Abstract
Abstract
In the study the effect of sodium chloride and quince seed gum solutions as immersion pretreatment to prevent enzymatic browning reaction in pear slices before freeze drying was evaluated. Four levels of gum concentrations (0%, 0.25%, 0.5% and 1%) and three concentrations of salt (0%, 0.25% and 0.5%) were used. Subsequently, freeze drying method was used to dry the pear slices and the qualitative, structural and sensory characteristics of dried pear slices were investigated. The moisture content of the dried pear slices decreased significantly with increasing sodium chloride concentration, while increasing the concentration of the gum significantly increased the moisture content (P < 0.05). Use of both immersion pretreatments were effective in maintaining the antioxidant activity and reducing browning index of the slides and with increasing concentration of the immersion solutions, the antioxidant activity loss decreased in the slices (P < 0.05). Electron microscopy study revealed that the microstructural changes of the drying process on the slices are very slight, although gum pre-treatment at the high levels made a layer on the surface of the pear slices which slowed down the transfer of water vapor molecules. By considering the results of qualitative, structural and sensory evaluations, using of immersion pretreatment with 0.25% gum and 0.25% salt solution to prevent enzymatic browning along with preserving the quality properties of pear slices prior to the drying process is recommended.
Graphic abstract
Keywords: Dried pear slices, Antioxidant activity, Browning index, Microstructure
Introduction
Pear (Pyrus communis L.) is a popular fruit in the world because of its desirable taste and commercial value. It is also recognized as a good source of antioxidants and valuable nutrients in the human diet (Komes et al. 2013; Chen et al. 2007). This fruit is often eaten fresh, but is sometimes consumed in processed forms such as purées, juice, jams and dried (Kolniak-Ostek 2016; Park et al. 2003). Drying is the oldest method used in food preservation which has been widely applied to increase the shelf life of fruits. Among of drying techniques, freeze drying is one of the most effective techniques for reducing moisture content and eliminating microbial activity and color deterioration as well as enzymatic browning (Bi et al. 2015).
Freeze drying (FD) is a dehydration method with the sublimation of ice from frozen material under vacuum for preserving the heat-sensitive foods such as fruits and vegetables. It has been used as a process to minimize the adverse quality changes associated with dried fruits (Pan et al. 2008; Gebczynski et al. 2017; Wang et al. 2010), and because of low-temperature applied allows the highest retention of bioactive compounds comparable with the unprocessed product it is regarded as the best method (Ratti 2001). Nowadays, freeze drying is combined with other methods and pretreatments such as infrared (Pan et al. 2008; Antal 2017) or microwave radiation (Duan et al. 2007), Osmotic dehydration (Aktas et al. 2007) and surface treatments (Moreira et al. 2015) to enhance shelf life quality of fresh cut fruits.
In the fresh cut fruit industry, enzymatic browning is very important and not only deteriorates color and reduces shelf life but also negatively affects the quality of the cut fruits (Shewfelt 1986; Huxsoll and Bolin 1989). As it has been stated by Lee and Eun (1999), enzymatic browning is due to the oxidation reaction of phenolic compounds through polyphenol oxidase (PPO) and results in the formation of o-quinone, which is polymerized to form dark pigment melanin. Therefore, browning can be prevented by inhibiting the activity of the enzyme by certain processes such as thermal processing, lowering the pH of the fruit surface, removal of oxygen or, Cu2+ from PPO, or by adding some inhibitory agents (Sun et al. 2002; Annese et al. 1997; Gorny 1997; Sapers et al. 1990). The most commonly used method to inhibit browning in fresh cut fruit industry is the application of natural anti-browning compounds. These compounds are antioxidant agent, chelating agent, firmness agent and acidifying agent (Bieganska-Marecik and Czapski 2007).
Various compounds are used as anti-browning agent, like citric acid, ascorbic acid, calcium chloride, sodium carbonate, ethanol, or cysteine, 4-Hexylresorcinol, cinnamic acid, P-coumaric acid, ferulic acid, sinapic acid, alginate, pectin, xanthan and gellan gum (El-Shimi 1993; Yildiz 2018; Arias et al. 2007; Nicoli et al. 1993; Jiang et al. 1999; Moreira et al. 2015; Lamikanra and Watson 2001; Sharma and Rao 2015). Gorny et al. (2002) reported that reported that the use of immersion solutions of 2% ascorbic acid, 0.5% cysteine and 1% calcium lactate by inhibiting the surface browning and preventing tissue deterioration extended the shelf life of Bartlett fresh-cut pear slices (P < 0.05). It was found that enrichment of xanthan gum based foods with cinnamic acid as an antioxidant in significantly (P < 0.05) reduces oxidative browning of fresh-cut pears (Sharma and Rao 2015).
Therefore, application of natural anti-browning agents as immersion treatments are considered to maintain freshness of fresh-cut fruits. It is reasonable to consider using immersion treatment before freeze drying to improve the color of dried pear slices. To our knowledge, no work is available the effect of effect of sodium chloride and quince seed gum pretreatments on the quality characteristics of freeze dried pear slices. Therefore, this work was undertaken to investigate the effects of sodium chloride and quince seed gum pretreatments on the quality and prevention of enzymatic browning of freeze-dried pear slices.
