Abstract
In this study the effect of gelan, xanthan and quince seed gum (QSG) on stability, probiotic viability and qualitative properties of Doogh using response surface methodology was determined. Three gums were used at three levels of 0, 0.25 and 0.5%. The results showed that the effect of QSG on viscosity and serum separation was significant (P < 0.05). The effect of QSG on viability of B. bifidum was significant (P < 0.05). Sensory evaluation showed that effect of using of QSG was significant and the score of taste, odor and overall acceptance increased by increasing of concentration of QSG. At the optimum point, the stability, viscosity pH, probiotic load and overall acceptance were 92.24%, 11.852 mPa s, 3.87, 8.37 log cfu/mL and 4.42, respectively. Using of combination of gums at the optimal concentration prevented phase separation and maintained the probiotic viability of Doogh. Therefore, the optimization of formulation can be used for production of stabilized probiotic Doogh in dairy industry.
Keywords: Doogh, Phase separation, Optimization, Gums, Stabilization
Introduction
Doogh, as drinkable dairy product, is prepared by mixing water, yoghurt and salt, is a growing area of interest based on its convenience, portability, and ability to deliver all of the health and nutritional benefits of yogurt including providing daily requirement of calcium and vitamins B2, B6 and B12 (Ziaolhagh and Jalali 2017). As previously explained by Azarikia and Abbasi (2010), one of the technological issues of Doogh is separation in to two phases due to its high acidity (pH < 4) and aggregation of proteins in Doogh. Many researchers have studied the effect of adding different hydrocolloids such as gelan, pectin, guar, locust bean, cress seed gum and tragacanth on the stability of this drink to overcome this problem (Behnia et al. 2013; Hassan et al. 2015; Azarikia and Abbasi 2010).
In food industry, edible hydrocolloids like gums and mucilages are used as stabilizer, thickener, gelling agent, suspension stability agent or emulsifiers in some cases as prebiotic. They form slimy masses in water which are typically heterogeneous in composition (Nikoofar et al. 2013; Hassan et al. 2015). The effect of some polysaccharides as prebiotics for the survival and growth of probiotic bacteria such as LAB in dairy products has been proven in many studies. Among these gums, inulin, fructoligosaccharide, gum wort, guar gum and gum arabic are more commonly known (Ranadheera et al. 2010; Gibson et al. 2004; Yilmaz-Ersan et al. 2017). As it has been defined by Gibson et al. (2004), a prebiotic as a non-digestible food ingredient can usefully stimulate the growth or activity of living microorganisms that provide health advantages in the host colon.
Quince seed gum, as a natural thickening compound, is a set of a cellulosic fraction with a more hydrolyzed polysaccharides. Quince seed gum is obtained from quince (Cydonia oblonga Miller, Rosaceae family) seeds when they soak in water and a transparent gel forms around the whole seed (Jouki et al. 2014a). Based on our previous findings, this gum with remarkable antioxidant activity can be used as a natural antioxidant in the food industry (Jouki et al. 2013). As we previously reported, this hydrocolloid is of interest as a gelling agent because of its unique colloidal properties, high viscosity, low production cost (in comparison with most mucilages) and easy extraction (Jouki et al. 2014b). Therefore, Quince seed gum has a good potential to use as an antioxidant stabilizing agent in the food systems.
Xanthan, as one of the most popular gums has been widely used as a stabilizer, is produced through fermentation by microorganisms, Xanthonmonas and also found on cruciferous vegetables such as cabbage and cauliflower (El-Sayed et al. 2002). The gum is made up of the main body of the β-d-glucose compound, and each second glucose unit is attached to a mannose, glucuronic acid, and mannose triglyceride. El-Sayed et al. (2002) reported that this gum is widely used because of its special properties such as solubility in cold water as well as hot water, consistency at low concentrations, good thermal stability and stability at freezing temperatures. Xanthan gum is an anionic and hygroscopic polysaccharide which is applied as a setting agent for daily products in low concentrations (Sikora et al. 2008). Many studies have been done about xanthan and its effective mechanism of stabilization (Ziaolhagh and Jalali 2017; El-Sayed et al. 2002; Park et al. 2019). It has been suggested that xanthan prevents phase separation in daily products (El-Sayed et al. 2002).
Gellan, as an extracellular anionic hydrocolloid, is produced by bacterium Sphingomonas elodea in aerobic condition and as previously reported Gibson and Sanderson (1997), it is a linear polysaccharide with a repeat of four units consisting of glucose, glucuronic acid and rhamnose. This gum is widely used in dairy products, jellies, jams, spreads, sauces, puddings, toppings and structured foods (Karlton-Senay and Ibrahim 2013).
