descending cortical information to the amygdala, allows for significant associative learning to occur within the BLA, especially fear-related associations (Benarroch, 2015). The BLA is often implicated in emotional learning through consolidation of fear memories and threat estimation in both rodents (Fanselow and Ledoux, 1999) and humans (Klumpers et al., 2015), with the BLA playing a key role in integrating and computing the valence of stimuli via emotional cues (Hortensius et al., 2016). The hyperconnectivity between the BLA and the thalamus observed in both male and female youths suggests that greater anxious-depressed symptoms may be related to an increased or biased orientation toward sensory and emotional information, perhaps leading to overestimation of threat – a key component of anxiety (Grupe and Nitschke, 2013). Interestingly, when age was included as a covariate in the model, the relationship between BLA-thalamic connectivity and anxious-depressed symptomatology was no longer significant. Prior longitudinal work demonstrated that connectivity between the BLA and thalamus increases with age (Gabard-Durnam et al., 2014), and future longitudinal investigations might help uncover the interaction between age and internalizing symptoms on this circuit. No other symptom-related modulation specific to the BLA was observed, which is surprising given that rsFC of the BLA has been shown to be altered in adults with generalized anxiety disorder (Etkin et al., 2009), related to state anxiety in healthy adults (Baur et al., 2013), and even predictive of trait anxiety severity in healthy children (Qin et al., 2014). Nevertheless, our findings are consistent with the notion that hyperarousal to threat in response to sensory and emotional stimuli seen in anxiety disorders may be linked to altered thalamic-amygdala circuitry.
With increasing anxious-depressed symptoms, females, compared to males, also showed greater connectivity between the whole amygdala – as well as the centromedial amygdala (CMA) and superficial amygdala (SFA) – with the pMCC, the SMA, and the precentral gyri, similar to prior work examining adolescents with subclinical depression (Scheuer et al., 2017). Interestingly, the CMA, SFA, and BLA in adults all tend to show connectivity with the pMCC and surrounding motor regions (Kerestes et al., 2017). Our results similarly demonstrate connectivity between the pMCC and all examined amygdala subnuclei, suggesting that this amygdala-cingulate network develops prior to adulthood. The mid-cingulate cortex is associated with salience processing and allocation of attentional and motor resources toward behaviorally relevant stimuli (Vogt, 2005). Specifically, the pMCC monitors the environment for salient stimuli and, through connections with the cingulate motor area and regions such as the precentral gyri and SMA, can coordinate bodily responses in the early anticipation of pain (Vogt, 2005). Tract tracing studies in rhesus monkeys (Morecraft et al., 2007) and diffusion tensor imaging studies in humans (Grèzes et al., 2014) have demonstrated direct anatomical connections between the amygdala and motor circuitry within the cingulate. This circuit might underlie motor behaviors in socio-emotional contexts to promote either approach or avoidance behaviors. Indeed, these motor regions are also shown to be consistently activated during successful emotion regulation (Kohn et al., 2014). Thus, the observation of hyperconnectivity between the amygdala and the pMCC, as well as other early motor regions, with increasing anxious-depressed symptoms may reflect greater engagement of this limbic-motor circuit in youth expressing higher symptomatology. Given that these regions are involved in emotion regulation, perhaps this network is especially primed and over-active in these children and adolescents who may regularly attempt to regulate their emotions and suppress feelings of anxiety, albeit perhaps unsuccessfully given their heightened symptomatology.
While internalizing symptoms modulated amygdala connectivity to a lesser degree in males than in females, males did show reduced functional connectivity between the SFA and subcallosal cortex as a function of increasing anxious-depressed symptomatology. This result was unexpected given that the subcallosal cortex tends to be hyperconnected to the amygdala in both male and female adolescents with clinical depression relative to controls (Connolly et al., 2013), and that positive coupling between the whole amygdala and the subcallosal cortex in neurotypical youth 8-29 years old tends to remain steady over time (Duijvenvoorde et al., 2019). Here the opposite pattern of connectivity was observed in that males endorsing fewer anxious-depressed symptoms showed stronger connectivity between the SFA and subcallosal cortex, whereas males with higher symptoms showed weaker connectivity. There are some potential explanations for these divergent findings. First, prior research demonstrating hyperconnectivity between the amygdala and the subcallosal cortex examined the whole amygdala, whereas our analyses identified hypoconnectivity only with the SFA (Connolly et al., 2013). The seemingly contradictory findings may thus reflect a finer level of analysis and/or degree of specificity within amygdalar subnuclei. Second, our sample consisted of typically-developing males expressing subclinical symptomatology; accordingly, this pattern of weaker SFA-subcallosal connectivity could reflect a compensatory or protective mechanism. Indeed, hyperactivity in the subcallosal cortex in depression is thought to index ruminative and self-referential processing (Nejad et al., 2013) and this hyperactivity has been shown to diminish after treatment in adults with depression (Hamani et al., 2011). Thus, despite the endorsement of some anxious-depressed symptoms, the hypoconnectivity between SFA and subcallosal cortex observed in our sample of neurotypical youth could actually help down-regulate activity in this region and guard against the onset of more severe symptoms. Of note, we also found that, when controlling for age, the relationship between SFA-subcallosal connectivity and anxious-depressed symptomatology was no longer significant, suggesting that age may account for a portion of the variance relating anxious-depressed symptoms to amygdala connectivity. Indeed, a moderation analysis revealed that weaker SFA-subcallosal connectivity as a function of anxious-depressed symptomology was more pronounced in younger, relative to older, males. A longitudinal follow-up would be required to determine whether connectivity within this SFA-subcallosal cortex circuit may confer resilience versus risk for the emergence of clinically meaningful symptomatology.