Materials and methods
Materials
Pears (Pyrus communis L. cv. Shah-miveh) used in this study were harvested at Kamal-Abad collection orchard (Albourz, Iran) and stored at 4 °C until used. All chemicals including sodium chloride, DPPH, ethanol and methanol were prepared from Sigma-Aldrich (St. Louis, USA).
Extraction of gum from quince seed
The quince seed gum was prepared based on the method suggested by Jouki et al. (2014c). Sequential processes were employed to extract gum from quince seed. Briefly, aqueous quince seed gum was extracted from quince seeds using distilled water (time 15 min, temperature 45 °C and water to seed ratio 30:1). The swelled seeds were stirred with a rod paddle blender to scrape the gum layer off the seed surface. The collected gum was filtered with cheese cloth and dried in an oven at 45 °C, overnight. The dried extracted gum was packed and put at dry and cool conditions.
Immersion in pre-treatments
Prior to drying, the pears were first peeled and cut into 5 mm thick slices using a hand-operated slicer. The samples were divided into twelve groups and each group contained 20 slices. The fresh-cut pear slices were put into immersion pre-treatments (Table 1) for 1 min before drying process to avoid surface enzymatic browning and then they were placed in plastic petri dishes.
Table 1.
Immersion pre-treatments formulations before freeze drying process
| Pre-treatments | Quince seed gum (%) | Sodium chloride (%) | Pre-treatments | Quince seed gum (%) | Sodium chloride (%) |
|---|---|---|---|---|---|
| P1 | 0.00 | 0.00 | P7 | 0.50 | 0.00 |
| P2 | 0.00 | 0.25 | P8 | 0.50 | 0.25 |
| P3 | 0.00 | 0.50 | P9 | 0.50 | 0.50 |
| P4 | 0.25 | 0.00 | P10 | 1.00 | 0.00 |
| P5 | 0.25 | 0.25 | P11 | 1.00 | 0.25 |
| P6 | 0.25 | 0.50 | P12 | 1.00 | 0.50 |
Freeze drying process
The pear slices were dried using a laboratory scale vacuum freeze-dryer (Armfield FT-33, Ltd., Ringwood, UK) according to the methods of Antal (2017). Before the FD process, the pear slices were spread uniformly in a single layer and were frozen at − 18 °C for 6 h in fast freezing chamber to prepare for drying stage. Then, the samples were freeze-dried for a period of 24 h at 22–40 Pa with a chamber temperature of 25 °C and a condenser temperature of − 52 °C. The qualitative, structural and sensory characteristics of dried pear slices were investigated.
Moisture content and dehydration
To determine the moisture content of the fresh and freeze-dried pears, pear samples of 10 g were placed in a vacuum oven (Model No. V01218A, Asheville, NC) at 70 °C for 48 h, under vacuum (AOAC 2000). The weight of pear slices before and after freeze-drying was used to measure the moisture loss (dehydration). The amount of moisture loss was calculated using Eq. (1):
| 1 |
where Mb is the moisture content of pear slices before freeze drying, Ma is the moisture content after freeze drying.
Color analysis and browning index (BI)
The color values of the dried pear slices were determined by a Minolta Chromameter (Model CR-400, Japan) from the surface of the slices (Yildiz 2018). Briefly, the optical receiver portion of colorimeter was placed in front of banana surface in non-central areas containing black dots. The color parameters were reported by CIE L (lightness/darkness), a (redness/greenness), and b (yellowness/blueness) coordinates. White standard plate (L = 97.94, a = − 0.13, b = 0.94) was used for the calibration of the device. Six replications were set up for each treatment. According Perez-Gago et al. (2006) the browning index can be calculated by the following Eqs. (2, 3):
| 2 |
| 3 |
DPPH radical-scavenging activity of dried pear slices
The DPPH method is used for the determination of antioxidant activity of freeze-dried pear slices (Brand-Williams et al. 1995). Briefly, 5 g of pear slice was extracted using a homogenizer (IKA T25-Digital Ultra Turrax, Germany) in 45 mL methanol: water (80:20) solvent mixture. The mixture was vortexed for 30 s, sonicated for 4 min and centrifuged (Ultra Centrifuge Sigma 8 K, Germany) for 15 min (10.000 × g) and then 0.1 mL of the extract was added to 3.9 mL of methanolic DPPH solution (0.1 mM). After 30 min incubation at 20 °C in the dark, the absorbance was measured at 517 nm by using an UV–Vis spectrophotometer (Unico 2100 A, USA). The scavenging activity of DPPH radicals was calculated according to the following Eq. (4):
| 4 |
The amount of antioxidant-activity loss (AAL) calculated using Eq. (5):
| 5 |
where AAb is the antioxidant activity of pear slices before freeze drying and the antioxidant activity after freeze drying is AAa.
Scanning electron microscopy analysis
AIS-2300 scanning electron microscope (SEM, Korea) was used to study the morphology of freeze-dried pear slices. The samples were cut by liquid nitrogen (Jouki et al. 2014b). Afterward, the samples were mounted on aluminum stubs (holder), coated with gold in BAL-TEC SCD 005 sputter coater under vacuum conditions for 30 s, and then observed on a scanning electron microscope for outer surface using an accelerating beam at a voltage of 6.0 kV.