Response surface methodology (RSM) is a commonly used technique for optimizing a multivariate process that can predict untested points in addition to reducing the number of treatments required in the range studied (Jouki et al. 2014c). The technique provides mathematical and statistical procedures to study relationships between one or more responses (dependent variables) and a number of factors (independent variables) (Joglekar and May 1987). The effect of adding various hydrocolloids on the stability of dairy drinks has been investigated in many studies (Ziaolhagh and Jalali 2017; Azarikia and Abbasi 2010; Koksoy and Kilic 2004). In several studies, other polysaccharide gums has been used to enhance viability of probiotics (Azarikia and Abbasi 2010; Karlton-Senay & Ibrahim 2013; Ghasempour et al. 2012). No such study has been carried out on optimization of probiotic Doogh formulation and its physico chemical characteristics. Therefore, the purpose of this study was to optimize Doogh formulation with the highest probiotic viability, stability and the lowest phase separation with fluid gels using response surface methodology.
Materials and methods
Materials
Low fat milk was purchased from a local milk producer in Tehran (Iran). The milk contained 4.7% lactose and 3.1% protein. Gellan and xanthan gums were purchased from Sigma Aldrich (St Louis, MO, USA). The quince seeds were purchased from a local market in Tehran, Iran. All chemicals were obtained from Merck (Germany).
Preparation of cultures
B. bifidum (BB12) and commercial lyophilized starter culture (YF-3331) containing mixed culture of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus were supplied by Christian Hansen (Horsholm, Denmark).
Gum extraction from quince seed
A sequential process was employed to extract gum from quince seeds using the method described by Jouki et al. (2013).
Doogh preparation
The milk used in the production of Doogh was pasteurized in a water bath at 90 °C for 15 min. After the cooling to 40 °C, it was inoculated by the commercial starter culture (YF-3331) and transferred to plastic cups, incubated at 42 °C for 5–6 h until the pH reached 4.3. After fermentation, the prepared yoghurt was held in the refrigerator (4 °C). The yogurt was diluted by drinking water (50% v/v and NaCl 0.7% w/v) to produce Doogh. Xanthan, gelan and quince seed gum (0, 0.13, 0.25, 0.38 and 0.50% w/v) were added to the mixture and stirred. Then, they were filled into 250-mL PET bottles and to achieve full hydration, the mixture was kept at room temperature overnight. Then, Doogh samples were pasteurized at 80 °C for 15 min. After cooling down of the Doogh samples up to the fermentation temperature (37 ± 1 °C), they inoculated with probiotic culture for 6 h. The necessary inoculum, to give approximately 9 log10 cfu/mL in Doogh after inoculation, was calculated (Haji Ghafarloo et al. 2020). Doogh samples were stored at 4 °C until measurements were made.
Probiotic enumeration
Enumeration of B. bifidum was fulfilled in MRS-agar with lithium chloride 2% and sodium propionate 3%, 37 °C, 72 h and aerobic condition using anaerobic jars (Haji Ghafarloo et al. 2020; Ghasempour et al. 2012). For microbiological analyses, 1 mL of a Doogh sample was suspended in 0.1% sterile peptone water and serially diluted to the desired levels; 1 mL of the appropriate dilutions was pour plate cultured (Ghasempour et al. 2012). The result was expressed as colony forming units per gram (cfu/mL) of Doogh sample.
Titratable acidity and pH
The titratable acidity (TA, g/L of lactic acid) was evaluated for all Doogh samples, using 0.1 N NaOH and 0.5% phenolphthalein solution. Briefly, 10 mL of sample was diluted with 10 mL distilled water and titrated against NaOH (Purwandari et al. 2007). The pH was measured using a digital pH meter which was calibrated with buffer 4, 7 and 10. The acidity of the Doogh samples was calculated as percent (%) lactic acid.
Phase separation
Phase separation of yogurt drink samples was measured using gravity separation. In order to determine stability of the Doogh samples, they were transferred to 10 mL sterilized and graduated test tubes with cap kept at 4 °C. Phase separation was expressed based on volume percent of the total sample (Shariati et al. 2020). Equation (1) was used to evaluate and calculate the Serum separation of Doogh samples:
| 1 |
Apparent viscosity
Apparent viscosity of Doogh samples was evaluated using a Brookfield viscometer (DVII + Pro, Middleboro, USA) equipped with a number 2 (LV2) spindle and head rotating at 30 rpm. The spindle head started moving and the apparent viscosity value was recorded at the 25th second as mPa s (Ghasempour et al. 2012). Measurements were made using 16 mL of Doogh samples at 20 °C. After primary experiments, the appreciate torque value found between 10 and 100% of the measuring range to obtain reliable results.
Sensory evaluation
Sensory attributes of probiotic Doogh samples including taste, odor, color and overall acceptability were evaluated using a 5-point hedonic test (1 = dislike extremely, 2 = dislike moderately, 3 = neither like nor dislike, 4 = like moderately and 5 = like extremely) by a 9 member trained panel from the food laboratory staff who were familiar with the characteristic qualities of daily products. Doogh samples were taken for the analysis after 1 days of storage.
