Abstract
The objective of this study was to investigate the effects of linolenic acid (LA) on oxidation stability of rapeseed oils. Four kinds of rapeseed were harvested by unified cultivation and management in the same geographical conditions, and then four rapeseed oils with different contents of LA were obtained. The effects of linolenic acid and antioxidants (tocopherols and phytosterols) on oxidation stability of rapeseed oils were evaluated. Results showed that rapeseed oil with 5.9% LA was the most stable among four rapeseed oils, followed by commercial rapeseed oil, rapeseed oil with 8.4% LA and rapeseed oil with 10.8% LA. The oxidation stability was negatively correlated with the contents of LA (r = − 0.931, p < 0.01), the polyunsaturated fatty acids (r = − 0.932, p < 0.01), and unsaturated fatty acids (r = − 0.766, p < 0.05). It had no correlation with tocopherols and phytosterols (p > 0.05). In addition, according to the European Union Standards, shelf-life of four rapeseed oils was longer than 30 days in the shelf-life test. Therefore, increasing the LA content in rapeseed oils can be considered as an efficient approach to solve the problem of insufficient LA intake globally.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04349-x) contains supplementary material, which is available to authorized users.
Keywords: Rapeseed oil, Linolenic acid, Oxidation stability, Shelf-life test, Fourier transform infrared spectroscopy
Introduction
Rapeseed is the second most important oilseed crop in the world after soybean (Mortuza et al. 2006). At present, traditional rapeseed breeding aims at reducing the contents of erucic acid, linolenic acid (LA) and at increasing the contents of oleic acid, linoleic acid. The breeders believe that rapeseed oils with a high content of LA are easily oxidized and deteriorate after exposure to air, light and heat; therefore, efforts have been made to decrease LA content from 9% to 3%. However, LA, which is an essential fatty acid, is reported to have neuroprotective, anti-cancer, antioxidant, anti-inflammatory, anti-hypertensive effects (Bassett et al. 2011; Kim et al. 2014). World Health Organization (WHO) identified LA as the only essential fatty acid in the ω-3 system and required that LA need additional replenish. In China, the daily intake of LA per person is only 0.4 g, which is far less than half of the recommended amount of WHO. Thus, producing edible oils rich in LA can benefit individuals whose LA consumption is deficient. Rapeseed oils are a highly widespread edible oils in the world and classified as one of the healthiest vegetable oils (Yang et al. 2013). Raising LA content in rapeseed oils and recognizing rapeseed oils as a main sources of LA is highly feasible. Therefore, relationship between the content of LA and oxidation stability of rapeseed oil must be evaluated and explored.
Several studies reported the effects on oxidation stability of rapeseed oil. The effect of fatty acids on stability was believed the major factor, and it depends mainly on their degree of unsaturation and the position of double bond (Bhatnagar et al. 2009; Kamal-Eldin 2006). Antioxidants, especially tocopherols and phytosterols, in rapeseed oil exert a certain inhibitory effect on oil oxidation by protecting polyunsaturated fatty acids from degradation (Braunrath et al. 2009; Isnardy et al. 2003). There are many traditional methods for evaluating oxidation stability of rapeseed oil: Schaal oven test, an oven storage test used to simulate the real-time aging of oils at approximately 63 °C (Dimic et al. 2015; Michotte et al. 2011); Rancimat method, a method use to judge the stability of oil and the degree of oxidation by determining the induction period of oil oxidation (Kowalska et al. 2013); shelf-life test (Symoniuk et al. 2017), a test to simulate the actual situation of edible oil by placing oil samples on shelves and evaluate its basic quality features at regular time intervals. With continuous development of detection technology, many new technologies have been proposed, including pressure differential scanning calorimetry (Ciemniewska-Żytkiewicz et al. 2014), electronic nose (Xu et al. 2016), near infrared transmission spectroscopy and Fourier transform infrared (FTIR) spectroscopy. Our group has developed quantitative FTIR oil analysis methods based on stainless steel mesh cell and polyethylene films, and these rapid analysis of oxidation stability of fats and oils (Xu et al. 2015; Yu et al. 2015). The methods and techniques mentioned above can be used to study various effects on oxidation stability of rapeseed oils.
To investigate effect of LA content on oxidation stability of oils, we nurtured and selected the rapeseed oils with different contents of LA. The Schaal oven test, Rancimat test, mesh-cell test and shelf-life test were conducted, and effects of fatty acid composition and endogenous antioxidants (tocopherols and phytosterols) on rapeseed oils were assessed.
Materials and methods
Materials and reagents
Four rapeseed with different LA contents were obtained from the Hybrid Rape Research Center of Shaanxi Province, China (108° 4.5′ E, 34° 16.92′ N). In the same geographical conditions, four kinds of rapeseed (three different seed sources and one commercial available variety) were harvested by unified cultivation and management in isolated chambers and used to prevent cross-contamination of pollen from other rape varieties.
Glacial acetic acid, isooctane, diethyl ether, ethanol, isopropanol, phenothalin, potassium iodide, sodium thiosulfate, potassium hydroxide were purchased from Tianjin Chemical Company, Ltd. 37 types of fatty acid methyl ester mixture standard products and triglyceride standard solution were purchased from Shanghai Anpel, China. Tocopherol standards were purchased Sigma-Aldrich, USA.