Sensory evaluation
The sensory analysis of freeze-dried pear slices was assessed accordingly as per the method described by Moreira et al. (2015) with slight modifications. Briefly, dried slices were served in individual plates, codified with a three-digit number, to 30 untrained panelists which were composed of students and researchers (being 14 men and 16 women). The panelists were trained to evaluate taste, color, odor and overall acceptability of dried pear slices. The panelists have judged the quality and ranked each pear slice sample by following hedonic scale of 5 points (1 = dislike very much, 2 = dislike a little, 3 = neither like nor dislike, 4 = like a little, and 5 = like very much).
Statistical analysis
The results were evaluated by analysis of variance (ANOVA) and comparison of mean values by Duncan’s multiple range test, applied at 5% level of significance (P < 0.05), using SPSS software version 21.0.
Results and discussion
Moisture content and dehydration rate of dried slices
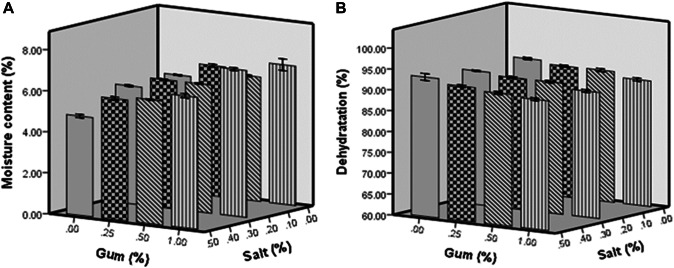
The moisture content (% wet basis) of fresh pear slices was 79.15%. Although, the salt concentration was not a critical parameter in the levels of 0 to 0.25%. It can be seen that after freeze drying, the sodium chloride (salt) pre-treated samples showed the lowest amount of moisture (4.84% w.b), followed by the control samples (5.69% w.b) and the gum dip pretreated slices (6.80% w.b). The effect of salt concentration on the reduction of moisture content in pear slices illustrated that the increase in osmotic salt concentration increases diffusional changes and the osmotic pressure exerted on the pear fruit cell structure which consequently results in greater moisture reduction in more concentrated salt solutions. The low moisture content in salt dried slices could be due to the leaching effect of the sodium chloride, which affected the pear slice tissue, making it easier for water to spread though during drying. In similar study Abano et al. (2013) illustrated that salt pre-treatment effected on the mango tissues and made it easier to diffuse water when the fruit was dried. Fuente-Blanco et al. (2006) stated that the pretreatments affect fruit tissues to remove water more easily during the drying process. Evaporation of salt solution can also reduce the moisture content of the slides in the drying process (Abano et al. 2013). They also stated that salts like sodium chloride evaporate rapidly on drying process. This could be related to the higher moisture loss (dehydration) in salt-pre-treated pear slice samples.
Comparing Fig. 1a, b, it is observed that in salt concentrations of 0, 0.25 and 0.50%, the reduction of moisture content (dehydration) was much higher in the pear slices congaing 0 and 0.25% gum in comparison to those containing 0.5 and 1% gum. The high moisture content (lower dehydration) retention in the gum dip pre-treated pear slices could be due to the hygroscopic nature of quince seed gum. In fact, the gum acts as a protective layer against moisture outflow and reduces mass transfer. This trend is consistent with the findings of Abano et al. (2013), that the casehardening effect for slowing down of moisture transport was enhanced by sugar pretreatment. They showed that the hygroscopic solution forms a sticky gel layer around fruit slices that are impermeable to moisture removal. This layer causes the viscose tissue to retain water and prevent moisture from being released, which is confirmed by microstructural analysis and sensory evaluation as shown in the following sections. During the freeze drying process, the moisture comes to the surface by moisture diffusion mechanism and evaporates. So, the rate of moisture transfer can be changed by any change in the surface of the fresh-cut fruit.
Fig. 1.
