Abstract
Background
Women with a BRCA1 or BRCA2 mutation have high lifetime risks of developing breast and ovarian cancers. We sought to estimate the prevalence of cancer-related distress and to identify predictors of distress in an international sample of unaffected women with a BRCA mutation.
Methods
Women with a BRCA1/2 mutation and no previous cancer diagnosis were recruited from the United States, Canada, the United Kingdom, Australia and from a national advocacy group. Using an online survey, we asked about cancer risk reduction options and screening, and we measured cancer-related distress using the Impact of Event Scale.
Results
Among 576 respondents, mean age was 40.8 years (SD = 8.1). On average 4.9 years after a positive test result, 16.3% of women reported moderate-to-severe cancer-related distress. Women who had undergone risk-reducing breast and ovarian surgery were less likely to have (moderate or severe) cancer-related distress compared to other women (22.0% versus 11.4%, P value = 0.007). Women recruited from the advocacy group were more likely to have cancer-related distress than other women (21.6% versus 5.3%, P value = 0.002).
Conclusions
Approximately 16% of women with a BRCA1 or BRCA2 mutation experience distress levels comparable to those of women after a cancer diagnosis. Distress was lower for women who had risk-reducing surgery.
Subject terms: Cancer prevention, Predictive markers, Breast cancer
Background
Since testing for BRCA1 and BRCA2 began in the 1990s, there has been keen interest in the psychosocial consequences of genetic testing.1–6 The risk of developing breast cancer by age 80 years is ~72% for BRCA1 mutation carriers and 69% for BRCA2 mutation carriers; the risk for ovarian cancer is 44% for BRCA1 carriers and 17% for BRCA2 carriers.7 Healthy women with a mutation are given information about these risks and have the options of risk-reducing surgery and screening. It is anticipated that receipt of this genetic information will be stressful for many women, but it is not clear how many experience significant distress, nor the duration of distress.4,6 The majority of psychosocial research in women with a BRCA1 or BRCA2 mutation suggests that distress increases immediately following the receipt of positive genetic test results, but return to baseline levels or below with time.4,6 However, the majority of research has focussed on women from academic genetic testing clinics, has included women with negative and positive BRCA results and has included women with and without a cancer diagnosis.
It is important to assess psychosocial functioning in the period following genetic testing for women without a cancer diagnosis. At this time, women face difficult decisions about cancer risk reduction. Several surveillance and prevention options are available with the goals of early detection and of reducing cancer incidence and mortality. Furthermore, the practice of genetic testing for BRCA1 and BRCA2 has changed over the past 20 years. Genetic testing can now be obtained directly by the consumer or ordered by the treating physician without genetic consultation. Further, there is increasing interest in population-based genetic testing for BRCA1 and BRCA2 with the goal of identifying women with a mutation prior to cancer diagnosis.8 In these situations, the genetic counsellor is often bypassed. It is important to assess the benefits and the risks of new genetic testing protocols, in particular among healthy women who receive a positive test result. These risks many include acute and chronic psychological distress. In the current study, we report on frequency and predictors of cancer-related distress in a large international cohort of unaffected women with BRCA1 or BRCA2 mutations aged 25 to 55 years.
Methods
Participants and procedures
Eligible women were unaffected by cancer, aged 25 to 55 years, and were BRCA1 or BRCA2 mutation carriers. All were able to read and understand English and consented to participate in an international patient preferences study around risk-reducing strategies for familial/genetic risk of developing breast and ovarian cancer.
Recruitment was between January 2015 and March 2016 via six sources across the United States, Canada, United Kingdom and Australia, including the patient advocacy group Facing Our Risk of Cancer Empowered (FORCE), clinical research registries at Creighton University (USA), Women’s College Hospital (Canada), The Royal Melbourne Hospital (Australia), the Kathleen Cunningham Foundation Consortium for Research into Familial breast cancer (kConFab) at Peter MacCallum Cancer Centre (Australia) and Manchester Centre for Genomic Medicine (UK). FORCE respondents provided a self-reported BRCA1/2 status and were recruited through its website, newsletters and social media. Clinical sites identified respondents who met the inclusion criteria and mailed them invitation letters with the URL of the online survey and a unique password. Institutional review boards at RTI International and all participating sites approved the study. All participants provided informed consent prior to their inclusion in the study.