Experimental design and statistical analysis
A central composite rotatable design (CCRD) was employed for designing the experimental data to find out the best Doogh formulation. In this study, actual-level and coded levels encoded design variables with response variables were selected. This experimental plan with star and six central points were designed to calculate reproducibility (Jouki et al. 2014c). The RSM was applied to determine the effect of three independent variables (Gelan, x1; Xanthan, x2 and Quince seed gum concentrations, x3) at three levels of each variable on the pH (Y1), acidity (Y2), viscosity (Y3), phase separation (Y4), probiotic viability (Y5), taste (Y6), color (Y7), odor (Y8) and overall acceptability (Y9) using a commercial statistical package, Design-Expert version 7.1.6 (Stat-Ease Inc., Minneapolis, MN, USA). The following second-order polynomial was used to relate the coded variables (xi, i = 1, 2 and 3) and the values by the following equation [Eq. (2)]:
| 2 |
The constant term (b0), linear effects (b1, b2 and b3), quadratic effects (b11, b22 and b33) and interaction effects (b12, b13 and b23) were used to show the polynomial coefficients. To illustrate the synchronous interaction of the two factors and the responses, the optimal laboratory variables location the mentioned plots are used (Bas and Boyaci 2007). The RSM outputs such as contour and 3D graphic surface plots provide the optimum and most influential variables for Doogh formulation. In the regression equations the statistical significance of the terms was examined. The significant terms in the model were found by analysis of variance (ANOVA) for each response. The adequacy of the models was determined using model analysis, lack-of fittest, coefficient of determination (R2) and adjusted-R2 analysis. As suggested by Joglekar and May (1987), the minimum R2 value for good model fit should be 0.8. As the value of t increases and P decreases, the relevant variables become more significant (Atkinson and Donev 1992). The significant factors were used to obtain the final reduced model and those which statistically non-significant were removed to refitted the experimental data.
Optimization and verification
By performing the experiments under the recommended optimum formulation, the experimental data for the acidity, viscosity, phase separation, probiotic viability, taste, odor and overall acceptability of Doogh were obtained. To verify the response surface model, the empirical value obtained from an independent set of samples is compared with the predicted value obtained from the optimized model with linear regression, which was estimated by MS Excel.
Results and discussion
Model fitting
Each response was evaluated as a function of linear, quadratic and interaction terms of the independent variables including gelan, xanthan and quince seed gum concentrations. ANOVA and regression analysis were used to fit the model and test the statistical significance of the terms. Table 1 shows the regression coefficients for the quadratic polynomial models and corresponding coefficients of determination (R2) for each dependent variable. A high R2 was obtained for all regression models. The R2 values were 0.91, 0.95, 0.94, 0.90, 0.92, 0.90 and 0.91, for acidity, viscosity, phase separation, probiotic viability, taste, odor and overall acceptability, respectively. As previously reported by Koocheki et al. (2009), the higher value of R2 shows the desirability of the model to elucidate the relationships between variables.
Table 1.
Response surface models for Doogh optimum formulation after backward elimination
| Responses | Quadratic polynomial model | R2 | p value |
|---|---|---|---|
| Y1 | NS | – | 0.1771 |
| Y2 | Y2 = + 0.67504 + 0.12338X1 − 0.038618X2 + 0.19178X3 − 0.39600X2X3 − 0.48436X12 − 0.48436X32 | 0.9134 | 0.0002 |
| Y3 | Y3 = 64.72411 + 57.58527X3 + 234.46000X1X3 + 410.01455X32 | 0.9466 | 0.0005 |
| Y4 | Y4 = 11.60114 − 11.27273X1 − 5.52273X2 − 2.02273X3 | 0.9417 | 0.0141 |
| Y5 | Y5 = 8.67545 + 27.65091X3 | 0.9212 | 0.0479 |
| Y6 | Y6 = 4.58295 − 9.09091003X3 | 0.9244 | 0.0003 |
| Y7 | NS | – | 0.0565 |
| Y8 | Y8 = 3.96636 − 0.46727X3 | 0.9034 | 0.0354 |
| Y9 | Y9 = 4.10000 − 4.00000X22 − 8.00000X32 | 0.9051 | 0.0001 |
Y1 (pH), Y2 (acidity), Y3 (viscosity), Y4 (phase separation), Y5 (probiotic viability), Y6 (taste), Y7 (color), Y8 (odor) and Y9 (overall acceptability)
In this study, regression models were appropriate to explain their behavior because R2 values for these response variables were above 0.80. To fit the actual data for the empirical model better, the R2 value data should be closer together. As previously has been stated to adjust the number of explanatory terms in a model, Adjusted R2 (modification of R2) was used. As Koocheki et al. (2009) reported, if the model with the new face is improved than expected, the adjusted R2 will only increase. The values of adjusted R2 were 0.90, 0.93, 0.92, 0.87, 0.90, 0.85 and 0.88, for acidity, viscosity, phase separation, probiotic viability, taste, odor and overall acceptability, respectively. Comparing R2 and adj-R2 values for the models did not show dramatic difference. This harmony indicated that non-significant terms have not been included in the model. It has also been mentioned that significant lack of fit shows that the models failed to represent the data in the experimental domain at which points were not included in the regression (Jouki et al. 2014c).