Rapeseed oil extraction
Four rapeseed crude oils were extracted using a small household oil extractor (Westinghouse Inc., USA) in cold press mode after cleaning and removing rapeseed impurities. Then, the following rapeseed oils were obtained by centrifugation: rapeseed oil with LA content of about 6% (RO1), rapeseed oil with LA content of about 8% (RO2), rapeseed oil with LA content of about 10% (RO3) and commercial rapeseed oil (CRO). The oils were stored in refrigerator at 4 °C.
Antioxidants determination
Tocopherols and phytosterols were considered as endogenous antioxidants and their determination was as follows:
The tocopherol content of rapeseed oils was determined by normal-phase high- performance liquid chromatography (HPLC, silica gel column) in accordance with the American Oil Chemists' Society (AOCS) Official Method (AOCS Ce 8-89). A HPLC (Waters Corporation, USA) equipped with a scanning fluorescence detector (mode 2475) on a Spherisorb silica column was utilized. The chromatographic conditions were as follows: the particle size of silica column was 25 cm × 4.6 mm × 5 μm with a temperature of 30 °C and the detection wavelength was set as 295 nm; the mobile phase consisted of n-hexane:isopropanol (v:v = 98.5:1.5) (ultrasonic bubble removal, flow rate: 0.6 mL/min, operating time: 20 min).
In accordance with the ISO method (ISO 12228), phytosterol content was determined by gas chromatography (GC) using an instrument equipped with a flame ionization detector (6890 N, Agilent Technologies, Palo Alto, CA, USA). The GC parameters were as follows: column, stationary phase SE-54 (50 m long × 0.25 mm internal diameter); hydrogen as the carrier gas; flow rate, 36 cm/s; split ratio, 1: 20; and detector temperature and sample inlet temperature, 320 °C. The temperature was programmed to increase from 240 °C to 255 °C at a speed of 4 °C/ min, with an injection volume of 1 µL.
Fatty acid composition determination
Fatty acid composition (FAC) was determined by GC through an Agilent Technologies 6890 N GC system (Agilent Technologies, Palo Alto, CA, USA) equipped with an autosampler injector, a flame ionization detector and a HP-INNOWAX capillary column (0.25 mm × 0.25 μm × 30 m). The injector (split ratio 75:1) and detector were held at 250 °C and sample inlet was held at 250 °C. The column temperature was programmed at 180 °C for 2 min and increased from 10 to 240 °C for 10 min. High-purity nitrogen, at a flow rate of 1.1 mL/min, was used as the carrier gas, and nitrogen, hydrogen and air were used as combustion gas. The content of fatty acids was calculated using an internal standard method through the retention time of the standard fatty acid methyl ester and sample peak. Oil samples from Schaal oven test and the shelf-life test were determined at regular intervals.
Oxidation stability analysis
Rancimat test: The accelerated oxidation test of rapeseed oil was carried out through ISO 6886:1997 at 110 ± 0.1 °C. The rapeseed oils were measured in a test tube matched with the oxidation stabilizer. Additional water (50 mL) was poured into the measuring tank, The pump was opened, and the flow rate was accurately set to 10 L/h at 110 °C. The measurement was finished until the signal reached the recording scale; the results were read directly from the oil oxidation stability tester.
Schaal oven test: 200 ± 0.1 g samples were weighed into beakers of 250 mL capacity and placed in an oven maintained at 63 ± 1 °C. The peroxide value (PV) and acid value (AV) were measured every 12 h until the PV reached 100 meq/kg.
Mesh-cell test was carried out on the basis of previous literature (Yu et al. 2015). The absorbance was used to calculate the real PV and AV, and the relationship between the absorbance and PV or AV followed the established model. In brief, at ambient temperature, samples (200 μL) were deposited onto the surface of a sieve mesh (100 mesh) using a micropipette and then the oil was spread uniformly with the tip of the micropipette. An oil film was formed on the stainless steel mesh and was oxidized at room temperature. The spectra of the oil films were collected by using a Bruker VERTEX 70 series FTIR spectrometer (Bruker Optics, Germany) and corrected using the mesh cell background spectrum. By using the software of OMINIC 7.3 and TQ analyst 7.2, the optical path of oil samples was corrected by 4334/4549 cm−1 and unified to 0.15 mm. All spectra were collected by co-adding 16 scans at a resolution of 4 cm−1 and a gain of 1.0 over the spectral range of 6000 to 400 cm−1 at 25 °C every 12 h. PV and AV models were established: PV model: y = 0.0011 x + 0.1693; R2 = 0.9816 (where y is the absorbance at 3471/3518 cm−1; x is the PV, meq/kg); AV model: y = 0.2503x + 1.4905; R2 = 0.9623 (where y is the absorbance at 1712/1600 cm−1; x is the AV, mg/g).
Shelf-life test: Four oils (300 ± 0.1 g) were placed into plastic bottles and prepared in triplicate. At room temperature and normal lighting conditions, a certain amount of rapeseed oil was withdrawn at regular intervals for three to five times a day to simulate the use of cooking oil in household. The PV and AV of the oils were measured every six days until PV reached 20 meq/kg.
PV and AV of rapeseed oils were determined using AOCS Official Method Cd 8b-90 and AOCS Official Method Cd 3a-63, respectively.