Participation involved completing an anonymous online survey developed following good research practices.9 The questions were developed with input from clinicians who treat women with BRCA1/2 mutations. The survey instrument was pretested in 14 one-on-one interviews with women in the United States who met the study inclusion criteria to assess respondent comprehension, the relevance of the questions to respondents and survey flow.10 Questionnaires assessed demographic, clinical (uptake of cancer screening, risk-reducing surgery and chemo-prevention) and genetic data (BRCA1/2 mutation status, date of testing). In addition, participants were asked to complete a family history questionnaire and the Impact of Event Scale (IES).11 The family history questionnaire asked about first- and second-degree relatives (with definitions) diagnosed with breast cancer before age 50 years, ovarian cancer at any age, male breast cancer, bilateral breast cancer and three or more breast cancer at any age, a combination of breast, ovarian and/or pancreatic cancer on the same side of the family.
The IES was used to measure cancer-related distress. The event was “Being at increased risk of cancer because of a confirmed mutation in the BRCA1 or BRCA2 genes”. For each item, respondents were asked to indicate how frequently each item was true for them during the past 7 days, with the answer choices being “Not at all”, “A Little Bit”, “Moderately” and “Quite a Bit”. Total distress scores can range between 0 and 75. Scores between 0 and 8 are considered sub-clinical, between 9 and 25 indicate mild distress, between 26 and 43 indicate moderate distress and scores >43 indicate severe distress.
Statistical methods
Descriptive statistics were used to characterise the sample. Multivariable logistic regression models were used to assess differences between women with sub-clinical or mild distress and women with moderate-to-severe psychological distress. Regression modelling was conducted for all respondents. Covariates included were based on a priori understanding of cancer-related distress work: age (40 years and older or younger than 40 years), whether a first-degree relative has ever been diagnosed with breast cancer and ovarian cancer (yes or no), higher education level (4-year college or higher or no 4-year college degree), marital status (yes or no), years since gene identification (continuous years), whether the respondent has children (yes or no), whether the respondent has had risk-reducing surgery (risk-reducing bilateral mastectomy (RRBM), bilateral salpingo oophorectomy (BSO), both or none) and recruiting source (online through FORCE or through a clinic). Tests of association between respondents who were recruited through clinics and respondents who were recruited through FORCE were calculated using two-sample t tests for sample means, χ2 tests (frequencies >5 in each category) and Fisher’s exact test (frequencies <5 in at least one category) for categorical variables. The multivariable logistic regression models were generated using the SAS software, version 9.4 (SAS Institute Inc., Cary, NC). Summary statistics and associated P values were generated using the Stata, version 15 software (StataCorp, College Station, TX). All P values < 0.05 (two-tailed) were considered to be statistically significant.
Patient sample
Between January 2015 and March 2016, subjects were recruited through international clinical sites and online through the advocacy group (FORCE). The clinical sites mailed 1163 letters to potentially eligible women, 383 women accessed the survey, and 338 met the inclusion criteria. Of the women who met the inclusion criteria, 303 completed the IES questions in the survey and provided data on the covariates used for analysis. Through FORCE (advocacy group), 1374 women accessed the survey, and 494 met the inclusion criteria. Of the women who met the inclusion criteria, 273 completed the IES questions in the survey and provided data on the covariates used for analysis. Combining the women recruited through clinics (n = 303) and recruited online through FORCE (n = 273), the final sample size was 576.
Results
Of the 576 study participants, 52.3% had a BRCA1 mutation, 45.1% had a BRCA2 mutation, and 1.4% had both a BRCA1 and BRCA2 mutation (1.2% were unsure of which gene). The mean age at the time of questionnaire completion was 40.8 years (SD = 8.1) and the mean time elapsed since genetic testing was 4.9 years (SD = 4.4, range 0–23 years). The majority of participants were from the United States (54.0%), but others were from the United Kingdom (20.3%), Australia (20.3%) and Canada (5.4%) (Table 1). Of the 311 USA participants, 273 were recruited from FORCE and 38 were recruited from Creighton University. Table 1 presents the characteristics of women recruited through a clinic and through FORCE separately. Looking at some of the larger differences, the FORCE sample had a higher percentage of college educated women and women employed full time. The average time since diagnosis was shorter in the FORCE sample.