In reduced models, no sign of significant lack of fit was observed (P ≥ 0.05) and hence, the fit of response surface model to the effect of significant independent variable (P < 0.05) was guaranteed. The results suggest that the models used in this research were able to identify optimization for selective formulation of Doogh samples. Table 1 shows the equations of the appropriate models after eliminating the effect of non-significant factors for the process variables.
Effects of gums on pH and acidity
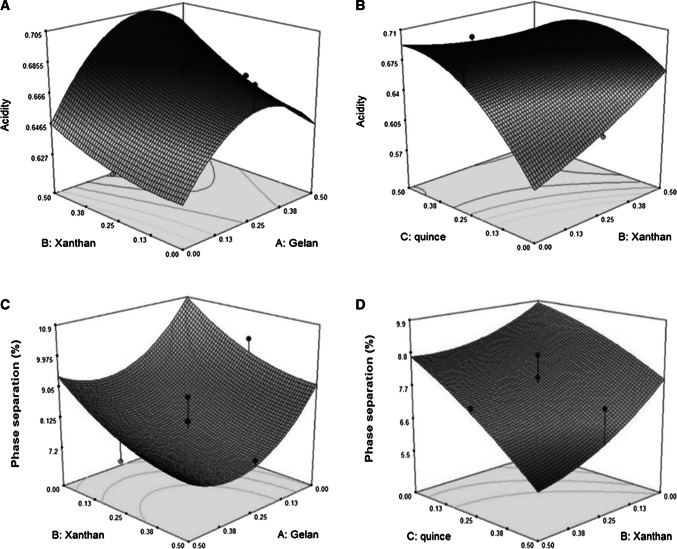
The results showed that the linear, quadratic and interaction effects of gum adding on the pH values of Doogh samples were not significant (P > 0.05) (Table 1). The pH values were decreased by increasing of the levels of gums (not shown), although this effect was not significant (P ≥ 0.05). This is probably due to the buffer effect of used gums. Similar trend has been reported by Ziaolhagh and Jalali (2017) for bio-Doogh samples. They reported that the samples containing 0.15% of xanthan gum illustrated no significant pH changes. Table 1 also illustrates that the effect of gums on the Acidity of Doogh samples, all the linear, quadratic and interaction effects of xanthan, gelan and quince seed gum were significant. Generally, the Doogh samples containing higher concentration of used gums had higher titratable acidity and lower pH as compared to control samples, which is due to the presence of probiotic and gums as prebiotics (Fig. 1).
Fig. 1.
3D plots for the interaction effects of independent variables on the acidity (a, b) and phase separation (c, d) of Doogh
Effects of gums on phase separation
Table 1 illustrates that he linear effect of xanthan and gelan and quince seed gum on the phase separation of Doogh were significant (P < 0.05). It can be seen that the variable with the largest effect on the stability was the linear effect of gelan concentration, followed by the linear effects of gelan and quince seed gum (Table 1). Increasing concentration of quince seed gum up to 0.5% decreased the phase separation of Doogh (Fig. 1) Azarikia and Abbasi (2010). reported that phase separation was not observed in the dough samples containing gellan gum during 28 days because of the formation of gel particles due to the accumulation of gel molecules. In addition, Sworn (2000) reported that gellan molecules can be affected by positively charged proteins. So, in this study we tryed to find the best Doogh formulation with the highest stability and lowest phase separation using RSM. Some recent researches have shown that some hydrocolloids can interact with casein micelles through calcium ions to prevent their accumulation, sedimentation and hence serum separation in daily drinks (Ziaolhagh and Jalali 2017; Lucey et al. 1999).