Statistical analyses
All values were expressed as mean ± standard deviation from three independent measurements. By using SPSS statistics, the Duncan multiple range test, which compare differences among means, and the Pearson correlation coefficient analysis of test data were conducted. Spectral data processing and statistical analysis were conducted using OMNIC 7.3, TQ Analyst 7.2 (Thermo Electron Inc., Madison, WI), and OriginPro 8 (Originlab, Northhampton, MA).
Results and discussion
FACs of rapeseed oils
FACs of the four rapeseed oils are shown in Table 1. The major fatty acids of rapeseed oils were C16:0, C18:0, C18:1, C18:2, C18:3, which was consistent with previous studies (Eder and Brandsch 2002; Velasco and Becker 1998). No significant differences in the contents of C16:0, C18:0, C18:1, C18:2 among four rapeseed oils were observed, except for C18:3 (LA, p < 0.05). The LA content of RO3, accounting for 10.8% (10.13 ± 0.06 g/100 g) of the total fatty acids, was the highest among the four rapeseed oils, followed by RO2 (8.4%), CRO (7.50%), RO1 (5.9%). Given the above analysis, the main fatty acid contents of four rapeseed oils showed no significant difference except for the LA content.
Table 1.
FACs of four rapeseed oils with various LA contents (g/100 g)
| Fatty acids | RO1 | RO2 | RO3 | CRO |
|---|---|---|---|---|
| C16:0 | 5.59 ± 0.05a | 5.65 ± 0.34a | 5.48 ± 0.03a | 5.66 ± 0.11a |
| C18:0 | 3.51 ± 0.03a | 3.53 ± 0.09a | 3.31 ± 0.08a | 3.42 ± 0.10a |
| C18:1 | 58.17 ± 0.14a | 58.66 ± 0.75a | 57.13 ± 0.15a | 58.42 ± 0.89a |
| C18:2 | 18.11 ± 0.06a | 17.49 ± 0.32a | 17.63 ± 0.14a | 17.40 ± 0.09a |
| C18:3 | 5.36 ± 0.04d | 7.86 ± 0.04b | 10.13 ± 0.06a | 6.92 ± 0.15c |
| ∑SFAA | 9.11 ± 0.03a | 9.18 ± 0.62a | 8.80 ± 0.05a | 9.08 ± 0.03a |
| ∑PUFAB | 23.47 ± 0.14d | 25.35 ± 0.51b | 27.77 ± 0.28a | 24.33 ± 0.33 cd |
| ∑UFAC | 81.64 ± 0.01b | 84.01 ± 1.26ab | 84.90 ± 0.13a | 82.75 ± 1.59ab |
The superscript letters correspond to the significant analysis results, and different letters in the same line indicate a significant difference (p < 0.05) between samples
FAC, Fatty Acid Composition; LA, linolenic acid; RO1, rapeseed oil with 5.9% LA; RO2, rapeseed oil with 8.4% LA; RO3, rapeseed oil with 10.8% LA; CRO, commercial rapeseed oil; α-T, α-tocopherols; β-T, β-tocopherols; δ-T, δ-tocopherols; γ-T, γ-tocopherols
ASum of saturated fatty acids
BSum of polyunsaturated fatty acids
CSum of unsaturated fatty acids
Endogenous antioxidants analysis
Table 2 shows contents of tocopherols and phytosterols in rapeseed oils.
Table 2.
Contents of tocopherols and phytosterols in four rapeseed oils
| Samples | RO1 | RO2 | RO3 | CRO |
|---|---|---|---|---|
| Tocopherols (mg/100 g) | ||||
| α-T | 29.38 ± 0.57a | 30.13 ± 0.61a | 29.09 ± 0.44a | 30.30 ± 0.89a |
| β-T | 5.44 ± 0.11b | 6.05 ± 0.13a | 3.94 ± 0.14c | 4.19 ± 0.02c |
| γ-T | 64.00 ± 1.52a | 63.30 ± 1.15a | 65.46 ± 1.84a | 63.78 ± 1.17a |
| δ-T | 1.07 ± 0.17ab | 0.52 ± 0.45b | 0.13 ± 0.20c | 0.51 ± 0.03b |
| Total tocopherols | 99.50 ± 2.73a | 100.00 ± 2.08a | 98.77 ± 1.95a | 98.78 ± 2.00a |
| Phytosterols (mg/g) | ||||
| Brassicasterols | 1.03 ± 0.05a | 0.99 ± 0.04a | 1.01 ± 0.04a | 1.04 ± 0.01a |
| Campesterols | 1.82 ± 0.03a | 1.82 ± 0.10a | 1.80 ± 0.001a | 1.81 ± 0.10a |
| Stigmasterols | 0.24 ± 0.03a | 0.21 ± 0.02a | 0.21 ± 0.01a | 0.22 ± 0.01a |
| β-Sitosterols | 2.25 ± 0.02a | 2.22 ± 0.02a | 2.20 ± 0.03a | 2.17 ± 0.04a |
| Total phytosterols | 5.34 ± 0.08a | 5.24 ± 0.11a | 5.22 ± 0.002a | 5.25 ± 0.07a |
Superscripts with a different letter in the same line are significantly different (p < 0.05)
RO1, rapeseed oil with 5.9% linolenic acid; RO2, rapeseed oil with 8.4% linolenic acid; RO3, rapeseed oil with 10.8% linolenic acid; CRO, commercial rapeseed oil; α-T, α-tocopherols; β-T, β-tocopherols; δ-T, δ-tocopherols; γ-T, γ-tocopherols
Tocopherols including α-tocopherols (α-T), β-tocopherols (β-T), γ-tocopherols (γ-T), and δ-tocopherols (δ-T), are widely distributed in oil crop seeds. Tocopherols are important endogenous antioxidants in plants by trapping the hydroperoxides intermediates and preventing the oxidation chain reaction (Colombo 2010; Tuberoso et al. 2007). Table 2 shows no significant difference in the contents of total tocopherols, α-T and γ-T among four rapeseed oils (p > 0.05). Consistent with earlier results (Bonvehi et al. 2000; Fang et al. 2017), α-T and γ-T were the major tocopherols in rapeseed oils; γ-T accounted for more than 60% of total tocopherols and α-T over 24%. The antioxidant capacity of tocopherols mainly depends on major tocopherol isomers in oils (Tuberoso et al. 2007). Brigelius-Flohe et al. (1999) reported that α-T exhibits the strongest physiological activity among various vitamin E, and γ-T holds the strongest antioxidant capacity. Evans et al. (2002) believed that the order of antioxidant ability of several monomers is α-T > γ-T > δ-T at mild temperature, whereas the antioxidant activity of several monomers is opposite at high temperature (Wagner et al. 2001). Let et al. (2005) speculated that γ-T is the most important tocopherol isomer present in rapeseed oil regarding antioxidation. The authors concluded that antioxidant capacity of tocopherols in rapeseed oils is closely related to α-T and γ-T.