Table 1.
Summary statistics.
| Source of recruitment | Summary statistics | |||
|---|---|---|---|---|
| Number of respondents (%)a, except where noted | ||||
| Clinic (n = 303) | FORCE (n = 273) | Test of difference, P value | All respondents (N = 576) | |
| Age (years) | ||||
| Min, max | 25, 55 | 25, 55 | 0.599 | 25, 55 |
| Mean (SD) | 40.68 (8.11) | 41.04 (8.16) | 40.85 (8.13) | |
| Median | 40 | 41 | 41 | |
| Age category | ||||
| 25‒39 | 137 (45.2%) | 117 (42.9%) | 0.569 | 254 (44.1%) |
| 40‒55 | 166 (54.8%) | 156 (57.1%) | 322 (55.9%) | |
| Ethnicity | ||||
| White or Caucasian | 282 (93.1%) | 257 (94.1%) | 0.136 | 539 (93.6%) |
| Black or African decent | 0 (0.0%) | 3 (1.1%) | 3 (0.5%) | |
| Hispanic or Latino | 1 (0.3%) | 3 (1.1%) | 4 (0.7%) | |
| Asian | 5 (1.7%) | 3 (1.1%) | 8 (1.4%) | |
| Other | 15 (5.0%) | 7 (2.6%) | 22 (3.8%) | |
| Higher education (4-year college and higher) | 170 (56.1%) | 217 (79.5%) | <0.001 | 387 (67.2%) |
| Marital status | ||||
| Married/living as married/civil partnership | 233 (76.9%) | 207 (75.8%) | 0.834 | 440 (76.4%) |
| Single/never married | 42 (13.9%) | 43 (15.8%) | 85 (14.8%) | |
| Divorced/separated/widowed/other | 28 (9.2%) | 23 (8.4%) | 51 (8.9%) | |
| Have child or children | 59 (19.5%) | 56 (20.5%) | 0.755 | 398 (69.1%) |
| Employment status | ||||
| Employed full time | 159 (52.5%) | 170 (62.3%) | 0.003 | 329 (57.1%) |
| Employed part time | 74 (24.4%) | 32 (11.7%) | 106 (18.4%) | |
| Self-employed | 28 (9.2%) | 28 (10.3%) | 56 (9.7%) | |
| Homemaker | 23 (7.6%) | 32 (11.7%) | 55 (9.5%) | |
| Other | 19 (6.3%) | 11 (4.0%) | 30 (5.2%) | |
| Income (above median income in respective country) | 136 (44.9%) | 75 (27.5%) | <0.001 | 211 (36.6%) |
| Country | ||||
| US | 38 (12.5%) | 273 (100.0%) | ─ | 311 (54.0%) |
| UK | 117 (38.6%) | ─ | 117 (20.3%) | |
| Australia | 117 (38.6%) | ─ | 117 (20.3%) | |
| Canada | 31 (10.2%) | ─ | 31 (5.4%) | |
| Place of recruitment | ||||
| Clinic | 303 (100.0%) | ─ | ─ | 303 (52.6%) |
| Online through FORCE | ─ | 273 (100.0%) | 273 (47.4%) | |
| Mutation | ||||
| BRCA1 | 154 (50.8%) | 147 (53.8%) | 0.066 | 301 (52.3%) |
| BRCA2 | 136 (44.9%) | 124 (45.4%) | 260 (45.1%) | |
| BRCA1 and BRCA2 | 7 (2.3%) | 1 (0.4%) | 8 (1.4%) | |
| Do not know or not sure | 6 (2.0%) | 1 (0.4%) | 7 (1.2%) | |
| Time in years since gene mutation identified; mean (SD), median (range) |
6.11 (4.7) 6 (0‒23) |
3.49 (3.5) 2 (0‒16) |
<0.001 |
4.87 (4.35) 4 (0‒23) |
| IES total score by category | ||||
| Sub-clinical (0‒8) | 166 (54.8%) | 144 (41.8%) | 0.002 | 280 (48.6%) |
| Mild (9‒25) | 102 (33.7%) | 100 (36.6%) | 202 (35.1%) | |
| Moderate (26‒43) | 27 (8.9%) | 47 (17.2%) | 74 (12.8%) | |
| Severe (≥ 44) | 8 (2.6%) | 12 (4.4%) | 20 (3.5%) | |
| Family historyb | ||||
