The authors of the recent publication “Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19” [1] appreciate the questions that were submitted as a letter to the editor [2] and the opportunity to address them to the best of our ability here.
The reported clinical trial was performed by the authors independently of corporate involvement under institutional review board (IRB) protocol review and approval. All patients or their legal health care proxies were provided informed consent as described in the original article.
Some of the questions presented in the letter to the editor required the authors to reach out to the manufacturer of ExoFlo™ (Direct Biologics LLC, Austin, TX) for a more detailed response to some of their questions.
The scientific team at Direct Biologics welcomes and agrees with the points made by Lim et al. In light of the current COVID-19 pandemic, transparency should be provided to enable appropriate evaluation of new technology and therapies. In the spirit of comradery requisite to conquer the challenges posed by the SARS-CoV2 virus, we wish to address the questions posed by the authors. We have provided responses to questions 1–4 and 7.
-
1.
Per FDA guidance for current Good Manufacturing Practices (cGMP) manufacturing, all raw materials, and supplies, vendors and manufacturing organizations involved in the manufacture of ExoFlo product are qualified and approved to ensure all regulatory standards are met. Our cGMP manufacturer is registered with the FDA to manufacture extracellular vesicles (EV) isolates. All processes are controlled under Quality Management System and have lot-specific master batch records and specified release criteria.
-
2.
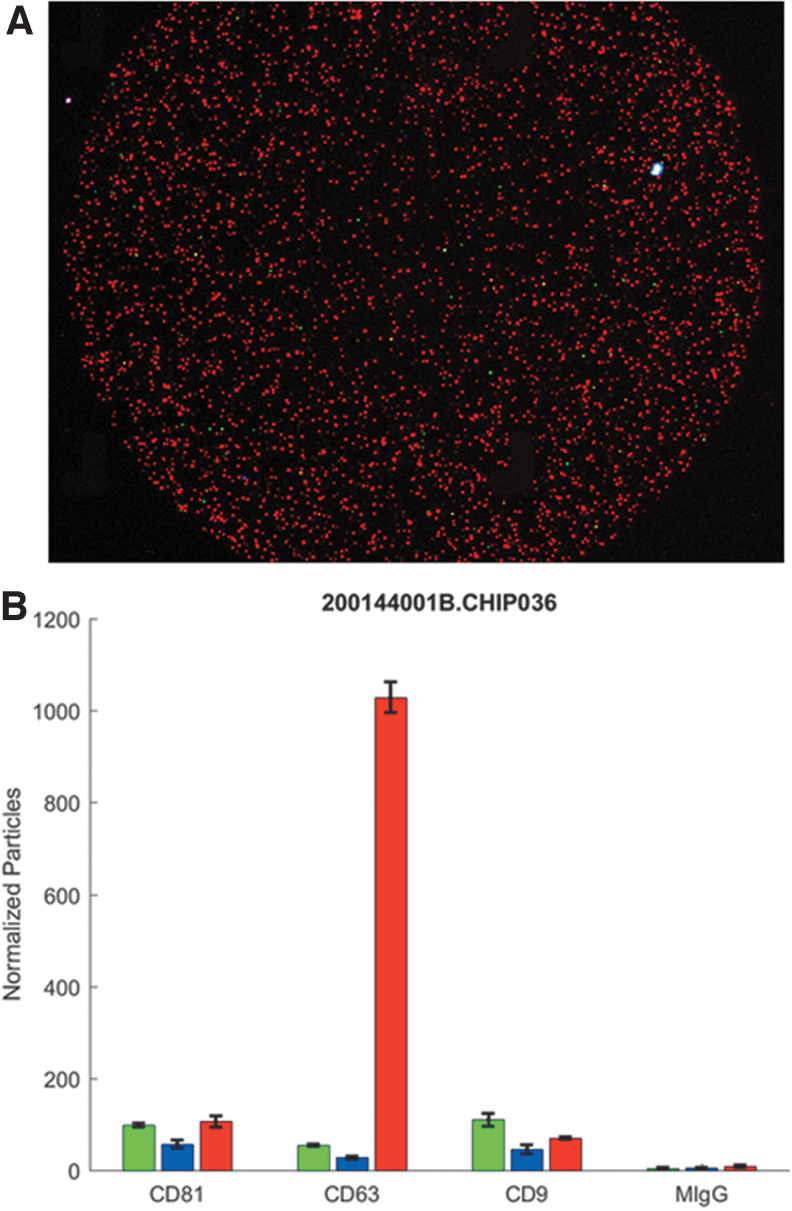
Lot-specific exosome characterization of ExoFlo is performed as product specification release criteria. These characterizations are done using in-house and third-party analyses, by traditional nanoparticle tracking analysis under both light scatter and fluorescence evaluation (NanoSight, Malvern Panalytical Ltd., United Kingdom) and single particle interferometric reflectance imaging sensor technology to visualize and quantify fluorescent antibody-labeled particles (NanoView Biosciences, Boston, MA). These methods collectively support that the concentration, size distribution, and protein identity of the nanoparticles within ExoFlo are an EV exosome population. The NanoView analysis of ExoFlo determined that the primary EV population has a phenotype of CD63+, CD9−, and CD81− and comprised >95% of the particles detected (Fig. 1). We do not dismiss the possibility that there may be a small population of cell membrane-derived EVs present. Ongoing studies will further clarify the identity of this population if it exists.
