Abstract
With an increase in the number of cochlear implant surgeries there is bound to be an increase in the number of complications. A dreaded problem in any implant procedure is the implant exposure and infection. Explantation of the implant leads to an unpleasant situation to the patient and the surgeon owing to the high cost of the device. There are reports in the literature favouring the mandatory relocation or removal of the infected implants. On the other hand, there are convincing reports of implant salvage using skin, muscle or fascial flaps. In this paper we have analysed a series of cases referred to us from the departments of E.N.T for the management of implant exposure/infection. We have also reviewed similar case series reported in the literature. From 2014 to 2017 we operated six cases of exposed cochlear implant. We salvaged the implant in five cases, where we could do two layer coverage consisting of the inner temporoparietal fascial flap and outer scalp skin flap. In one case where the temporoparietal fascial flap could not be done as superficial temporal vessels were found to be injured in the previous surgery, the implant was removed due to persistent infection. All these cases were administered appropriate antibiotics for a minimum period of 3 weeks. Early double layer closure with inner temporoparietal fascial flap and outer scalp rotation flap coupled with appropriate antibiotics can salvage an infected, exposed implant.
Keywords: Exposed cochlear implant, Infected cochlear implant, Cochlear implant salvage, Temporoparietal fascial flap
Introduction
With a reduction in the cost of the cochlear implants, but with no such reduction in the incidence of congenital sensorineural hearing loss, cochlear implant surgery has become a common procedure in select centres. In places where the implants and the surgical procedures are funded by the state, there is an exponential increase in the number of centres offering cochlear implant surgery. Hence, logically there is bound to be an increase in the number of complications.
A dreaded problem in any implant procedure is the implant exposure or infection necessitating the removal of the implant. Infection of the implant invariably occurs once it gets exposed due to wound dehiscence or skin necrosis. Though early flap cover effectively salvages the cochlear implant, saving it is a big ordeal once the infection gets established. In resistant cases, the implant has to be removed which leads to a very unpleasant situation to the patient and the surgeon owing to the high cost of the device. There are reports in the literature favouring the mandatory relocation or removal of the infected implants [1, 2]. On the other hand, there are convincing reports of implant salvage using skin, muscle or fascial flaps [3, 4].
In this paper, we have analysed a series of cases referred to us from the departments of E.N.T for the management of implant exposure/infection. We have also reviewed similar case series reported in the literature.
Patients and Methods
From 2014 to 2017 we operated six cases of exposed cochlear implant (Table 1). In two cases, there were primary wound dehiscence and infection. In these cases, local scalp flaps were done elsewhere. Though the flaps were viable, the implants were exposed due to persistent infection and wound break down.
Table 1.
Details of the patients
| No | Age | Side | Diagnosis | Duration of problem | Previous flap | Wound culture | Procedure | Result |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 year | Rt | Exp implant | 6 weeks | Post scalp rotation | MRSA | Tpff, post scalp rotation | Successful |
| 2 | 3 years | Lt | Exp implant | 4 weeks | Nil | Staph epidermidis | Tpff, post scalp rotation | Successful |
| 3 | 3 years | Rt | Exp implant | 6 weeks | Sup scalp rotation | Staph epidermidis | Post scalp rotation | Explantation |
| 4 | 7 years | Rt | Keloid | 1 year | Nil | No organisms | Tpff, skin flap advancement | Successful |
| 5 | 2 years | Rt | Exp implant | 3 weeks | Nil | No organisms | Tpff, post scalp rotation | Successful |
| 6 | 2 years | Rt | Exp implant | 3 weeks | Nil | Staph aureus | Tpff, post scalp rotation | Successful |
Rt right, Lt left, Exp exposed, Post posterior, Sup superior, MRSA methicillin resistant staphylococcus aureus, Staph staphylococcus, Tpff temporoparietal fascial flap
In one case, the post aural wound initially healed well. Wound infection occurred 3 weeks after the surgery, resulting in the exposure of the implant.
In two cases, the surgical wounds healed well and the patients were undergoing rehabilitation. There were progressive thinning of the scar over the implants which eventually gave way.
One case developed a huge keloid in the post aural scar interfering with the placement of the external device. As there were pain and itching, the keloid was excised resulting in the exposure of the implant.
In our first case, we did a scalp rotation flap to cover the implant. Initially, the flap settled well. But after 4 weeks, there was discharge from the implant site resulting in wound dehiscence and the exposure of the implant. Debridement of the wound and temporoparietal fascial flap cover were done to cover the implant. Post operatively, antibiotics as per the culture and sensitivity was given for 3 weeks. The wound healed well with no recurrence of infection during the 3 years follow up (Fig. 1a, b). For all our subsequent cases we used temporoparietal fascial flap to cover the implant. Scalp rotation flap was used to close the wound (Fig. 2a–d). Antibiotics as per the culture and sensitivity report were administered for 3 weeks.