| First-degree relative with breast cancer before age 50 years | 221 (72.9%) | 197 (72.2%) | 0.835 | 418 (72.6%) |
| First-degree relative with ovarian cancer at any age | 144 (47.5%) | 126 (46.2%) | 0.742 | 270 (46.9%) |
| ≥2 family members with breast cancer on the same side of the family | 202 (66.7%) | 181 (66.3%) | 0.926 | 383 (66.5%) |
| Male relative with breast cancer | 26 (8.6%) | 15 (5.5%) | 0.150 | 41 (7.1%) |
| Breast, ovarian, and/or pancreatic cancer on the same side of the family | 112 (37.0%) | 111 (40.7%) | 0.363 | 223 (38.7%) |
| ≥3 relatives with breast cancer at any age | 140 (46.2%) | 126 (46.2%) | 0.990 | 266 (46.2%) |
| None of the above | 14 (4.6%) | 9 (3.3%) | 0.418 | 23 (4.0%) |
| Risk-reducing strategies (completed/current) | ||||
| RRBM (only) | 46 (15.2%) | 44 (16.1%) | 0.757 | 90 (15.6%) |
| BSO (only) | 60 (19.8%) | 47 (17.2%) | 0.426 | 107 (18.6%) |
| RRBM and BSO | 95 (31.4%) | 98 (35.9%) | 0.249 | 193 (33.5%) |
| Neither RRM or BSO | 102 (33.7%) | 84 (30.8%) | 0.458 | 186 (32.3%) |
RRBM risk-reducing bilateral mastectomy, BSO bilateral salpingo oophorectomy.
aPercentage of respondents who answered the question, does not account for missing observations.
bFirst-degree relative: mother, daughter, sister, father, son or brother.
The mean cancer-related distress score, as measured by the IES, was 12.7 (SD = 13.1). Ninety-four participants (16.3%) scored within the moderate to severe range of total cancer-related distress and 280 participants (48.6%) scored in the sub-clinical range (no distress). The prevalence of moderate or severe cancer-related distress is presented in Table 2 for various subgroups.
Table 2.
Prevalence of moderate or severe cancer-related distress.
| Category | Prevalence, n (%) |
|---|---|
| Total | 94 (16.3%) |
| Age (years) | |
| 25–39 (n = 254) | 46 (18.1%) |
| 40–55 (n = 322) | 48 (14.9%) |
| Country | |
| US, FORCE (n = 273) | 59 (21.6%) |
| US, Creighton University (n = 38) | 2 (5.3%) |
| UK (n = 117) | 18 (15.4%) |
| Canada (n = 31) | 2 (6.5%) |
| Australia (n = 117) | 13 (11.1%) |
| Recruitment source | |
| Online through FORCE (n = 273) | 59 (21.6%) |
| Clinic (n = 303) | 35 (11.6%) |
| Risk-reducing surgery | |
| None (n = 186) | 41 (22.0%) |
| RRBM only (n = 90) | 14 (15.6%) |
| BSO only (n = 107) | 17 (15.9%) |
| RRBM and BSO (n = 193) | 22 (11.4%) |
| Time since genetic testing (years) | |
| 0–1 (n = 148) | 39 (26.4%) |
| 2–4 (n = 176) | 31 (17.6%) |
| 5+ (n = 252) | 24 (9.5%) |
Sixteen per cent of the participants had a previous RRBM only, 18.6% had undergone BSO only and 33.5% had both RRBM and BSO. Thirty-two per cent of the participants had neither preventive surgery. The prevalence of moderate or severe cancer-related distress was 22.0% in women without preventive surgery compared to 11.4% in those who had undertaken both surgeries (P value = 0.007).