Finally, females also displayed hyperconnectivity of the whole amygdala – as well as the CMA and SFA – in relation to somatic complaints, compared to males. More specifically, females showed stronger CMA connectivity with somatosensory cortices with greater somatic complaints. The CMA is involved in salience detection and is well connected with sensorimotor regions (LeDoux, 2007, 2000; Roy et al., 2009). The observed hyperconnectivity between the CMA and somatosensory cortices might reflect greater allocation of attentional resources to the processing of somatosensory information/interoceptive stimuli in female youth with heightened somatic complaints. Similarly, females also showed stronger connectivity between the SFA and the right STG, left central operculum, posterior insula, and pMCC as a function of increased somatic complaints; as compared to males, females also displayed connectivity between the SFA and the left anterior STG and SMA. Of note, the increased SFA-insula connectivity was strongest with the posterior insula whose resting-state network is prominently associated with sensorimotor integration (Cauda et al., 2011). In this network, the posterior insula demonstrates connectivity with the amygdala as well as SMA and right STG (Cauda et al., 2011), which is considered a part of the “social brain” (Blakemore, 2008). This heightened SFA connectivity observed in females who express more somatic complaints is likely related to atypical sensorimotor and socio-emotional processing; however, future studies using a longitudinal design are needed to determine whether this hyperconnectivity reflects a compensatory mechanism or increased risk for worsening symptoms over time.
To our knowledge, this study offers the first look into sex differences in amygdala functional connectivity profiles in relation to subclinical internalizing symptoms in typically-developing children and adolescents. However, there are important limitations and future directions to consider. Though we were able to investigate the impact of subclinical symptomatology on functional connectivity of the amygdala, the cross-sectional nature of this study prevents a full characterization of the likely bi-directional nature of the relationship between the emergence of internalizing symptoms and developmental changes in functional connectivity. While we found that the majority of our findings did not change when age was included as a covariate in our models, age effects were most prominent in analyses using the anxious-depressed subscale; this suggests that the relationship between amygdala connectivity and this class of symptoms might be the most impacted by age. Longitudinal studies investigating the relationship between amygdala connectivity, age, and anxious-depressed symptomatology are crucial to further understanding this circuitry in developmental samples. Future research might also utilize a larger sample size with different age-cohorts to more fully address developmental issues, such as examining developmental changes in amygdala connectivity in relation to amygdala volume. Furthermore, since our sample consisted of typically-developing youth, the observed range of internalizing symptoms was limited. While this is expected in a sample of children and adolescents without a clinical diagnosis of any internalizing disorder, it does limit our ability to examine how functional connectivity might vary as a function of a broader range of internalizing symptoms. Future investigations incorporating more clinically enriched samples with a wider range of symptom severity are needed to better understand the relationship between subclinical internalizing symptoms and amygdala connectivity. Nevertheless, our findings highlight the importance of considering individual variability within samples of putatively neurotypical youth, especially in the context of comparisons with a clinical sample. Future studies involving developmental samples may also want to consider the moderating effect of factors such as socioeconomic status and presence of other comorbidities.