The amounts of moisture (a) and dehydration (b) in the control and pre-treated freeze-dried pear slices
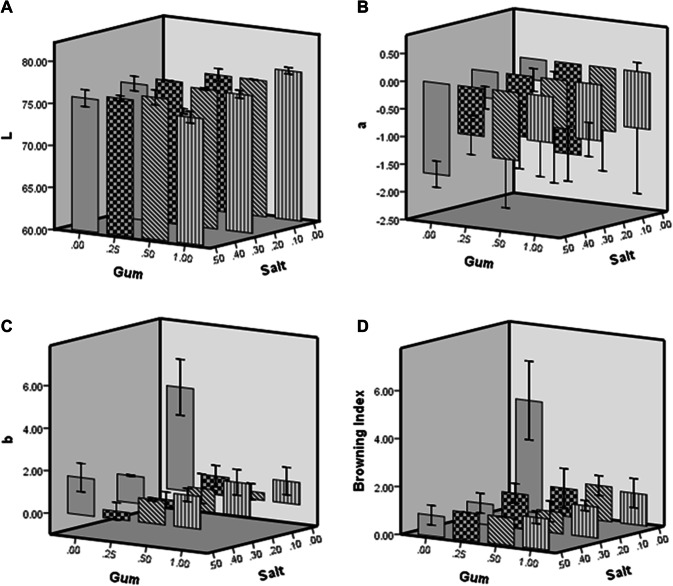
Color analysis and browning index (BI)
Figure 2 shows the variation of the color parameters and browning index of freeze-dried pear slices. Color parameters of control dried pear slices (untreated) was: L* = 71.40, a* = − 0.38, b* = 4.82, respectively. Compared to the control samples, the L* values increased and the a* and b* values decreased significantly for pre-treated dried pear slices. Similar result was observed by Antal (2017). The pre-treated freeze-dried slices were lighter (+ L*) and less yellow (− b*) than untreated dried slices. The browning index (BI) was used to describe the variation of colors that indicate the enzymatic intensity browning in the dried pear slices. The BI of freeze-dried pear was in a range between 0.87 and 4.65. As it has been stated in several researches, the browning reaction could be slowed down or inhibited by inactivating the enzyme by adding natural preservative like calcium chloride, sodium carbonate, sodium chloride, alginate, pectin, xanthan and gellan gum (Yildiz 2018; Nicoli et al. 1993; Sharma and Rao 2015).
Fig. 2.
Variation of the color parameters L (a), a (b), b (c) and browning index (d) of freeze-dried pear slices
Yildiz (2018) showed that sodium chloride could reduce the amount of enzymatic activity in banana slices by half (from 695 to 288 U/ml). Sapers and Miller (1998) also found that sodium erythorbate, in combination with 4-HR, had a highly effect on browning inhibition of pear slices. These results were also observed by Lu et al. (2006) and Lu et al. (2007), who reported that sodium chlorite effects on fresh-cut apples browning reaction. Sharma and Rao (2015) also found that when cinnamic acid combined with xanthan gum showed greater inhibition of browning and higher L* values in fresh cut pears. As it has been reported by Li et al. (2015), in terms of delaying the browning of apple slices by salts, the advantages were as follows: chloride = phosphate > sulfate > nitrate and there was no difference between potassium, sodium, and calcium ions.
The amount of browning in the pear slices also decreased with increasing concentration of gum or salt. These results were in agreement with the results of Lim and Wong (2018), who reported that as the concentration of anti-browning agents increased, the percentage of inhibition increased. They reported that sodium chloride shows the high effect in inhibiting enzymatic browning in ginger. Antibrowning effects of quince seed gum can be due to their antioxidant activity, which allows them to interact directly with the enzyme or react with oxidized bed molecules (Arias et al. 2007). The antioxidant activity of seed gum has been stated previously. According to the report of Jouki et al. (2014a), the antioxidant activity of quince seed gum at 1% concentration is equal to 29.88%.
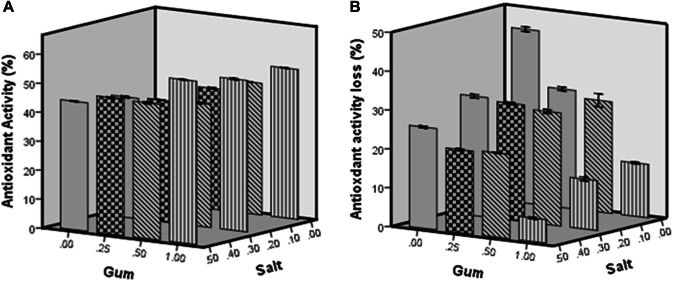
Antioxidant activity of dried pear slices
Figure 3 shows the results of antioxidant activity and reduction of antioxidant activities based on DPPH free radical scavenging test. The antioxidant activity of fresh-cut pear slices before dying was 59.6%. A decrease in radical scavenging activity in freeze-dried pear slices was observed as compared to its value for fresh slices. The reduction of antioxidant activity in dried pear slices was 6.05, 13.75, 26.06 and 43.73% for samples pre-treated by maximum levels of gum and salt, pre-treated by 1% gum, pre-treated with 0.5% salt and untreated (control), respectively (Fig. 3b). As it has been stated, the antioxidant activity correlated well with phenolic content in fruits (Salta et al. 2010; Oms-Oliu et al. 2008; Sharma and Rao 2015). Therefore, the decrease in the antioxidant activity can be due to a decrease in the total phenolic content.
Fig. 3.
Antioxidant activity (a) and Antioxidant activity loss (b) of treated and untreated freeze-dried pear slices
The reduction of phenolic compounds and antioxidant activity in fruits during the processing and drying stages has been reported in many studies (Sharma and Rao 2015; Wojdylo et al. 2014; Coklar et al. 2018). The lower reduction of antioxidant activity in pear slices due to the use of pre-treatment solutions prior to the freeze drying could be related to the protective effect of gum and salt on the target food. As reported by Lim and Wong (2018), Sodium chloride, as a sodium salt, acts as agent of firmness to inhibit enzymatic browning effects. They reported that sodium chloride shows the high effect in inhibiting ginger polyphenol oxidase (PPO) as it exhibited 10.91–28.18% inhibition at 1–5 mM. Therefore, by increasing the antioxidant capacity and inhibiting the enzyme activity by gum and sodium chloride, it is possible to preserve the antioxidant properties of pear slices and reduce its enzymatic browning.