Phytosterols are important micronutrients in oils. They can effectively reduce the content of serum cholesterol and prolong the shelf-life of oils (Damirchi et al. 2005; Thanh et al. 2006). Table 2 shows that the main phytosterols in rapeseed oil are brassicasterol, campesterol, β-sitosterol and stigmasterol. No significant difference was observed in content of total phytosterols in four rapeseed oils (p > 0.05). Moreover, the content of phytosterols was not consistent with previous reports, because the phytosterol content in different rapeseed oils significantly differs and mainly depend on many factors, such as varieties, growing conditions, oil production methods (pressing, refining), product storage conditions, saponification temperature and extract amount (Azadmard-Damirchi et al. 2010; Phillips et al. 2002).
Oxidation stability analysis
The induction period of oil samples at 110 °C was determined. The result of Rancimat test is presented in Table 3. Significant differences (p < 0.05) were observed in the induced rancidity time of rapeseed oils. The induction period of RO1 was the longest (10.10 h), followed by RO2 (9.49 h), CRO (9.07 h) and RO3 (7.39 h). The different induction periods primarily indicated the degree of oxidation stability of fats and oils. Thus, the oxidation rates of four rapeseed oils varied as follows: RO3 > RO2 > CRO > RO1.
Table 3.
Induced period of rapeseed oils during Rancimat test
| Samples | RO1 | RO2 | RO3 | CRO |
|---|---|---|---|---|
| Induced period (h) | 10.10 ± 0.07a | 9.49 ± 0.03b | 7.39 ± 0.06d | 9.06 ± 0.38c |
Superscripts with a different letter in the same line are significantly different (p < 0.05)
RO1, rapeseed oil with 5.9% linolenic acid; RO2, rapeseed oil with 8.4% linolenic acid; RO3, rapeseed oil with 10.8% linolenic acid; CRO, commercial rapeseed oil
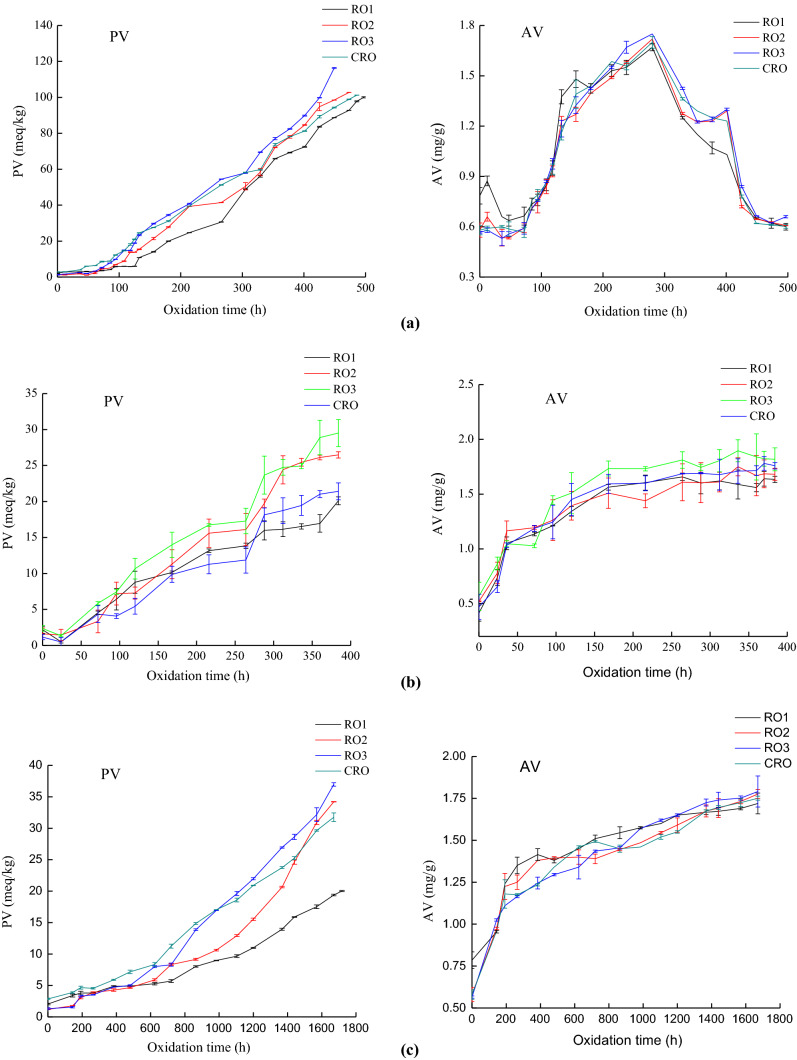
PV determines the concentration of hydroperoxides formed through oxidation and AV indicatives the free fatty acid content of the oil expressed as oleic acid (%). The changes of PV and AV of the four rapeseed oils under Schaal oven test, mesh-cell test and shelf-life test during oxidation are shown in Fig. 1. It shows that PV of four rapeseed oils increased significantly with time in three methods.