In conclusion, this work highlights the shared and distinct functions of the amygdala and its subnuclei as hubs of neural integration of salience, action, emotion, and sensory processing. Compared to males, females displayed greater internalizing symptoms and greater modulation of amygdala circuits in relation to these symptoms. In accordance with taking a dimensional approach toward psychiatric disorders, this work demonstrates that the effects of subclinical symptomatology on neural circuitry can be readily detected in neurotypical populations. This lays the groundwork for future research investigating whether these network atypicalities could be predictive of worsening symptoms and/or of a future clinical diagnosis. These early alterations in amygdala functional connectivity may reflect risk for the onset of an internalizing disorder in the future but they could also reflect neuroplasticity that could promote resilience. Longitudinal investigations with large samples, such as the Adolescent Brain Cognitive Development Study (ABCD Study; http://abcdstudy.org), will help further elucidate the complex nature of these brain-behavior relationships and ultimately inform early screening, diagnosis, and interventions for psychiatric disorders that emerge during adolescence.
Declaration of Competing Interest
The authors report no conflicts of interest.
Acknowledgments
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (under Grant no. 1650604 to N.T.P.), the National Institute of Child Health and Human Development (award number 1T32HD091059 to N.T.P.), and the National Institute of Mental Health (award number R01MH100028 to M.D.). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Science Foundation or the National Institutes of Health. We are grateful for the generous support from the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Capital Group Companies Charitable Foundation, William M. and Linda R. Dietel Philanthropic Fund, and Northstar Fund. We would additionally like to thank Hilary Bowman and members of the GENDAAR Consortium for assistance in data collection and coordination. We would also like to thank the participants and their families for generously sharing their time for this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100797.
Appendix A. CBCL Instructions and Questions
CBCL Instructions:
Below is a list of items that describe children and youths. For each item that describes your child now or within the past 6 months, please circles the 2 if the item is very true or often true of your child. Circle the 1 if the item is somewhat or sometimes true of your child. If the item is not true of your child, circle the 0. Please answer all the items as well as you can, even if some do not seem to apply to your child.
0 = Not True (as far as you know)
1 = Somewhat or Sometimes True
2 = Very True or Often True
CBCL Questions – separated by subscale:
Anxiety-Depression:
14. Cries a lot
29. Fears certain animals, situations, or places, other than school (describe): ___
30. Fears going to school
31. Fears he/she might think or do something bad
32. Feels he/she has to be perfect
33. Feels or complains that no one loves him/her
35. Feels worthless or inferior
45. Nervous, high strung, or tense
50. Too fearful or anxious
52. Feels too guilty
71. Self-conscious or easily embarrassed
91. Talks about killing self
112. Worries
Somatic Complaints:
47. Nightmares
49. Constipated, doesn’t move bowels
51. Feels dizzy or lightheaded
54. Overtired without good reason
56. Physical problems without known medical causes:
-
a
Aches or pains (not stomach or headaches)
-
b
Headaches
-
c
Nausea, feels sick
-
d
Problems with eyes (not if corrected by glasses): describe __
-
e
Rashes or other skin problems
-
f
Stomachaches
-
g
Vomiting, throwing up
-
h
Other (describe): __
Withdrawn-Depressed
5. There is very little he/she enjoys
42. Would rather be alone than with others
65. Refuses to talk
69. Secretive, keeps things to self
75. Too shy or timid
102. Underactive, slow moving, or lacks energy
103. Unhappy, sad, or depressed
111. Withdrawn, doesn’t get involved with others
Appendix B. Supplementary data
The following is Supplementary data to this article:
References
- Achenbach T.M. University of Vermont Department of Psychiatry; Burlington: 1991. Manual for the Child Behavior Checklist 4–18 and 1991 Profile. [Google Scholar]
- American Psychiatric Association . 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and statistical manual of mental disorders. [DOI] [Google Scholar]
- Amaral D.G., Price J.L., Pitkanen A., Carmichael S.T. The Amygdala: Neurobiological Aspects of Emotion. 1992. Anatomical organization of the primate amygdaloid complex; pp. 1–66. [Google Scholar]
- Alarcón G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., Habel U., Schneider F., Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl). 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Angold A., Erkanli A., Silberg J., Eaves L., Costello E.J. Depression scale scores in 8-17-year-olds: effects of age and gender. J. Child Psychol. Psychiatry. 2002;43:1052–1063. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- Baur V., Hänggi J., Langer N., Jäncke L. Resting-State Functional and Structural Connectivity Within an Insula-Amygdala Route Specifically Index State and Trait Anxiety. BPS. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Lau J.Y.F., Guyer A.E., McClure-Tone E.B., Monk C.S., Nelson E.E., Fromm S.J., Goldwin M.A., Wittchen H.-U., Leibenluft E., Ernst M., Pine D.S. Common and Distinct Amygdala-Function Perturbations in Depressed vs Anxious Adolescents. Arch. Gen. Psychiatry. 2009;66:275. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. The amygdala: Functional organization and involvement in neurologic disorders. Neurology. 2015;84:313–324. doi: 10.1212/WNL.0000000000001171. [DOI] [PubMed] [Google Scholar]