Effects of gums on viscosity
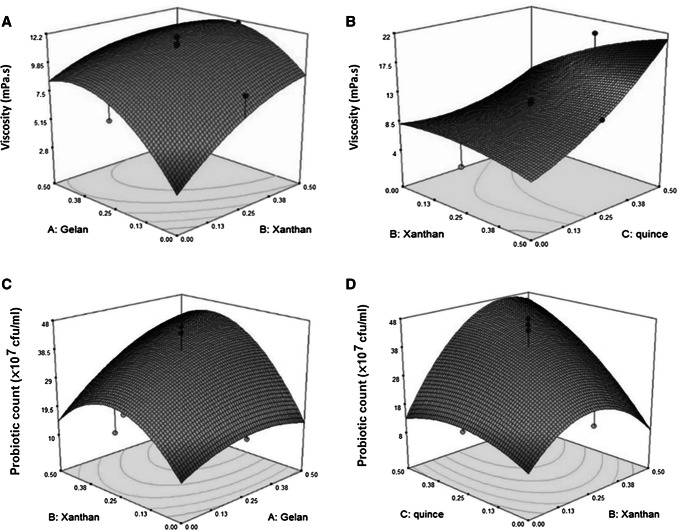
As Table 1 shows, the linear regression equation was fitted (P < 0.001) for prediction of independent variables effect on viscosity change. The results illustrated that the linear, quadratic and interaction effects of quince seed gum adding on the viscosity of Doogh samples were significant (P < 0.001), while linear and quadratic effects of xanthan and gelan were not significant (P > 0.05). Increasing concentration of quince seed gum up to 0.5% increased the viscosity of Doogh (Fig. 2). This fact is probably because of the quince seed gum ability to induce formation of a weak network structure in Doogh. It can be seen that the variable with the largest effect on the viscosity was the quadratic effect of quince seed gum concentration, followed by the interaction effects of xanthan and gelan and linear effect of quince seed gum (Table 1). It can be concluded that type of gum substantially affected the apparent viscosity due to the differing in the constituent and structure of the gel (Jouki et al. 2014c). It has been also stated that other factors such as neutral sugars, uric acid, configuration, methoxyl group content, position of glycosidic bonds and conformation have important role on rheological properties of hydrocolloid (Jouki et al. 2014c). As we previously reported, QSG induced a high viscosity after dispersing in water and its viscosity is higher than xanthan and gelan gums (Jouki et al. 2014c). This might be due to the high molecular weight (Mw) of quince seed gum as a natural organic seed constituent with a molecular weight of 200,000 or greater (Hoffmann 2003).
Fig. 2.
3D plots for the interaction effects of independent variables on the viscosity (a, b) and probiotic count (c, d) of Doogh
Effects of gums on probiotic viability
The effect of gelan, xanthan, and quince seed gum concentrations on survival of B. bifidum was investigated after 1 day of cold storage at 4 °C. Figure 2 show the changes in cell counts of B. bifidum in Doogh. After 1 day of manufacture, the number of B. bifidum colonies was present at log10 8.3 cfu/mL in the control sample (without gums). The minimum required level of probiotic bacteria daily products is 106–108 cfu/mL of living bacteria (Lourens-Hattingh and Viljoen 2001; Granato et al. 2010). The level in the present study was found to be > 108, that is regarded beneficial for the consumers.
As Table 1 shows, the linear, effect of quince seed gum adding on the probiotic count of Doogh samples was significant (P < 0.05). Doogh fortified with gums showed good viability at the first of the storage period compared to the control sample. The number of B. bifidum colonies were significantly increased by addition of quince seed gum, but increasing the levels of gelan and xanthan gums had no significant effect (P ≥ 0.05), although Moreira et al. (2015) claimed that gellan gum could be introduced as prebiotic. Quince seed gum as a prebiotic, enhanced viability of B. bifidum significantly (P < 0.05) in Doogh sample, so it can be regarded as a prebiotic like inulin, gum arabic and guar gums (Ranadheera et al. 2010; Yilmaz-Ersan et al. 2017). The utilization of prebiotics like inulin by probiotic bacteria has been reported (Desai et al. 2004). They found that these compounds were the favored carbon source for Lactobacillus strains. These compounds improve the viability of the probiotic bacteria because they supply additional nutrients for increasing culture growth (Makras et al. 2005).
Effects of gums on sensory parameters
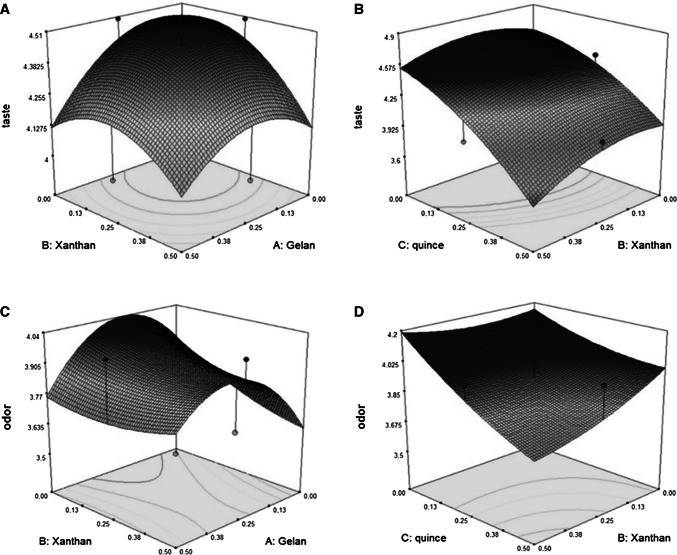
The linear regression equation was fitted to predict the effects of xanthan, gelan and quince seed gum on the variation of sensory parameters leading to achieve the optimum taste value (Fig. 3b). The linear effect of quince seed gum on the taste of Doogh was significant (P < 0.001).