Fig. 1.
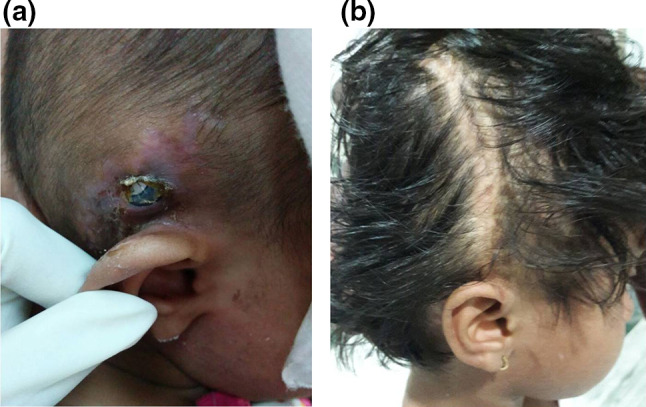
a Exposed implant with surrounding inflamed skin. b Implant covered with TPFF and rotation flap. Healed wound 2 years later
Fig. 2.
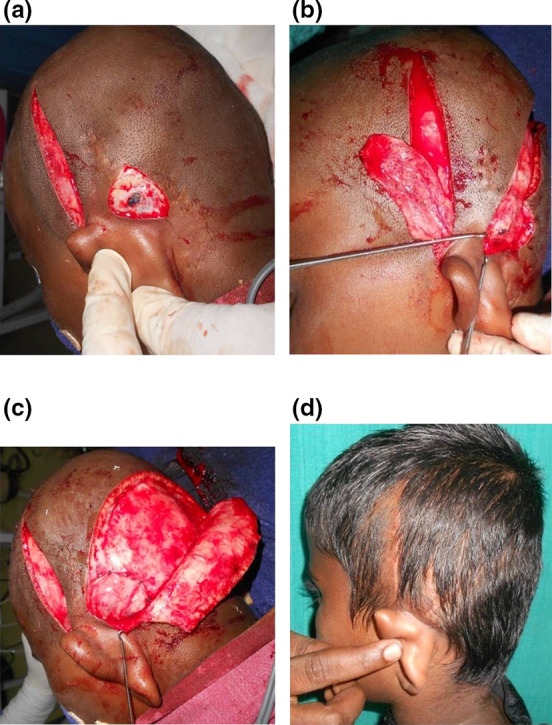
a After debridement part of the implant got exposed. Also seen in the picture is the incision for elevation of the temporoparietal fascial flap.(TPFF). b Elevated TPFF. The superficial temporal vessels at the pedicle of the flap can be well appreciated. c Implant well covered with TPFF. Posterior scalp rotation flap was elevated for skin closure. d Healed wound with well settled scar
In the case of keloid, the exposed implant was covered with the temporoparietal fascial flap and the wound was closed with flap advancement. Post operative therapy included silastic gel sheet compression for 3 months and weekly intra scar injections of kenocort 10 mg for 5 weeks. The wounds healed well with no recurrence during 2 years follow up (Fig. 3a–d).
Fig. 3.
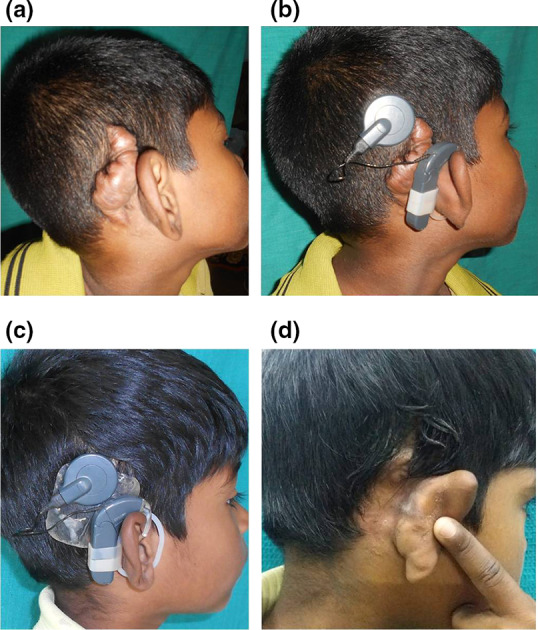
a Huge keloid post aural region. b Improper and insecure placement of the external device due to keloid. c After keloid excision, TPFF and flap advancement. External device in position. Silastic gel sheet was used for 3 months to prevent the recurrence of the keloid. d Well covered implant with no recurrence of the keloid
In one case, temporoparietal fascial flap could not be used, as the superficial temporal artery was injured during the mobilization of the superiorly based scalp rotation flap which was done elsewhere. As the reach of the temporalis muscle flap was found to be inadequate, the implant was partially covered with the local fascial flap. The wound was closed using scalp rotation flap. The wound healed and the flap settled well. But wound discharge started after 6 weeks. Despite adequate antibiotics for 6 weeks, the discharge was persistent and the device was exposed necessitating removal of the implant (Fig. 4a–d).