Fig. 1.
PV and AV versus time of rapeseed oils in a Schaal oven test, b mesh-cell test, and c shelf-life test. AV, acid value; PV, peroxide value; RO1, rapeseed oil with 5.9% linolenic acid; RO2, rapeseed oil with 8.4% linolenic acid; RO3, rapeseed oil with 10.8% linolenic acid; CRO, commercial rapeseed oil
In Schaal oven test, it took 497.4 h for the PV of RO1 to reach 100 meq/kg; CRO, 485.3 h; RO2, 461.2 h; and RO3, 425 h. The increased rate of PV reflects the degree of hydroperoxide generation in rapeseed oil, so the result indicates that the oxidation rate of four rapeseed oils followed the order: RO3 > RO2 > CRO > RO1.
In mesh-cell test, the PV of RO1 reached 20 meq/kg after sample oxidized for 384 h; RO4, 21 meq/kg; RO2, 26 meq/kg; and RO3, 29 meq/kg. RO1 was observed with a strong oxidation stability, while CRO, RO2 and RO3 had a poor oxidation stability.
To simulate the oxidation of rapeseed oil in the household, the shelf-life test was conducted and showed that the order of oxidation stability of the four rapeseed oils is RO3 > RO2 > CRO > RO1, consistent with the results of the Rancimat test, Schaal oven test, and mesh-cell test. In addition, the limiting PVs critical for acceptability in GB 1536–2004, AOCS Official Standards, and the European Union Standards are 20, 10, and 10 meq/kg, respectively. When the PV reached 10 meq/kg, RO1, RO2, RO3, and CRO had been oxidized for 50, 41, 33, and 30 days, respectively. When the PV reached 20 meq/kg, RO3 had been oxidized for 48 days, followed by CRO for 50 days, RO2 for 57 days, and RO1 for 71 days. Thus, according to the China National standard, AOCS Official Standards, and the European Union Standards, the four rapeseed oils can be stored for at least 30 days before deterioration.
In the three methods, no significant difference in AV was observed among the four rapeseed oils during oxidation, and the AVs of all samples were below the limits (AV ≤ 6.0 mg KOH/g) specified by the European Union Standards. In addition, the AVs of the four rapeseed oils decreased sharply after 330 h of oxidation in the Schaal oven test, which may be ascribed to the drastic oxidation conditions. The free fatty acid produced by oil decomposition will be polymerized with the small molecular acid produced by hydroperoxide during oxidation, and thus, free fatty acid will be reduced and lead to the decline of AV in oil.
Given the results of the four oxidation methods, the oxidation rates of the four rapeseed oils followed the order: RO3 > RO2 > CRO > RO1. The oxidation rate ratio of oleic acid, linoleic acid, and LA is 1:22:77, which means that fats and oils with high contents of unsaturated fatty acids are more prone to oxidize (Kapich et al. 2010; Pennisi Forell et al. 2010). Therefore, the experimental results and previous theories collectively suggest that oxidation is closely related to the content of unsaturated fatty acids, especially the content of LA. However, our results demonstrated that slightly improving LA content is acceptable.
FAC analysis
The FACs of oil samples were measured at intervals during Schaal oven test and shelf-life test, and the results are presented in supplementary Table S1 and Table S2.
Significant changes were observed in the main fatty acids of rapeseed oils (Table S1). With time, the LA and linoleic acid levels decreased obviously (p < 0.05). The content of LA in RO1 was significantly decreased by 8.40% from 5.36 g/100 g to 4.91 g/100 g, and those in RO2, RO3, and CRO decreased by 16.41%, 15.40%, and 12.28%, respectively. The contents of linoleic acid in RO1, RO2, RO3, and CRO were reduced by 4.69%, 3.89%, 5.84%, and 5.75%, respectively. Meanwhile, the contents of oleic acid, palmitic acid, and stearic acid in the four rapeseed oils increased (p < 0.05). A similar trend was found by Kim et al. (2013) During oxidation, partial cis-double bonds in PUFAs were oxidized to produce a range of compounds derived from hydroperoxides, and further oxidation allowed hydroperoxides to decompose into secondary products, such as esters, aldehydes, alcohols, ketones, lactones, and hydrocarbons, which resulted in decreases in LA and linoleic acid contents. Meanwhile, the production of hydrogen peroxides and polymers by lipid oxidation and thermal polymerization increased the saturated fatty acid (palmitic and stearic acids) and oleic acid concentrations. Moreover, the FAC changes in the rapeseed oils indicated that the rapeseed oils with high LA contents exhibited a low oxidation stability, which is in agreement with the experimental results of the Schaal oven test.