Distress levels were similar across countries. In the USA, 61 of 311 women (19.6%) experienced moderate to severe distress. Distress was more common among American women recruited through the online advocacy group FORCE compared to women recruited through a cancer genetics clinic in Omaha (21.6% versus 5.3%, P value < 0.001).
In the multivariable analysis higher education was protective of moderate or severe distress (odds ratio (OR) = 0.57, 95% confidence interval (CI): 0.34–0.96, P value = 0.036) (Table 3). Women who had a sister or mother with cancer were not more likely to have moderate or severe distress compared to women with no affected first-degree relative. Women with both RRBM and BSO were less likely to have moderate or severe cancer-related distress compared to women without either surgery (OR = 0.37, 95% CI: 0.18–0.76, P value = 0.007); however, those with a single surgery were not different than those with neither surgery. Women recruited through FORCE were more than twice as likely to have moderate or severe levels of cancer-related distress compared to women recruited through cancer genetics clinics in the entire study group (OR = 2.26, 95% CI: 1.34–3.82, P value = 0.002). Outside the FORCE group, only one clinic in the Midwest USA provided study subject and at this clinic distress was very low (5.3%), but the sample size was small (n = 38).
Table 3.
Multivariate logistic model evaluating predictors of cancer-related moderate-to-severe cancer-related distress, full sample.
| Variables | All women (n = 576) | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Intercept | 0.30* | (0.14, 0.67) | 0.0030 |
| Age | |||
| <40 years | 1.00 | ||
| 40 years or over | 1.03 | (0.56, 1.91) | 0.9222 |
| First-degree relative with breast cancer | |||
| No | 1.00 | ||
| Yes | 1.09 | (0.66, 1.79) | 0.7306 |
| First-degree relative with ovarian cancer | |||
| No | 1.00 | ||
| Yes* | 1.07 | (0.60, 1.90) | 0.8298 |
| Higher education | |||
| No | 1.00 | ||
| Yes | 0.57* | (0.34, 0.96) | 0.0356 |
| Married | |||
| No | 1.00 | ||
| Yes | 0.85 | (0.47, 1.54) | 0.5904 |
| Time since genetic test (years) | 0.92* | (0.85, 0.98) | 0.0158 |
| Have children | |||
| No | 1.00 | ||
| Yes | 1.69 | (0.93, 3.08) | 0.0863 |
| Cancer risk-reducing surgery | |||
| None | 1.00 | ||
| RRBM only | 0.60 | (0.30, 1.21) | 0.1537 |
| BSO only | 0.54 | (0.25, 1.14) | 0.1055 |
| RRBM and BSO | 0.37* | (0.18, 0.76) | 0.0069 |
| Recruitment source | |||
| Clinic | 1.00 | ||
| Online through FORCE | 2.26* | (1.34, 3.82) | 0.0023 |
*P value < 0.05.
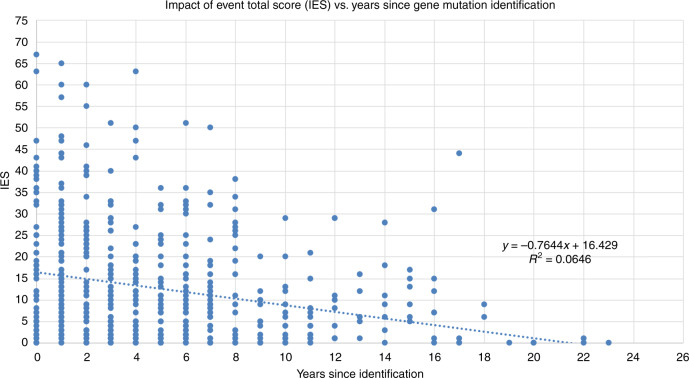
More years since genetic testing were associated with lower levels of cancer-related distress (Fig. 1). For each year elapsed since testing, the probability of experiencing moderate or severe distress declined by 8% (OR = 0.92, 95% CI: 0.854–0.984, P value = 0.016).