Fig. 4.
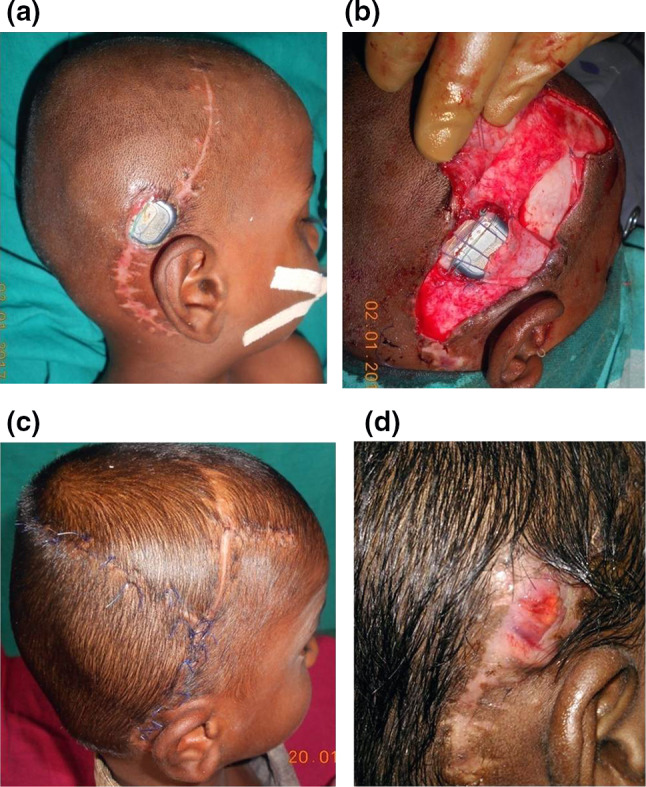
a Exposed cochlear implant after coverage with the rotation flap. Scar of the superior scalp rotation flap is visible. b Only part of the implant is covered with the fascial flap due to injury to the superficial temporal vessels. c Posterior scalp rotation flap for skin closure. d In this case, despite delayed suture removal and antibiotics for 6 weeks the implant had to be removed due to persistent infection
Procedure
Temporoparietal fascia is superficial to the deep temporal fascia extending well beyond the margins of the temporalis muscle. Superficial temporal vessels lie within the fascial layers and hence, the fascia can be elevated as a well vascularised flap based on the narrow vascular pedicle. At first, the axis of the parietal branch of the superficial temporal artery was marked by palpation or hand held doppler. It roughly corresponds to a vertical line of 10 cms length from the tragus towards the parietal region. Skin incision is made 1 cm behind this axis and the skin flaps are dissected at the sub dermal plane leaving the superficial temporal artery and vein in the fascial bed. Care is taken to include the hair follicles in the skin flap. The required dimensions of the fascial flap is marked and elevated after incising the margins. There is always a good avascular cleavage plane between the flap and the deep temporal fascia. The flap based on the vascular pedicle is turned over to cover the implant and fixed by absorbable sutures. For the coverage of cochlear implants, dissection proximal to the root of the helix to trace the superficial temporal vessels is never needed (Fig. 5). Scalp flap in the form of rotation was then mobilised to cover the wound
Fig. 5.
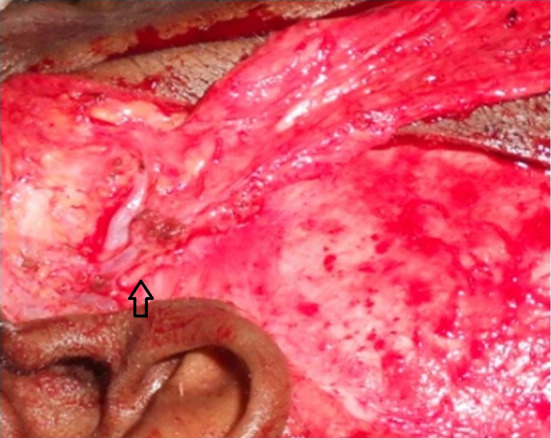
Proximal dissection of the TPFF showing the superficial temporal vessels. The arrow is pointing the auriculotemporal nerve
Results
Of the six cases, we could salvage the implant in 5 cases. They were undergoing speech rehabilitation with the well functioning implant in place. There was no recurrence of infection during 1–3 years follow up. In one case, though two attempts were made for wound closure with skin flaps the implant had to be removed due to recurrent and resistant infection. However, in this case complete coverage of the implant with the fascial flap could not be done, as the implant was placed in a more postero superior position and the superficial temporal vessels were injured in the previous surgery, limiting the territory of the temporoparietal fascial flap.