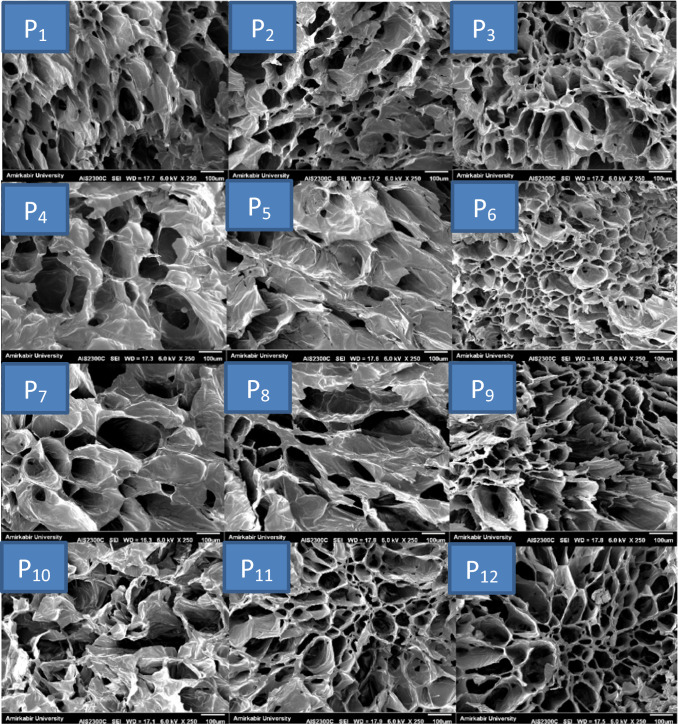
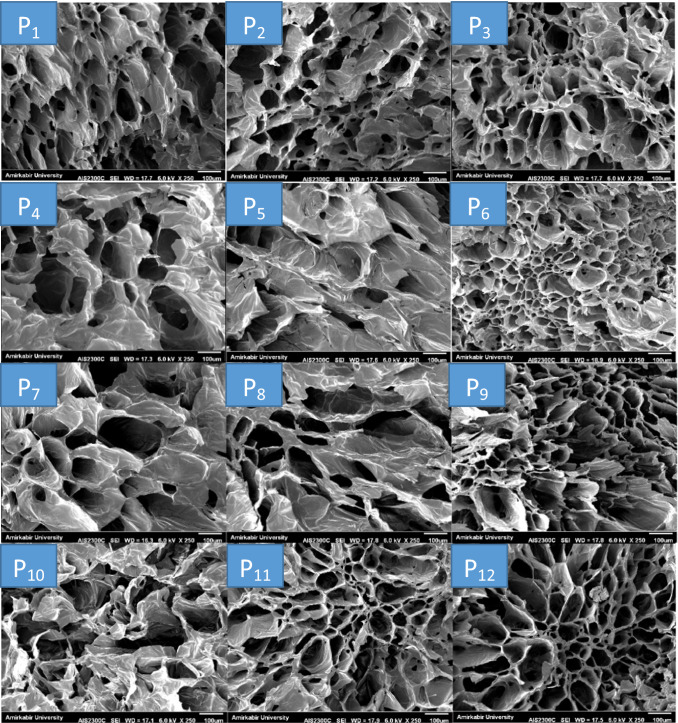
Microstructure analysis
The microstructure images of pears slices dried with freeze dryer after immersion pre-treatment are shown in Fig. 4. As can be seen, the freeze-dried pear slices had uniform and small porous structure. Sodium chloride and quince seed gum pretreatments increased the porosity greatly and created a dried slices with a massive and porous tissue. As mentioned in the moisture result section, sodium chloride treatment prior to the freeze drying process improved drying conditions to remove more moisture from fruit tissue compared to untreated samples. This could be due to the sodium chloride cleanses some sugar, protein or starch from the surface of pear slices, which more porous tissue is formed (Central and right images). These findings were consistent with the results of Askari et al. (2008), who coated the fresh cut apple slices with the pectin, starch, calcium chloride and carboxymethyl cellulose prior to hot air and microwave drying. They reported that coating pre-treatment would increase the porosity greatly and makes a product with massive and porous texture. In another study, Pan et al. (2008) investigated the effect of ascorbic acid and citric acid pre-treatments on structure of dried banana slices. They reported that dipping with acid solution prior to infrared radiation drying can help to create a more porous texture and thus reduce the drying time of the slices. They also stated that the effects of improving the drying rate mainly occur during the initial drying steps.
Fig. 4.
SEM of cross section of dried pear slices after processing
SEM images show that immersion in the gum solution prior to the freeze drying process did not improve the microstructural properties of the pear slices. A closer look shows that the gum as a layer on the surface of the slices tends to reduce porosity and cavities, which may be the reason why the moisture content of slices after drying was higher in these samples. This layer is seen with increasing concentration of gum (left side, from top to bottom) so that at the highest concentration (1%), seen in Fig. 4 (P10), it causes some kind of collapse in the cavities and microstructure of the slices. In other words, the gum retains more moisture by keeping water in its structure and creating a layer on the surface of pear slices. These samples had softer texture with lower crispness than other samples, which that was considered by the panelists in sensory evaluation test. Immersion with solutions containing both gum and salt combinations, as shown in Fig. 4 (P5, P6, P8, P 9, P11 and P12), caused porous and massive tissue formation and no significant change in microstructure was observed with increasing gum concentration in the presence of sodium chloride. However, further mass transfer and microstructure studies should be performed to better explain the effect of gum immersion on the drying of fruit slices.