- Bickart K.C., Hollenbeck M.C., Barrett L.F., Dickerson B.C. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J. Neurosci. 2012;32:14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch J.D., Ansari T.L., Smith S., Gauld O., Zika O., Boessenkool S., Browning M., Reinecke A., Bishop S.J. 2018. Stratification of MDD and GAD patients by resting state brain connectivity predicts cognitive bias. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford J.U., Pine D.S. Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child Adolesc. Psychiatr. Clin. N. Am. 2012;21:501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. Is Adolescence a Sensitive Period for Sociocultural Processing? Annu. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Burghy C.A., Stodola D.E., Ruttle P.L., Molloy E.K., Armstrong J.M., Oler J.A., Fox M.E., Hayes A.S., Kalin N.H., Essex M.J., Davidson R.J., Birn R.M. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Laird A.R., Zilles K., Fox P.T., Eickhoff S.B. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum. Brain Mapp. 2013;34:3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annu. Rev. Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Heller A.S., Gee D.G., Cohen A.O. Development of the emotional brain. Neurosci. Lett. 2019;693:29–34. doi: 10.1016/J.NEULET.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., D’Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/J.NEUROIMAGE.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Center for Collegiate Mental Health . 2015. Annual Report. [Google Scholar]
- Connolly C.G., Wu J., Ho T.C., Hoeft F., Wolkowitz O., Eisendrath S., Frank G., Hendren R., Max J.E., Paulus M.P., Tapert S.F., Banerjee D., Simmons A.N., Yang T.T. Resting-State Functional Connectivity of Subgenual Anterior Cingulate Cortex in Depressed Adolescents. Biol. Psychiatry. 2013;74:898–907. doi: 10.1016/J.BIOPSYCH.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J.N., Gruber C. Second Edition. Western Psychological Services; Torrance, CA: 2012. Social Responsiveness Scale. (SRS-2) [Google Scholar]
- Costello E.J., Angold A.C. Developmental Epidemiology. In: Sameroff A.J., Lewis M., Miller S.M., editors. Handbook of Developmental Psychopathology. Springer; Boston, MA: 2000. [Google Scholar]
- Cox R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crick N.R., Zahn-Waxler C. The development of psychopathology in females and males: Current progress and future challenges. Dev. Psychopathol. 2003;15:719–742. doi: 10.1017/S095457940300035X. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Publ. Gr. 2012:13. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cummings C.M., Caporino N.E., Kendall P.C. Comorbidity of Anxiety and Depression in Children and Adolescents: 20 Years After NIH Public Access. Psychol Bull. 2014;140:816–845. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan R.K., Vuilleumier P. Amygdala Automaticity in Emotional Processing. Ann. N. Y. Acad. Sci. 2006;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Drysdale A.T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y., Fetcho R.N., Zebley B., Oathes D.J., Etkin A., Schatzberg A.F., Sudheimer K., Keller J., Mayberg H.S., Gunning F.M., Alexopoulos G.S., Fox M.D., Pascual-Leone A., Voss H.U., Casey B., Dubin M.J., Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvenvoorde A.C.K., Westhoff B., Vos F., Wierenga L.M., Crone E.A. A three‐wave longitudinal study of subcortical–cortical resting‐state connectivity in adolescence: Testing age‐ and puberty‐related changes. Hum. Brain Mapp. 2019;40:hbm.24630. doi: 10.1002/hbm.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton N.R., Keyes K.M., Krueger R.F., Balsis S., Skodol A.E., Markon K.E., Grant B.F., Hasin D.S. An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample. J. Abnorm. Psychol. 2012;121:282–288. doi: 10.1037/a0024780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman J., Linnman C., Dijk Van K.R.A., Milad M.R. Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology. 2016;63:34–42. doi: 10.1016/j.psyneuen.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. Disrupted Amygdalar Subregion Functional Connectivity and Evidence of a Compensatory Network in Generalized Anxiety Disorder. Arch. Gen. Psychiatry. 2009;66:1361. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M.S., Ledoux J.E. Why we think plasticity underlying viewpoint pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/S0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Hare T., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/J.NEUROIMAGE.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J., Weersing V.R. Comorbidity of Anxiety and Depression in Youth: Implications for Treatment and Prevention. Clin. Psychol. Sci. Pract. 2010;17:293–306. doi: 10.1111/j.1468-2850.