Fig. 3.
3D plots for the interaction effects of independent variables on the taste (a, b) and odor c, d) of Doogh
The linear, quadratic and interaction effects of gums on the color of Doogh were shown to be non-significant on color (Table 1). The color score was increased by increasing of the levels of gums (not shown), although this effect was not significant (P ≥ 0.05). The panelists also could not differentiate the various types of Doogh samples in color attribute. The mean color scores were not significantly different between the control and treated samples. The linear effect of quince seed gum on the odor of Doogh (Fig. 3) was significant (P < 0.001), while linear and quadratic effects of xanthan and gelan were not significant (P > 0.05).
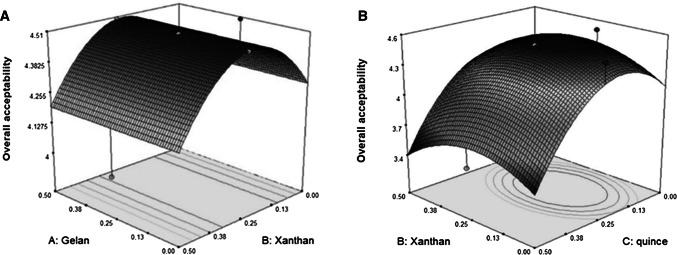
The quadratic effects of xanthan and quince seed gum on overall acceptability of Doogh samples were significant (P < 0.0001), while only linear effect of these gums were not significant. Increasing concentration of quince seed gum up to 0.5% showed higher scores in terms of overall acceptability of Doogh samples (Fig. 4). As shown in Table 1, the quadratic effect of quince seed gum concentration followed by the linear effect of xanthan had the largest effects on the overall acceptability of Doog. In a similar study, Azarikia and Abbasi (2010) showed that adding hydrocolloids such as xanthan gum had not negative organoleptic effects on sensory parameters of Doogh samples. The overall acceptability of the formulated Doogh samples under optimum condition was ideal for the panelists.
Fig. 4.
3D plots for the interaction effects of independent variables on the overall acceptability (a, b) of Doogh
Optimization and verification of formulation
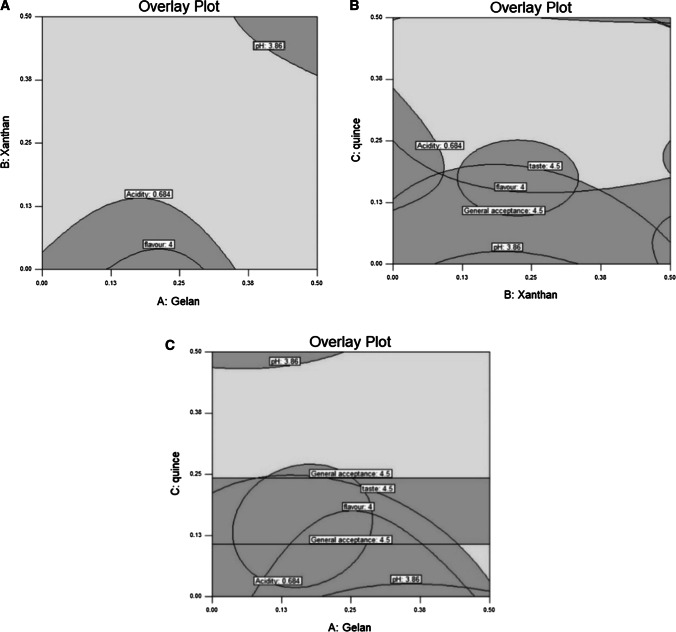
To predict the optimum level of independent variables (minimum content of acidity, phase separation, and maximum pH, viscosity, probiotic viability, taste, odor, color and overall acceptability scores), numerical and graphical optimization procedures were carried out. A graphical optimum formulation region is shown in Fig. 5. Figure 5a represent the gelan concentration of 0.00–0.50% and xanthan concentration of 0.13–0.50% at quince seed gum concentration of 0.30 as the best combinations. The gelan concentration of 0.00–0.50% and quince seed gum concentration of 0.25–0.50% at constant xanthan concentration of 0.17% are shown in Fig. 5b. It also represent the xanthan concentration of 0.00–0.50% and quince seed gum concentration of 0.00–0.50% at gelan concentration of 0.32 as the best combinations (Fig. 5c). However, the common area for seven variables was the optimum condition. Adequacy of the models for predicting the optimum response values was tested by Doogh formulation in the optimum levels of variables obtained by the RSM optimization this optimum condition (gelan: 0.32%, xanthan: 0.17% and quince seed gum: 0.30%) provided the highest value of viscosity of 11.852 mPa s, pH of 3.87, probiotic load of 8.37 log cfu/mL, stability of 92.24%, and overall acceptance of 4.42. Predicted and mean of experimental values for the acidity (0.671 and 0.678 ± 0.009 lactic acid), viscosity (11.852 and 12.021 ± 2.011 mPa s), probiotic count (8.37 and 8.22 ± 0.21 log cfu/mL taste (4.35 and 4.30 ± 0.25), color (4.36 and 4.40 ± 0.18), odor (3.91 and 4.00 ± 0.15) and overall acceptability (4.42 and 4.33 ± 0.17) showed that the experimental and predicted values were very close and were not statistically different at the 5% significance level. Analyzes showed that the experimental results were in good agreement with the and predicted values by software and also the models were satisfactory and accurate.