Sensory evaluation
Table 2 presents the sensory parameters of freeze-dried pear slices. As can be seen, the taste scores of the pear slices were not statistically significant (P ≥ 0.05). In other words, the panelists were not able to detect differences between samples regarding the taste evaluation. For the color parameter, the panelists gave the highest scores to samples pre-treated with medium concentrations of gum and salt (P5 and P6). These results were in agreement with the results of colorimetric test and browning index, where these samples showed the highest values of lightness (L) and had the lowest values of browning index. The lowest color score in the sensory test was related to the control sample (untreated) and the sample containing the highest concentration of gum and salt. In the case of the control sample, the development in enzymatic browning was the reason why the panelists gave the lowest color score. In the case of sample p12, the cause may be due to the formation of a layer by dried gum and salt on the surface of dried slices that caused the slices to become a bit opaque. The Texture scores of pre-treated pear slices significantly declined significantly with increasing gum concentration to 1% compared to other samples (P < 0.05). The dried pear slices pre-treated with sodium chloride had much higher crispness than the slices traded with gum before freeze-drying. The results were in agreement with the results of the moisture content and microstructure test, which the samples treated with gum solutions at higher concentrations showed higher moisture and lower porosity. As a result, these samples contain higher humidity and lower crispness, which was recognized by the panelists. A similar trend was observed for the overall acceptance scores of the samples and the panelists gave lower acceptance scores to the samples treated with the solution containing high concentration of gum. However, the combination of sodium chloride with gum reduced the negative effects of gum on the tissue of the slices and these samples received higher scores. This may explain why Samples P5 (0.25% Gum + 0.25% Salt) and P6 (0.25% Gum + 0.25% Salt) received the highest overall acceptance score among the samples.
Table 2.
Effects of pretreatments on the sensory attributes of freeze-dried pear slices
| Pre-treatments | Taste (Mean ± SD) | Color (Mean ± SD) | Texture (Mean ± SD) | Acceptability (Mean ± SD) |
|---|---|---|---|---|
| P1 (0.00% Gum + 0.00% Salt) | 4.10 ± .28a | 2.90 ± .57e | 3.90 ± .28abc | 3.65 ± .21g |
| P2 (0.00% Gum + 0.25% Salt) | 4.25 ± .35a | 4.10 ± .14abc | 3.90 ± .14abc | 4.05 ± .21bcdefg |
| P3 (0.00% Gum + 0.50% Salt) | 3.95 ± .21a | 3.65 ± .21cd | 4.35 ± .21ab | 3.95 ± .07cdefg |
| P4 (0.25% Gum + 0.00% Salt) | 4.30 ± .14a | 4.30 ± .14ab | 4.10 ± .14abc | 4.25 ± .07abcd |
| P5 (0.25% Gum + 0.25% Salt) | 4.15 ± .21a | 4.55 ± .07a | 4.20 ± .28abc | 4.40 ± .14ab |
| P6 (0.25% Gum + 0.50% Salt) | 4.15 ± .21a | 4.55 ± .07a | 4.20 ± .28abc | 4.45 ± .07a |
| P7 (0.50% Gum + 0.00% Salt) | 4.25 ± .35a | 4.30 ± .14ab | 3.90 ± .14abc | 4.20 ± .14abcde |
| P8 (0.50% Gum + 0.25% Salt) | 4.20 ± .28a | 4.40 ± .14a | 4.15 ± .21abc | 4.30 ± .14abc |
| P9 (0.50% Gum + 0.50% Salt) | 4.05 ± .35a | 4.10 ± .14abc | 4.40 ± .14a | 4.20 ± .14abcde |
| P10 (1.0% Gum + 0.00% Salt) | 3.95 ± .35a | 4.25 ± .07ab | 3.65 ± .07c | 3.90 ± .14defg |
| P11 (1.0% Gum + 0.25% Salt) | 3.95 ± .35a | 3.85 ± .07bcd | 3.80 ± .42bc | 3.85 ± .21efg |
| P12 (1.0% Gum + 0.50% Salt) | 3.80 ± .14a | 3.60 ± .14d | 3.80 ± .14bc | 3.80 ± .14fg |
Means within each column followed by different letters (a-e) show significant different (P < 0.05) between treatments at the same time
Conclusion
The results of this study indicated that application of both sodium chloride and quince seed gum pre-treatments caused significant changes in browning index, moisture and phenolic contents, color and antioxidant activity of freeze-dried pear slices. Treated-dried pear slices have higher dehydration rate, lightness and overall acceptability than the ones that were dried without pre-treatments. Also, sodium chloride and quince seed gum treatments reduced enzymatic browning by maintaining higher antioxidant activity and reducing moisture content in freeze-dried pear slices. Application of sodium chloride and gum pre-treatments at medium concentration used (P5 or P6) resulted in the best quality product in terms of browning index, total phenolic content, antioxidant activity, color parameters and sensory attributes when compared to other samples after freeze drying.