The FAC changes in the shelf-life test were similar to those from the Schaal oven test (Table S2). The contents of LA and linoleic acid in the four rapeseed oils increased after 70 days of oxidation. The LA content of RO1 was reduced by 7.09% from 5.36 g/100 g to 4.98 g/100 g, and the LA contents of RO2, RO3, and CRO were decreased by 11.07%, 12.04%, and 10.40%, respectively. The contents of linoleic acid of RO1, RO2, RO3, and CRO were reduced by 4.64%, 6.63%, 5.67%, and 4.25%, respectively. The contents of oleic acid, palmitic acid, and stearic acid in the four rapeseed oils were significantly increased (p < 0.05).
Correlation analysis
To analyze the effect of fatty acids and endogenous substances on the oxidation stability of rapeseed oils, the correlation of oleic acid, linoleic acid, LA, tocopherol, and phytosterol contents and oxidation stability of the rapeseed oils was determined (Tables 4, 5).
Table 4.
Correlation analysis of FAC with oxidation stability in rapeseed oils
| Index | C18:1 | C18:2 | C18:3 | SFA | PUFA | UFA |
|---|---|---|---|---|---|---|
| r | 0.540 | 0.288 | − 0.931** | 0.504 | − 0.932** | − 0.766* |
r represents correlation coefficient
*,**Significant differences at the 0.05, 0.01 levels, respectively
Table 5.
Correlation between tocopherols and phytosterols with oxidation stability in rapeseed oils
| Correlation coefficient | Tocopherols | Phytosterols | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-T | β-T | γ-T | δ-T | Total tocopherols | Brassicasterols | Campesterols | Stigmasterols | β-Sitosterols | Total phytosterols | |
| r | 0.442 | 0.840* | 0.541 | 0.775* | 0.321 | 0.101 | 0.200 | 0.384 | 0.576 | 0.437 |
r represents correlation coefficient
*Significant differences at the 0.05 level.r represents correlation coefficient
The UFAs in oils are easily affected by external factors (light, oxygen, etc.), which allow the oil to oxidize. Therefore, the effect of FAC on the oxidation stability of oil was determined by analyzing the correlation of SFA, PUFA, UFA, and the three main fatty acids (oleic acid, linoleic acid, and LA) with the induction period (Table 4). The results showed that the induction periods of the four rapeseed oils were negatively correlated with the LA content (r = − 0.931, p < 0.01,), indicating that the oxidation stability of oil was negatively correlated with LA content. Furthermore, the oxidation stability of oil was also negatively correlated with PUFA (r = − 0.932, p < 0.01), UFA (r = − 0.766, p < 0.05), suggesting that low PUFA and UFA content of oil had strong oxidation stability impact on storage. The results proved that FAC, especially LA content, has great effect on rapeseed oil oxidation.
The relationship among endogenous antioxidants in rapeseed oil and oxidation stability of oil was analyzed (Table 5). The results shows that no significant positive correlation was observed between tocopherol content and oil induction period (p > 0.05), whereas β-T and δ-T were significantly positively correlated with the induction period (r = 0.840, p < 0.05; r = 0.775, p < 0.05). The contents of β-T and δ-T are very low, and the substances that exert antioxidant effects in oil are mainly α-T and γ-T (Fang et al. 2017; Tuberoso et al. 2007). In addition, Fang et al. (2017) described that the oxidation stability of vegetable oil is not directly related to the content of tocopherols and they gradually lose efficacy as their concentrations increase. Huang et al. (1995) also found that when the content of α-T in corn oil exceeds 100 ppm, the content of hydrogen peroxide produced increase relatively. Lampi et al. (1999) found that α-T could promote the formation of lipid peroxide in corn oil and induce oxidation when the content of tocopherols exceeds 250 ppm. In conclusion, studies on the antioxidant activity of various tocopherols in oils are varied and cannot be uniformly evaluated. Seppanen et al. (2010) explained that experiments generally focus on different foods and used different methods for detecting of antioxidant activities. Hence, no direct relationship existed between oxidation stability and tocopherol contents of the four rapeseed oils, and this results are only valid for the experimental conditions used in the present study that analyzed groups of rapeseed oils with a similar variety.
The correlation between phytosterols and oxidation stability in rapeseed oils is shown in Table 5. The phytosterol content in rapeseed oils was negatively correlated with the induction period, but the relationship was not significant (p > 0.05). Wang et al. (2002) investigated the antioxidant activity of phytosterols, ferulic acid ester of sterols, corn fiber oil, and rice bran oil and reported that phytosterols does not improve the oxidation stability of oils at lower concentrations. This finding is consistent with result of our research.
Conclusion
On the basis of the Rancimat test, Schaal oven test, mesh-cell test, and shelf-life test, the order of oxidation stability of the four rapeseed oils is RO1 > CRO > RO2 > RO3, which is related to the content of LA. Compared with linoleic acid, the unsaturated bond of LA is closer to the end methyl group and is more easily oxidized upon air exposure. The endogenous antioxidants (tocopherols and phytosterols) in rapeseed oils have no effect on the oxidation of rapeseed oils.