The bone marrow (BM) cell source used in the manufacture of this ExoFlo lot is from the iliac crest aspiration of a single donor. The primary adherent cells were expanded and tested per FDA regulations under current Good Tissue Practices compliant banking conditions to generate a master cell bank (MCB). The MCB has a Master File recorded at the FDA as does the chemically defined xeno-free medium used to support the cell population during the manufacturing process. Characterization of the MCB cells confirm their identity as BM-derived mesenchymal stem cell (MSCs) and meet all of the criteria established by the International Society for Stem Cell Research and International Society of Cell and Gene Therapy societies to qualify the cells as BM-MSCs. This includes the capacity to undergo trilineage differentiation in vitro toward adipocyte, osteoblast, and chondrocyte phenotypes, positive expression of MSC marker proteins, (CD73, CD105, CD166, and CD90), and negative expression for CD markers associated with other marrow cell types (CD14, CD31, CD34, and CD45).
-
3.
Based upon the current BM MSC-derived EV literature, and internal characterization and potency studies, a primary mode of action for ExoFlo is to communicate changes in host immune response. Independent proteomic analysis evaluated ExoFlo for the presence of secreted or membrane-associated proteins using commercially available antibody arrays. The study identified that ∼40% of the proteins present within ExoFlo have functions associated with immunoregulation. The remaining proteins were associated with cell migration, regulation of apoptosis, extracellular matrix remodeling, angiogenesis, and cell differentiation. Consistent with the years of mechanism of action studies performed to assess the MSCs themselves, the secreted biomolecule milieu is complex, and the primary mode of action is dependent on the environment into which it is introduced into the body.
-
4.
The authors agree that the rationale for the study clinical dose requires clarification. The clinical dose for the trial was established based upon historic dose ranges reported in prior MSC and cell delivery clinical trials (eg, ClinicalTrials.gov, numbers NCT01775774 and NCT04338347). A single ExoFlo lot was utilized during the study. EV concentrations obtained during manufacturing were divided from a known number of source BM-MSCs to determine the average quantity of EVs generated per cell. This value was multiplied by the low and high range values of MSC concentrations (1 million to 10 million cells/kg), and a mid-range ExoFlo EV concentration equivalent to ∼40,000,000 cells/mL was determined. Based upon the weight and BMI range observed for adult populations, the delivered EV dose in a volume of 15 mL was predicted to fall within the cell equivalent dose range of 1 to 10 million cells/kg >95% of the time.
-
5.
Patient vital signs were monitored T = 5, T = 10, T = 15, T = 30, T = 45, and T = 60 min after infusion initiation, then hourly for the first 6 h postinfusion, every 3–4 h thereafter as per hospital standards. Patients in the ICU and on the floor were monitored with standard measures of continuous cardiac and SpO2 monitoring. As reported in the original article, the patients were all followed to a minimum of 14 days.
-
6.
The independent data safety monitoring board evaluated each case in its clinical context, and was able to reasonably attribute adverse events either to a clearly identifiable and temporally correlated provoking stimulus or to natural progression of processes that preceded the therapeutic intervention. The National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, which categorize adverse events as suspected or not suspected to be attributable to a therapeutic intervention solely on the basis of temporal correlation, were used as a starting point. The 72-h interval for attribution was chosen as a more rigorous time frame over the more commonly used 24-h window for intravenous therapies. Before study initiation, these endpoints were reviewed and approved by an experienced FDA regulatory consultant. Other sources for establishing this standard were studies in the critical care literature using intravenous MSCs to treat Acute Respiratory Distress Syndrome, most notably the Stanford START Trial cited in the article, and again here [3].
-
7.
A certificate of analysis is provided with each vial of ExoFlo product. Obviously, there are restrictions in communicating research and development data of a biotechnology product due to intellectual property and proprietary information. This often prevents full disclosure of information associated with the manufacturing of a company's product. Therefore, although we have performed most of the studies deemed essential by the International Society for Extracellular Vesicles minimal information for studies of extracellular vesicles statement, we have not yet made this information publicly available to the research community. Based upon the experimental categories listed on the EV track website you referenced, three of these categories would not be applicable for our product. We do have characterization data and information for each of the remaining six categories. As our data become unrestricted in the future, the Direct Biologics research and development team commits to sharing pertinent information on an ongoing basis with the academic community.
FIG. 1.
(A) Fluorescent image of ExoFlo™ that was triple stained with antibodies against tetraspanin proteins reported in the literature to be enriched in exosomes using the NanoView Biosciences NanoView system. Red fluorescence, CD63; blue fluorescence, CD9; green fluorescence, CD81. (B) Semiquantification analysis of ExoFlo nanoparticles captured on different Nanoview antibody capture arrays. Data demonstrate an exosome population highly enriched for CD63 expression is the dominant EV population. EV, extracellular vesicle.
The authors hope that the additional information provided by the responses to these questions supports and aids in interpretation of our very promising clinical results. The intention of this study was to provide humanitarian relief to the patients during this serious COVID-19 crisis and global pandemic. We hope that anticipated future investigational new drug applications and associated studies will support the use of this unique biologic technology.
Author Disclosure Statement
T.M. and K.H. serve as chief science officer and director of research and development, respectively, at Direct Biologics LLC, Austin, TX, from which they receive their paychecks and have a vested financial interest in the success of ExoFlo as a therapy. All other authors declare no competing financial interests exist.
Funding Information
This study was supported by Drs. Vikram and Sascha Sengupta in collaboration with Christ Hospital. None of the authors were compensated for this study. Thrivewell Infusion, LLC provided acquired, commercially available product from Direct Biologics, LLC, Austin, TX, storage, medical sup- plies, transportation, legal resources, as well as supportive administrative and clinical personnel. No governmental funding of any kind was used to support this study.
References
- 1. Sengupta V, Sengupta S, Lazo Jr A, Woods P, Nolan A and Bremer N (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 29:747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim SK, Giebel B, Weiss DJ, Witwer KW and Rohde E (2020). Re: “Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19” by Sengupta et al. Stem Cells Dev 29:877–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, et al. (2015). Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 3:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]