Discussion
The incidence of infection of the cochlear implant is stated to be 1.08% to 8.2% [3–5]. The exposure of the implant occurs as a result of infection or secondary to necrosis of flap margins. Once the infection sets in, the treatment is more complex than a simple infected wound. In the presence of an implant a relatively lower number of bacteria can initiate and sustain an infection. Further, penetrability of the antibiotics is poor due to the bio film formation [6].
Coverage of the exposed implant with a well vascularised tissue helps in treating the infection [7]. Though mobilising the adjacent scalp in the form of advancement, rotation or transposition to close the wound offers a straight forward solution, it often fails. Galea aponeurotica, the layer adjacent to the implant is relatively non adherent and there is no sub galeal vascular plexus. Hence, the scalp flaps do not bring in the much needed vascularity to the site of infection, though the scalp flaps are highly vascular at the skin margins. This fact explains the delayed wound dehiscence as the result of latent infection, even if the flaps initially healed well. Moreover, the suture line of the skin flaps is likely to lie over a part of the implant. The resultant scar is right over the implant with no tissue in between. Delayed exposure of the implant due to thinning and stretching of the scar is a distinct possibility.
Seo et al. [8] reported successful salvage of the exposed implants by nape of the neck rotation flaps in two cases. Gawęcki et al. could save only 52.6% of their exposed and infected cochlear implants by two layer coverage consisting of inner temporalis muscle fascia flap and outer rotation skin flap. They concluded that single layer closure with a rotation skin flap was not successful in their series [9]. Muscle flaps are generally preferred to treat implant related infections in the limbs. Studies documented that muscle or myocutaneous flaps could bring in more blood supply to the site of implants facilitating the eradication of the infection [7]. For osteomyelitis of the long bones sequestrectomy and coverage of the defect with muscle flaps effect a possible cure. Theoretically, increasing the vascularity by flaps at the site of infections can increase the concentration of the antibiotics effecting better penetration of the biofilm.
Though temporalis muscle flap can be used to cover the implant in the post aural region, the reach of the flap is limited. Temporalis is supplied by the deep temporal vessels which enter the deeper part of the muscle near it’s insertion into the coronoid process of the mandible. As the pedicle of this flap is far more anterior and deeper the flap easily reaches the orbit or oral cavity [10]. But, obviously there is restriction for using this flap in the post aural region. Moreover, the flap has to be pliable and mobile to cover a convex implant unlike in cases of a post aural mastoid cavity where muscle flaps are useful to pack the infected cavity. Further, the muscle flap together with the skin flap result in thick tissue cover over the impant which may interfere with the signal transmission [11–13].
There are documented case reports of successful coverage of an exposed cochlear implant with temporoparietal fascial flap [14, 15]. Karimnejad et al. [16] used temporoparietal fascial flap cover to prevent extrusion in two cases of reimplantation of cochlear implant.
Temporoparietal fascial flap is well vascularised and thin. As the fascia extends beyond the margins of the temporalis muscle and the pedicle consisting of superficial temporal vessels lie immediately adjacent to the root of the helix and the tragus, the posterior reach of the flap is much more than the temporalis muscle [17]. Hence, it easily covers the entire implant. These characteristics make this flap well suited for this problem.
Conclusion
Temporoparietal fascial flap due to its proximity, ease of reach, thinness and vascularity should be the flap of choice for the coverage of exposed/infected cochlear implants. Early double layer closure of the implants with inner temporoparietal fascial flap and outer scalp rotation flap coupled with appropriate antibiotics for a minimum period of 3 weeks can salvage an infected, exposed implant.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with Ethical Standards
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical Approval
As this paper was a case series involving only a modification of an accepted flap design, no specific ethical committee approval was obtained.