Fig. 5.
The optimum region, obtained by overlaying contour plots of the nine responses
Conclusion
In this study, the response surface methodology was used to determine the optimal formulation for the production of probiotic Doogh, which is stabilized by different types of gum. The optimum composition for stable Doogh production was found to contain (% wt) gelan, xanthan and quince seed gum was 0.32, 0.17 and 0.30, respectively. Sensory evaluation showed that effect of using of QSG was significant and the score of taste, odor and overall acceptance increased by increasing of concentration of QSG. These optimum conditions yielded the stability of 92.24%, viscosity of 11.852 mPa s, pH of 3.87, probiotic load of 8.37 log cfu/mL and overall acceptance of 4.42. Samples containing mixed gums showed higher survival probiotic rate than control sample, which was above therapeutic minimum level (> 106−7 CFU/g). This research revealed that the combination of these gums can be used in the production of probiotic yogurt drink with appropriate textural stability.
Acknowledgements
The authors would like to extend their appreciation for the financial support provided by the Islamic Azad University (Iran, Tehran).
Compliance with ethical standards
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethical standards
This study does not involve any human or animal testing.
Informed consent
All participants signed an informed consent form and were compensated for their time.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Atkinson AC, Donev AN. Optimum experimental designs. Oxford: Oxford University Press; 1992. pp. 132–189. [Google Scholar]
- Azarikia F, Abbasi S. On the stabilization mechanism of Doogh (Iranian yoghurt drink) by gum tragacanth. Food Hydrocolloid. 2010;24:358–363. doi: 10.1016/j.foodhyd.2009.11.001. [DOI] [Google Scholar]
- Bas D, Boyaci IH. Modeling and optimization I. Usability of response resurface methodology. J Food Eng. 2007;78:836–845. doi: 10.1016/j.jfoodeng.2005.11.024. [DOI] [Google Scholar]
- Behnia A, Karazhiyan H, Niazmand R, Nafchi ARM. Rheological properties of low fat yogurt containing cress seed gum. Agric Sci. 2013;4:29–32. [Google Scholar]
- Desai AR, Powell IB, Shah NP. Survival and activity of probiotic lactobacilli in skim milk containing prebiotics. J Food Sci. 2004;69:FMS57–FMS60. [Google Scholar]
- El-Sayed EM, El-Gawad IA, Murad HA, Salah SH. Utilization of laboratory-produced xanthan gum in the manufacture of yogurt and soy yogurt. Eur Food Res Technol. 2002;215:298–304. doi: 10.1007/s00217-002-0551-9. [DOI] [Google Scholar]
- Ghasempour Z, Alizadeh M, Rezazad Bari MR. Optimization of probiotic yogurt production containing Zedo gum. Int J Dairy Technol. 2012;65:118–125. doi: 10.1111/j.1471-0307.2011.00740.x. [DOI] [Google Scholar]
- Gibson W, Sanderson GR. Gellan gum. In: Lemson A, editor. Thickening and gelling agents for food. New York: Chapman & Hall; 1997. pp. 119–143. [Google Scholar]
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Granato D, Branco GF, Cruz AG, Faria JAF, Shah NP. Probiotic dairy products as functional foods. Comp Rev Food Sci Food Saf. 2010;9:455–470. doi: 10.1111/j.1541-4337.2010.00120.x. [DOI] [PubMed] [Google Scholar]
- Haji Ghafarloo M, Jouki M, Tabari M. Production and characterization of synbiotic Doogh, a yogurt-based Iranian drink by gum arabic, ginger extract and B. bifidum. J Food Sci Technol. 2020;57:1158–1166. doi: 10.1007/s13197-019-04151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan LK, Haggag HF, ElKalyoubi MH, Abd EL-Aziz M, El-Sayed MM, Sayed AF. Physico-chemical properties of yoghurt containing cress seed mucilage or guar gum. Ann Agric Sci. 2015;60:21–28. doi: 10.1016/j.aoas.2014.11.021. [DOI] [Google Scholar]
- Hoffmann D. Medical herbalism: the science and practice of herbal medicine. Rochester: Healing Arts Press; 2003. [Google Scholar]
- Joglekar AM, May AT. Product excellence through design of experiments. Cereal Food World. 1987;32:857–868. [Google Scholar]