Acknowledgements
The authors would like to extend their appreciation for the financial support provided by the Islamic Azad University (Iran, Tehran).
Compliance with ethical standards
Conflict of interest
We declare no potential conflict of interest related to this manuscript.
Ethics statement
We declare no ethical issue related with this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abano EE, Sam-Amoah LK, Owusu J, Engmann FN. Effects of ascorbic acid, salt, lemon juice, and honey on drying kinetics and sensory characteristic of dried mango. Croat J Food Sci Technol. 2013;5:1–10. [Google Scholar]
- Aktas T, Fujii S, Kawano Y, Yamamoto S. Effects of pretreatments of sliced vegetables with trehalose on drying characteristics and quality of dried products. Food Bioprod Process. 2007;85:178–183. [Google Scholar]
- Annese M, Manzano M, Nicoli MC. Quality of minimally processed apple slices using different modified atmosphere conditions. J Food Qual. 1997;20:359–370. [Google Scholar]
- Antal T. Drying characteristics and quality of pear under hybrid drying (mid-infrared-freeze drying) Hung Agric Eng. 2017;3:33–44. [Google Scholar]
- AOAC . Association of official analytical chemists. Official methods of analysis. 17. Rockville: AOAC; 2000. [Google Scholar]
- Arias E, González J, Oria R, Lopez-Buesa P. Ascorbic acid and 4-hexylresorcinol effects on pear PPO and PPO catalyzed browning reaction. J Food Sci. 2007;72:C422–C429. doi: 10.1111/j.1750-3841.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- Askari GR, Emam-Djomeh Z, Mousavi SM. Effects of combined coating and microwave assisted hot-air drying on the texture, microstructure and rehydration characteristics of apple slices. Food Sci Technol Int. 2008;12:39–46. [Google Scholar]
- Bi JF, Wang X, Chen QQ, Liu X, Wu X, Wang Q, Lv J, Yang A. Evaluation indicators of explosion puffing Fuji apple chips quality from different Chinese origins. LWT Food Sci Technol. 2015;10:1129–1135. [Google Scholar]
- Bieganska-Marecik R, Czapski J. The effect of selected compounds as inhibitors of enzymatic browning and softening of minimally processed apples. Acta Sci Pol Technol Aliment. 2007;6:37–49. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Chen J, Wang Z, Wu J, Wang Q, Hu X. Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 2007;104:268–275. [Google Scholar]
- Coklar H, Akbulut M, Kilinc S, Yildirim A, Alhassan I. Effect of freeze, oven and microwave pretreated oven drying on color, browning index, phenolic compounds and antioxidant activity of hawthorn (Crataegus orientalis) Not Bot Hortic Agrobot Cluj. 2018;46:449–456. [Google Scholar]
- Duan X, Zhang M, Mujumdar AS. Studies on the microwave freeze drying technique and sterilization characteristics of cabbage. Dry Technol. 2007;25:1725–1731. [Google Scholar]
- El-Shimi NM. Control of enzymatic browning in apple slices by using ascorbic acid under different conditions. Plant Foods Hum Nutr. 1993;43:71–76. doi: 10.1007/BF01088098. [DOI] [PubMed] [Google Scholar]
- Fuente-Blanco S, Sarabia ERF, Acosta-Aparicio VM, Blanco-Blanco A, Gallego-Juárez JA. Food drying process by power ultrasound. Ultrason Sonochem. 2006;44:e523–e527. doi: 10.1016/j.ultras.2006.05.181. [DOI] [PubMed] [Google Scholar]
- Gębczyński P, Skoczeń-Słupska R, Kur K. Effect of storage on the content of selected antioxidants and quality attributes in convection and freeze-dried pears (Pyrus communis L.) Italian. J Food Sci. 2017;29:454–462. [Google Scholar]
- Gorny JR. Summary of CA & MA requirements and recommendations for fresh-cut (minimally processed) fruits and vegetables. In: Gorny JR, editor. Proceedings of the seventh international controlled atmosphere conference. Davis, CA: Postharvest Outreach Program, University of California; 1997. pp. 30–66. [Google Scholar]
- Gorny JR, Hess-Pierce B, Cifuentes RA, Kader AA. Quality changes in fresh-cut pear slices as affected by controlled atmospheres and chemical preservatives. Postharvest Biol Technol. 2002;24:271–278. [Google Scholar]
- Huxsoll CC, Bolin HR. Processing and distribution alternatives for minimally processed fruits and vegetables. Food Technol. 1989;43:124–128. [Google Scholar]
- Jiang Y, Fu J, Zauberman G, Fuchs Y. Purification of polyphenol oxidase and the browning control of litchi fruit by glutathione and citric acid. J Sci Food Agric. 1999;79:950–954. [Google Scholar]
- Jouki M, Mortazavi SA, Yazdi FT, Koocheki A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int J Biol Macromol. 2014;66:113–124. doi: 10.1016/j.ijbiomac.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Jouki M, Mortazavi SA, Yazdi FT, Koocheki A. Characterization of antioxidant– antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr Polym. 2014;99:537–546. doi: 10.1016/j.carbpol.2013.08.077. [DOI] [PubMed] [Google Scholar]