In evaluating the oxidation stability of oils, their antioxidant capacity was compared; however, their shelf-lives were not measured. In household oil, the quality of edible oils is influenced by frequency of use, light, temperature, effects of packaging materials, and other factors. The shelf-life test can simulate the actual action of cooking oil in domestic situations. The results revealed that the four rapeseed oils can be stored for at least 30 days with a PV less than 10 meq/kg at room temperature. Therefore, increasing the LA content in rapeseed oils is an effective means to solve the problem of insufficient LA intake globally. Moreover, the susceptibility of LA to oxidation can be alleviated incrementally by the continuous improvement of edible oil packaging and antioxidant technology. In summary, from the perspective of nutrition and health, it is generally advisable to increase the content of LA in rapeseed oils during the breeding process.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was financially supported by the Key R&D Program Projects of Shaanxi Province, China (2019NY-120).
Conflict of interest The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Azadmard-Damirchi S, Habibi-Nodeh F, Hesari J, Nemati M, Achachlouei BF. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010;121:1211–1215. doi: 10.1016/j.foodchem.2010.02.006. [DOI] [Google Scholar]
- Bassett CMC, McCullough RS, Edel AL, Patenaude A, LaVallee RK, Pierce GN. The α-linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans fat. Am J Physiol Heart Circ Physiol. 2011;301:H2220–H2226. doi: 10.1152/ajpheart.00958.2010. [DOI] [PubMed] [Google Scholar]
- Bhatnagar AS, Prasanth Kumar PK, Hemavathy J, Gopala Krishna AG. Fatty acid composition, oxidative stability, and radical scavenging activity of vegetable oil blends with coconut oil. J Am Oil Chem Soc. 2009;86:991–999. doi: 10.1007/s11746-009-1435-y. [DOI] [Google Scholar]
- Braunrath R, Isnardy B, Solar S, Elmadfa I. Influence of α-, γ-, and δ-tocopherol on the radiation induced formation of peroxides in rapeseed oil triacylglycerols. Food Chem. 2009;117:349–351. doi: 10.1016/j.foodchem.2009.03.104. [DOI] [Google Scholar]
- Brigelius-FlohÉ R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. doi: 10.1096/fasebj.13.10.1145. [DOI] [PubMed] [Google Scholar]
- Ciemniewska-Żytkiewicz H, Ratusz K, Bryś J, Reder M, Koczoń P. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J Therm Anal Calorim. 2014;118:875–881. doi: 10.1007/s10973-014-3861-9. [DOI] [Google Scholar]
- Colombo ML. An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules. 2010;15:2103–2113. doi: 10.3390/molecules15042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damirchi SA, Savage GP, Dutta PC. Sterol fractions in hazelnut and virgin olive oils and 4,4″-dimethylsterols as possible markers for detection of adulteration of virgin olive oil. J Am Oil Chem Soc. 2005;82:717–725. doi: 10.1007/s11746-005-1133-y. [DOI] [Google Scholar]
- Dimic E, Premovic T, Takaci A, Vujasinovic V, Radocaj O, Dimic S. Effect of seed quality on oxidative stability of cold-pressed sunflower oil. Hem Ind. 2015;69:175–184. doi: 10.2298/HEMIND140216032D. [DOI] [Google Scholar]
- Eder K, Brandsch C. The effect of fatty acid composition of rapeseed oil on plasma lipids and oxidative stability of low-density lipoproteins in cholesterol-fed hamsters. Eur J Lipid Sci Technol. 2002;104:3–13. doi: 10.1002/1438-9312(200201)104:1<3::AID-EJLT3>3.0.CO;2-A. [DOI] [Google Scholar]
- Evans JC, Kodali DR, Addis PB. Optimal tocopherol concentrations to inhibit soybean oil oxidation. J Am Oil Chem Soc. 2002;79:47–51. doi: 10.1007/s11746-002-0433-6. [DOI] [Google Scholar]
- Fang B, Zhang M, Shen YM. Importance of the higher retention of tocopherols and sterols for the oxidative stability of soybean and rapeseed oils. J Food Sci Technol. 2017;54:1938–1944. doi: 10.1007/s13197-017-2628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-W, Frankel EN, German JB. Effects of individual tocopherols and tocopherol mixtures on the oxidative stability of corn oil triglycerides. J Agric Food Chem. 1995;43:2345–2350. doi: 10.1021/jf00057a006. [DOI] [Google Scholar]
- Isnardy B, Wagner KH, Elmadfa I. Effects of alpha-, gamma-, and delta-tocopherols on the autoxidation of purified rapeseed oil triacylglycerols in a system containing low oxygen. J Agric Food Chem. 2003;51:7775–7780. doi: 10.1021/jf0348525. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur J Lipid Sci Technol. 2006;108:1051–1061. doi: 10.1002/ejlt.200600090. [DOI] [Google Scholar]
- Kapich AN, Korneichik TV, Hatakka A, Hammel KE. Oxidizability of unsaturated fatty acids and of a non-phenolic lignin structure in the manganese peroxidase-dependent lipid peroxidation system. Enzyme Microb Technol. 2010;46:136–140. doi: 10.1016/j.enzmictec.2009.09.014. [DOI] [Google Scholar]