- Jouki M, Mortazavi SA, Yazdi FT, Koocheki A. Physical, barrier and antioxidant properties of a novel plasticized edible film from quince seed mucilage. Int J Biol Macromol. 2013;62:500–507. doi: 10.1016/j.ijbiomac.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Jouki M, Mortazavi SA, Yazdi FT, Koocheki A. Characterization of antioxidant–antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr Polym. 2014;99:537–546. doi: 10.1016/j.carbpol.2013.08.077. [DOI] [PubMed] [Google Scholar]
- Jouki M, Mortazavi SA, Yazdi FT, Koocheki A. Quince seed mucilage films incorporated with oregano essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocolloid. 2014;36:9–19. doi: 10.1016/j.foodhyd.2013.08.030. [DOI] [Google Scholar]
- Jouki M, Mortazavi SA, Yazdi FT, Koocheki A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int J Biol Macromol. 2014;66:113–124. doi: 10.1016/j.ijbiomac.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Karlton-Senay BD, Ibrahim SA. Impact of gums on the growth of probiotics. Agro Food Ind Hi Tech. 2013;24:10–14. [Google Scholar]
- Koksoy A, Kilic M. Use of hydrocolloids in textural stabilization of a yoghurt drink, ayran. Food Hydrocolloid. 2004;18:593–600. doi: 10.1016/j.foodhyd.2003.10.002. [DOI] [Google Scholar]
- Koocheki A, Taherian AR, Razavi SMA, Bostan A. Response surface methodology for optimization of extraction yield, viscosity, and hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocolloid. 2009;23:2369–2379. doi: 10.1016/j.foodhyd.2009.06.014. [DOI] [Google Scholar]
- Lourens-Hattingh A, Viljoen BC. Yoghurt as probiotic carrier food. Int Dairy J. 2001;11:1–17. doi: 10.1016/S0958-6946(01)00036-X. [DOI] [Google Scholar]
- Lucey JA, Tamehana M, Singh H, Munro PA. Stability of model acid milk beverage: effect of pectin concentration, storage temperature and milk heat treatment. J Texture Stud. 1999;30:305–318. doi: 10.1111/j.1745-4603.1999.tb00219.x. [DOI] [Google Scholar]
- Makras L, Van Acker G, De Vuyst L. Lactobacillus casei subsp. casei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerisation. Appl Environ Microbiol. 2005;71:6531–6537. doi: 10.1128/AEM.71.11.6531-6537.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira MR, Tomadoni B, Martín-Belloso O, Soliva-Fortuny R. Preservation of fresh-cut apple quality attributes by pulsed light in combination with gellan gum-based prebiotic edible coatings. LWT Food Sci Technol. 2015;64:1130–1137. doi: 10.1016/j.lwt.2015.07.002. [DOI] [Google Scholar]
- Nikoofar E, Hojjatoleslami M, Shariaty MA. Surveying the effect of quince seed mucilage as a fat replacer on texture and physicochemical properties of semi fat set yoghurt. Int J Farm Allied Sci. 2013;2:861–865. [Google Scholar]
- Park YW, Oglesby J, Hayek SA, Aljaloud SO, Gyawali R, Ibrahim SA. Impact of different gums on textural and microbial properties of goat milk yogurts during refrigerated storage. Foods. 2019;8:169–176. doi: 10.3390/foods8050169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwandari U, Shah NP, Vasiljevic T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yogurt. Int Dairy J. 2007;17:1344–1352. doi: 10.1016/j.idairyj.2007.01.018. [DOI] [Google Scholar]
- Ranadheera RDCS, Baines SK, Adams MC. Importance of food in probiotic efficacy. Food Res Int. 2010;43:1–7. doi: 10.1016/j.foodres.2009.09.009. [DOI] [Google Scholar]
- Shariati Z, Jouki M, Flora R. Flavored functional drinking yogurt (Doogh) formulated with Lactobacillus plantarum LS5, cress seed gum, and coriander leaves extract. Food Sci Nutr. 2020;8:894–902. doi: 10.1002/fsn3.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora MS, Kowalski P, Tomasik P. Binary hydrocolloids from starches and xanthan gum. Food Hydrocolloid. 2008;22:943–952. doi: 10.1016/j.foodhyd.2007.05.007. [DOI] [Google Scholar]
- Sworn G. Gellan gum. In: Phillips G, Williams P, editors. Handbook of hydrocolloids. Cambridge: Woodhead Publishing Ltd.; 2000. [Google Scholar]
- Yilmaz-Ersan L, Ozcan T, Akpinar-Bayizit A. Impact of some gums on the growth and activity of bifidobacterium Animalis subsp. lactis. Int J Food Eng. 2017;3:73–77. [Google Scholar]
- Ziaolhagh SH, Jalali H. Physicochemical properties and survivability of probiotics in bio-Doogh containing wild thyme essence and xanthan gum. Int Food Res J. 2017;24:1805–1810. [Google Scholar]