- Jouki M, Yazdi FT, Mortazavi SA, Koocheki A. Quince seed mucilage films incorporated with oregano essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014;36:9–19. [Google Scholar]
- Kolniak-Ostek J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC-PDA-Q/TOF-MS. J Food Compos Anal. 2016;49:65–77. [Google Scholar]
- Komes D, Belščak-Cvitanović A, Domitran Z, Opalić M. Content of saccharides, antioxidant and sensory properties of pear cultivar “Abate Feted” affected by ultrasound pre-treatment and air drying duration. J Food Nutr Res. 2013;52:239–250. [Google Scholar]
- Lamikanra O, Watson MA. Effects of ascorbic acid on peroxidase and polyphenoloxidase activities in fresh-cut cantaloupe melon. J Food Sci. 2001;66:1283–1286. [Google Scholar]
- Lee JC, Eun JB. Inhibition of enzymatic browning in precut lotus (Nelumbo Nucifera) roots by browning inhibitors and vacuum-packaging. ISHS Acta Hortic. 1999;483:349–356. [Google Scholar]
- Li Y, Wills RB, Golding JB, Huque R. Effect of halide salts on development of surface browning on fresh-cut ‘Granny Smith’ (Malus domestica Borkh) apple slices during storage at low temperature. J Sci Food Agric. 2015;95:945–952. doi: 10.1002/jsfa.6766. [DOI] [PubMed] [Google Scholar]
- Lim WY, Wong W. Inhibitory effect of chemical and natural anti-browning agents on polyphenol oxidase from ginger (Zingiber officinale Roscoe) J Food Sci Technol. 2018;55:3001–3007. doi: 10.1007/s13197-018-3218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Luo Y, Feng H. Inhibition of apple polyphenol oxidase activity by sodium chlorite. J Agric Food Chem. 2006;54:3693–3696. doi: 10.1021/jf0518103. [DOI] [PubMed] [Google Scholar]
- Lu S, Luo Y, Turner E, Feng H. Efficacy of sodium chlorite as an inhibitor of enzymatic browning in apple slices. Food Chem. 2007;104:824–829. [Google Scholar]
- Moreira MR, Cassani L, Martín-Belloso O, Soliva-Fortuny R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J Food Sci Technol. 2015;52:7795–7805. doi: 10.1007/s13197-015-1907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli MC, Anese M, Severini C. Combined effects in preventing enzymatic browning reactions in minimally processed fruit. J Food Qual. 1993;17:221–229. [Google Scholar]
- Oms-Oliu G, Soliva-Fortuny RC, Martín-Belloso O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol Technol. 2008;50:87–94. [Google Scholar]
- Pan Z, Shih C, McHugh TH, Hirschberg E. Study of banana dehydration using sequential infrared radiation heating and freeze-drying. LWT Food Sci Technol. 2008;41:1944–1951. [Google Scholar]
- Park KJ, Bin A, Brod FPR. Drying of pear d’Anjou with and without osmotic dehydration. J Food Eng. 2003;56:97–103. [Google Scholar]
- Perez-Gago M, Serra M, Del Rio M. Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biol Technol. 2006;39:84–92. [Google Scholar]
- Ratti C. Hot air and freeze-drying of high-value foods: a review. J Food Eng. 2001;49:311–319. [Google Scholar]
- Salta J, Martins A, Santos RG, Nenga NR, Nogueira JMF, Justino J, Rauter AP. Phenolic composition and antioxidant activity of Rocha pear and other pear cultivars e a comparative study. J Funct Foods. 2010;2:153–157. [Google Scholar]
- Sapers GM, Miller RL. Browning inhibition in fresh-cut pears. J Food Sci. 1998;63:342–346. [Google Scholar]
- Sapers GM, Garzarella L, Pilizota V. Application of browning inhibitors to cut apple and potato by vacuum and pressure infiltration. J Food Sci. 1990;55:1049–1053. [Google Scholar]
- Sharma S, Rao TVR. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT Food Sci Technol. 2015;62:791–800. [Google Scholar]
- Shewfelt RL. Flavor and color of fruits as affected by processing. In: Woodroof JG, Luh BS, editors. Commercial fruit processing. Westport: Avi Publishing Co.; 1986. pp. 481–529. [Google Scholar]
- Sun N, Lee S, Song KB. Effect of high-pressure treatment on the molecular properties of mushroom polyphenoloxidase. LWT Food Sci Technol. 2002;35:315–318. [Google Scholar]
- Wang R, Zhang M, Mujumdar AS. Effects of vacuum and microwave freeze drying on microstructure and quality of potato slices. J Food Eng. 2010;101:131–139. [Google Scholar]
- Wojdylo A, Figiel A, Lech K, Nowicka P, Oszmiański J. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014;7:829–841. [Google Scholar]
- Yildiz G. The effect of different chemical agents on the prevention of enzymatic browning in banana. J Food Sci Eng. 2018;7:791–796. [Google Scholar]