Fig. 1. Scatter plot and regression line for Impact of Event Scale (IES) total score and years since gene mutation identification.
y-axis: Impact of Event Scale total score. x-axis: Years since identification of gene mutation.
Discussion
In this large international study, we report that after an average of 5 years after genetic testing, most women with a BRCA mutation without cancer did not have elevated levels of distress. However, a significant proportion (16.3%) did have moderate or severe levels of cancer-related distress. Women with less education were more likely to experience distress than those with post-secondary education.
Since the introduction of genetic testing for BRCA1 and BRCA2 over 20 years ago, the psychosocial consequences of genetic testing have received considerable attention.1–6 The majority of research has focussed on short-term levels of distress and has suggested that distress may increase immediately following the receipt of positive genetic test results, but levels of distress return to baseline levels or below over time.4,6 In the current cross-sectional study in which women were surveyed on average of 5 years post-receipt of genetic test results, distress was significantly lower as time since genetic testing increased. This is consistent with a previous American study that measured long-term cancer-related distress in 107 unaffected BRCA mutation carriers.2 This suggests that support should be focussed in the time period immediately following receipt of test results. In an American study, psychosocial telephone counselling shortly after standard genetic counselling was shown to offer modest short-term benefits for distress and anxiety in women with a BRCA1 or BRCA2 mutation.12 Further research to evaluate psychosocial interventions is required to support women who continue to experience distress in both the short and long term.
Most previous studies have reported on predictors of cancer-related distress in women from single institutions and have included women with cancer.1–5 Both cancer and genetic status have been shown to raise cancer-related distress.13 We chose to focus on unaffected women with a positive BRCA1 or BRCA2 genetic test result to determine if there were modifiable predictors that could be the targets of interventions to reduce cancer-related distress in this subgroup. Although not modifiable, education level and time since testing may help identify women who may require additional support after receiving a positive BRCA genetic test result.
The National Comprehensive Cancer Network recommends that BRCA mutation carriers have BSO between the ages of 35 and 40 years or when child-bearing is complete, and that RRBM is discussed as an option. In the current study, women who elected for both BSO and RRBM were significantly less likely (OR = 0.37, 95% CI: 0.18–0.76, P value = 0.007) to have moderate or severe cancer-related distress compared to women with neither surgery; however, no significant reduction in distress was seen with only one surgery (either BSO or RRBM). We have previously reported on changes in cancer-related distress in unselected Jewish women who were found to have a BRCA1 or BRCA2 mutation.14 Cancer-related distress decreased significantly after uptake of both bilateral prophylactic mastectomy and BSO in women with a BRCA1 or BRCA2 mutation. The current study provides further evidence that risk-reducing surgery is beneficial in reducing cancer-related distress in women with a BRCA1 or BRCA2 mutation.
Much of the previous research reporting on psychosocial outcomes after genetic testing for BRCA1 and BRCA2 has enrolled women from academic cancer genetics clinics. It is important to evaluate outcomes in women who are recruited from outside cancer genetics clinics as this is increasingly common. In the current study, 21.6% of the women who were recruited through an advocacy group (FORCE) were experiencing moderate or severe levels of cancer-related distress; they were more than twice as likely to experience moderate or severe cancer-related distress than women recruited through cancer genetics clinics (OR = 2.26, 95% CI: 1.34–3.82, P value = 0.002). There were only 38 women enrolled from the USA from clinics other than FORCE and the comparison group of American women is small. The reason(s) for this difference are unclear, nor is the direction of the association. These women might not have received traditional genetic counselling and may remain with unresolved uncertainties that provoke anxiety. Alternatively, women who are experiencing cancer-specific distress may seek information about their condition. It is also possible that participating in the online group has the effect of reinforcing the woman’s awareness of her cancer vulnerability on a daily basis and thus changes the affect of the patient. We do not have the data to conclude that the increased levels of distress were the direct consequence of participating in the online forum and this is a topic of future study.