Informed Consent
Informed consent was obtained from all the patients. Additional informed consent was obtained from those patients whose identifiable information is included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
N. C. Hariharan, Email: drnchariharan@gmail.com
R. Muthukumar, Email: muthukumar.ramamurthy@gmail.com
R. Sridhar, Email: milosri@yahoo.com
B. Shankari, Email: drbshankari@gmail.com
V. S. Valarmathy, Email: vsvalar@gmail.com
References
- 1.Skrivan J, Drevinek P. A case report of a cochlear implant infection—A reason to explant the device? Cochlear Implants Int. 2016;17(5):246–249. doi: 10.1080/14670100.2016.1227019. [DOI] [PubMed] [Google Scholar]
- 2.Haberkamp TJ, Schwaber MK. Management of flap necrosis in cochlear implantation. Ann Otol Rhinol Laryngol. 1992;101(1):38–41. doi: 10.1177/000348949210100111. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham CD, Slattery WH, Luxford WM. Postoperative infection in cochlear implant patients. Otolaryngol Head Neck Surg. 2004;131(1):109–114. doi: 10.1016/j.otohns.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Leach J, Kruger P, Roland P. Rescuing the imperiled cochlear implant: a report of four cases. Otol Neurotol. 2005;26(1):27–33. doi: 10.1097/00129492-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gluth MB, Singh R, Atlas MD. Prevention and management of cochlear implant infections. Cochlear Implants Int. 2011;12(4):223–227. doi: 10.1179/146701011X12950038111576. [DOI] [PubMed] [Google Scholar]
- 6.Schierholz JM, Beuth J. Implant infections: a haven for opportunistic bacteria. J Hosp Infect. 2001;49(2):87–93. doi: 10.1053/jhin.2001.1052. [DOI] [PubMed] [Google Scholar]
- 7.Tan KJ, Lim CT, Lim AY. The use of muscle flaps in the salvage of infected exposed implants for internal fixation. J Bone Jt Surg Br. 2010;92(3):401–405. doi: 10.1302/0301-620X.92B3.22115. [DOI] [PubMed] [Google Scholar]
- 8.Seo BF, Park SW, Han HH, Moon SH, Oh DY, Rhie JW. Salvaging the exposed cochlear implant. J Craniofac Surg. 2015;26(8):749–752. doi: 10.1097/SCS.0000000000002259. [DOI] [PubMed] [Google Scholar]
- 9.Gawęcki W, Karlik M, Borucki Ł, Szyfter-Harris J, Wróbel M. Skin flap complications after cochlear implantations. Eur Arch Otorhinolaryngol. 2016;273(12):4175–4183. doi: 10.1007/s00405-016-4107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birt BD, Antonyshyn O, Gruss JS. The temporalis muscle flap for head and neck reconstruction. J Otolaryngol. 1987;16(3):179–184. [PubMed] [Google Scholar]
- 11.Mattingly JK, Greene NT, Jenkins HA, Tollin DJ, Easter JR, Cass SP. Effects of skin thickness on cochlear input signal using transcutaneous bone conduction implants. Otol Neurotol. 2015;36(8):1403–1411. doi: 10.1097/MAO.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elgandy MS, Mobashir MK, El-Sheikh E, Hansen M, Tyler R, Dunn C, Gantz B. Revision cochlear implant surgery. Int Tinnitus J. 2018;22(2):188–197. [Google Scholar]
- 13.Odabasi O, Mobley SR, Bolanos RA, Hodges A, Balkany T. Cochlear implantation in patients with compromised healing. Otolaryngol Head Neck Surg. 2000;123:738–741. doi: 10.1067/mhn.2000.111355. [DOI] [PubMed] [Google Scholar]
- 14.Beckenstein MS, Steenerson RL, Elliott LF, Hartrampf CR. Use of a superficial temporal fascia flap for coverage of an exposed cochlear implant. Otolaryngol Head Neck Surg. 1999;120(6):940–942. doi: 10.1016/S0194-5998(99)70343-8. [DOI] [PubMed] [Google Scholar]
- 15.Limasánchez JS, Berenguer B, Aránguez G, Gonzálezmeli BM, Marín CM, de Tomás P. Extruded cochlear implant magnet covered with a temporoparietal fascial flap. A case report E. Cir Pediatr. 2013;26(1):48–51. [PubMed] [Google Scholar]
- 16.Karimnejad K, Akhter AS, Walen SG. The temporoparietal fascia flap for coverage of cochlear reimplantation following extrusion. Int J Pediatr Otorhinolaryngol. 2017;94:64–67. doi: 10.1016/j.ijporl.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Jaquet Y, Higgins KM, Enepekides DJ. The temporoparietal fascia flap: a versatile tool in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2011;19(4):235–241. doi: 10.1097/MOO.0b013e328347f87a. [DOI] [PubMed] [Google Scholar]