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J., Valabrègue R., Gholipour B., Chevallier C. A direct amygdala-motor pathway for emotional displays to influence action: A diffusion tensor imaging study. Hum. Brain Mapp. 2014;35:5974–5983. doi: 10.1002/hbm.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Nitschke J.B. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Silk J.S., Nelson E.E. The neurobiology of the emotional adolescent: From the inside out. Neurosci. Biobehav. Rev. 2016;70:74–85. doi: 10.1016/j.neubiorev.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C., Mayberg H., Stone S., Laxton A., Haber S., Lozano A.M. The Subcallosal Cingulate Gyrus in the Context of Major Depression. Biol. Psychiatry. 2011;69:301–308. doi: 10.1016/J.BIOPSYCH.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hankin B.L., Abramson L.Y., Moffitt T.E., Silva P.A., Mcgee R., Angell K.E. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J. Abnorm. Psychol. 1998;107:128–140. doi: 10.1037/0021-843X.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hortensius R., Terburg D., Morgan B., Stein D.J., van Honk J., de Gelder B. The role of the basolateral amygdala in the perception of faces in natural contexts. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016:371. doi: 10.1098/rstb.2015.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M., Larsen B., Hallquist M.N., Foran W., Calabro F., Luna B. Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biol. Psychiatry. 2017;82:511–521. doi: 10.1016/J.BIOPSYCH.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage. 2002;17:825–841. doi: 10.1006/NIMG.2002.1132. [DOI] [PubMed] [Google Scholar]
- Kerestes R., Chase H.W., Phillips M.L., Ladouceur C.D., Eickhoff S.B. Multimodal evaluation of the amygdala’s functional connectivity. Neuroimage. 2017;148:219–229. doi: 10.1016/j.neuroimage.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D., Angstadt M., Sripada C.S. Reevaluating "cluster failure" in fMRI using nonparametric control of the false discovery rate. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E3372–E3373. doi: 10.1073/pnas.1614502114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F., Morgan B., Terburg D., Stein D.J., van Honk J. Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Soc. Cogn. Affect. Neurosci. 2015;10:1161–1168. doi: 10.1093/scan/nsu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Skouras S., Fritz T., Herrera P., Bonhage C., Küssner M.B., Jacobs A.M. 2013. The roles of superficial amygdala and auditory cortex in music-evoked fear and joy. [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation — An ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/J.NEUROIMAGE.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. The amygdala. Curr. Biol. 2007:17. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P.M., Rohde P., Seely J.R. Adolescent psychopathology: III. The clinical consequences of comorbidity. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34:510–519. doi: 10.1097/00004583-199504000-00018. [DOI] [PubMed] [Google Scholar]
- Mareckova K., Holsen L.M., Admon R., Makris N., Seidman L., Buka S., Whitfield-Gabrieli S., Goldstein J.M. Brain activity and connectivity in response to negative affective stimuli: Impact of dysphoric mood and sex across diagnoses. Hum. Brain Mapp. 2016;37:3733–3744. doi: 10.1002/hbm.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Hatzenbuehler M.L., Mennin D.S., Nolen-Hoeksema S. Emotion dysregulation and adolescent psychopathology: a prospective study. Behav. Res. Ther. 2011;49:544–554. doi: 10.1016/j.brat.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behav. Res. Ther. 2011;49:186–193. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K.R., He J., Burstein M., Swanson S.A., Avenevoli S., Cui L., Benjet C., Georgiades K., Swendsen J. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:980–989. doi: 10.1016/J.JAAC.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft R.J., McNeal D.W., Stilwell-Morecraft K.S., Gedney M., Ge J., Schroeder C.M., van Hoesen G.W. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J. Comp. Neurol. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Murphy K., Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. Self-Referential Processing, Rumination, and Cortical Midline Structures in Major Depression. Front. Hum. Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;171:415–436. doi: 10.1016/J.NEUROIMAGE.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat. Rev. Neurosci. 2010;11:773–782. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/J.NEUROIMAGE.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Qin S., Young C.B., Duan X., Chen T., Supekar K., Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol. Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2019. R: A language and environment for statistical computing. [Google Scholar]
- Raichle M.E. Two views of brain function. Trends Cogn. Sci. 2010;14:180–190. doi: 10.1016/J.TICS.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer H., Alarcón G., Demeter D.V., Earl E., Fair D.A., Nagel B.J. Reduced fronto-amygdalar connectivity in adolescence is associated with increased depression symptoms over time. Psychiatry Res. Neuroimaging. 2017;266:35–41. doi: 10.1016/j.pscychresns.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/J.NEUROIMAGE.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/J.NEUROIMAGE.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.