- Kim TS, Yeo J, Kim JY, Kim MJ, Lee J. Determination of the degree of oxidation in highly-oxidised lipids using profile changes of fatty acids. Food Chem. 2013;138:1792–1799. doi: 10.1016/j.foodchem.2012.11.119. [DOI] [PubMed] [Google Scholar]
- Kim KB, Nam YA, Kim HS, Hayes AW, Lee BM. α-Linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol. 2014;70:163–178. doi: 10.1016/j.fct.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Kowalska D, Kostecka M, Tarnowska K, Kowalski B. Oxidative stabilities of enzymatically interesterified goose fat and rapeseed oil blend by rancimat and PDSC. J Therm Anal Calorim. 2013;115:2063–2070. doi: 10.1007/s10973-013-3125-0. [DOI] [Google Scholar]
- Lampi A-M, Kataja L, Kamal-Eldin A, Vieno P. Antioxidant activities of α- and γ-tocopherols in the oxidation of rapeseed oil triacylglycerols. J Am Oil Chem Soc. 1999;76:749–755. doi: 10.1007/s11746-999-0171-7. [DOI] [Google Scholar]
- Let MB, Jacobsen C, Pham KA, Meyer AS. Protection against oxidation of fish-oil-enriched milk emulsions through addition of rapeseed oil or antioxidants. J Agric Food Chem. 2005;53:5429–5437. doi: 10.1021/jf047960f. [DOI] [PubMed] [Google Scholar]
- Michotte D, Rogez H, Chirinos R, Mignolet E, Campos D, Larondelle Y. Linseed oil stabilisation with pure natural phenolic compounds. Food Chem. 2011;129:1228–1231. doi: 10.1016/j.foodchem.2011.05.108. [DOI] [PubMed] [Google Scholar]
- Mortuza MG, Dutta PC, Das ML. Erucic acid content in some rapeseed/mustard cultivars developed in Bangladesh. J Sci Food Agric. 2006;86:135–139. doi: 10.1002/jsfa.2301. [DOI] [Google Scholar]
- Pennisi Forell SC, Ranalli N, Zaritzky NE, Andres SC, Califano AN. Effect of type of emulsifiers and antioxidants on oxidative stability, colour and fatty acid profile of low-fat beef burgers enriched with unsaturated fatty acids and phytosterols. Meat Sci. 2010;86:364–370. doi: 10.1016/j.meatsci.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Phillips KM, Ruggio DM, Toivo JI, Swank MA, Simpkins AH. Free and esterified sterol composition of edible oils and fats. J Food Compos Anal. 2002;15:123–142. doi: 10.1006/jfca.2001.1044. [DOI] [Google Scholar]
- Seppanen CM, Song Q, Saari Csallany A. The antioxidant functions of tocopherol and tocotrienol homologues in oils fats, and food systems. J Am Oil Chem Soc. 2010;87:469–481. doi: 10.1007/s11746-009-1526-9. [DOI] [Google Scholar]
- Symoniuk E, Ratusz K, Ostrowska-Ligęza E, Krygier K. Impact of selected chemical characteristics of cold-pressed oils on their oxidative stability determined using the rancimat and pressure differential scanning calorimetry method. Food Anal Methods. 2017;11:1095–1104. doi: 10.1007/s12161-017-1081-1. [DOI] [Google Scholar]
- Thanh TT, Vergnes M-F, Kaloustian J, El-Moselhy TF, Amiot-Carlin M-J, Portugal H. Effect of storage and heating on phytosterol concentrations in vegetable oils determined by GC/MS. J Sci Food Agric. 2006;86:220–225. doi: 10.1002/jsfa.2322. [DOI] [Google Scholar]
- Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007;103:1494–1501. doi: 10.1016/j.foodchem.2006.08.014. [DOI] [Google Scholar]
- Velasco L, Becker HC. Estimating the fatty acid composition of the oil in intact-seed rapeseed (Brassica napus L.) by near-infrared reflectance spectroscopy. Euphytica. 1998;101:221–230. doi: 10.1023/A:1018358707847. [DOI] [Google Scholar]
- Wagner KH, Wotruba F, Elmadfa I. Antioxidative potential of tocotrienols and tocopherols in coconut fat at different oxidation temperatures. Eur J Lipid Sci Technol. 2001;103:746–751. doi: 10.1002/1438-9312(200111)103:11<746::AID-EJLT746>3.0.CO;2-P. [DOI] [Google Scholar]
- Wang T, Hicks KB, Moreau R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J Am Oil Chem Soc. 2002;79:1201–1206. doi: 10.1007/s11746-002-0628-x. [DOI] [Google Scholar]
- Xu L, Fei T, Li Q, Yu X, Liu L. Qualitative analysis of edible oil oxidation by FTIR spectroscopy using a mesh “cell”. Anal Methods. 2015;7:4328–4333. doi: 10.1039/C5AY00438A. [DOI] [Google Scholar]
- Xu L, Yu X, Liu L, Zhang R. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016;202:229–235. doi: 10.1016/j.foodchem.2016.01.144. [DOI] [PubMed] [Google Scholar]
- Yang M, Zheng C, Zhou Q, Huang F, Liu C, Wang H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J Food Compos Anal. 2013;29:1–9. doi: 10.1016/j.jfca.2012.08.009. [DOI] [Google Scholar]
- Yu X, Li Q, Sun D, Dong X, Wang T. Determination of the peroxide value of edible oils by FTIR spectroscopy using polyethylene films. Anal Methods. 2015;7:1727–1731. doi: 10.1039/C4AY02718C. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.