There are several limitations to the current study. This study was a convenience sample and employed a cross-sectional design, and as a result, we were not able to measure changes in distress over time. We only measured distress using one instrument (IES); however, this measure has demonstrated concurrent and discriminative validity in women at an increased risk of developing breast cancer.15 In addition, as expected with a survey study, the participation rate was not optimal and we only surveyed women between the ages of 25 and 55 years, so the results may not be generalisable to women over the age of 55 years. Furthermore, we did not collect data about access to care, including insurance status, rural versus urban or access to a genetic counsellor. All the factors should be considered in future research to determine if these factors have an impact on cancer-related distress after receipt of positive BRCA genetic test results.
Those of us who have counselled unaffected women with mutations have noted the wide range of emotions expressed by these women and will have encountered a small number of women who become fixated on their genetic status. Often, these women seek the input of multiple counsellors and physicians. It is not clear if any current cognitive therapies are effective in reducing life-altering levels of distress, but there is some evidence that preventive surgery is therapeutic in this regard. These women may require more targeted support than is available through an online support group or through classical genetic counselling. Further research is required to determine if any additional interventions are effective in this vulnerable subgroup of patients.
Acknowledgements
We dedicate this research study in honour of Melanie Price who made significant contributions to further the field of psycho-oncology. Melanie was a tireless advocate for individuals affected by cancer, and their families. We, as well as the cancer genetics community as a whole, are saddened by the passing of our co-author Henry T. Lynch, M.D., a renowned researcher and true pioneer in the study of hereditary cancer. Henry was a mentor and friend to several of us and will be greatly missed.
Author contributions
K.A.M.: conceptualisation, visualisation, methodology, project administration, formal analysis, supervision, data curation, writing—original draft, review and editing. M.A.P.: data curation, writing—original draft, review and editing. C.M.: conceptualisation, visualisation, methodology, project administration, formal analysis, data curation, writing—original draft, review and editing. D.C.H.: data curation, writing—original draft, review and editing. G.J.L.: data curation, writing—original draft, review and editing. A.F.: data curation, writing—original draft, review and editing. J.P.: data curation, writing—original draft, review and editing. S.F.: data curation, writing—original draft, review and editing. C.S.: data curation, writing—original draft, review and editing. H.T.L.: data curation, writing—original draft, review and editing. D.G.E: data curation, writing—original draft, review and editing. S.A.N.: conceptualisation, visualisation, methodology, project administration, formal analysis, supervision. A.L.: conceptualisation, visualisation, methodology, project administration, formal analysis, supervision, funding acquisition, data curation, writing—original draft, review and editing.
Ethics approval and consent to participate
The following institutional review boards approved the study: RTI International Institutional Review Board, Research Triangle Park, USA; Social Behavioral Institutional Review Board, Creighton University, Omaha, USA; Research Office, Central Manchester University Hospitals NHS Foundation Trust, Manchester, UK; The Peter MacCallum Cancer Centre Ethics Committee, Melbourne, Australia; Women’s College Hospital Research Ethics Board, Toronto, Canada. All participants provided informed consent prior to their inclusion in the study. This study was performed in accordance with the Declaration of Helsinki.
Consent to publish
None.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
D.C.H. discloses salary compensation and stock ownership with Amgen Inc. (Thousand Oaks, CA). A.L. discloses salary and stock ownership with Amgen Inc. during the time of study conduct; salary and stock ownership with AbbVie Inc. (North Chicago, IL) as of April 2019. C.M. is a current and J.P. is a former employee of RTI Health Solutions that were contracted by Amgen Inc. for the conduct of this study. S.A.N. is an Editorial Board Member of the British Journal of Cancer. No other conflicts of interest are declared for remaining authors.
Funding information
RTI Health Solutions received funding from Amgen Inc. (Thousand Oaks, CA, USA) for this study (study no. 20140153). This publication was also supported by revenue from Nebraska’s excise tax on cigarettes awarded to Creighton University through the Nebraska Department of Health & Human Services (DHHS). Its contents represent the views of the authors and do not necessarily represent the official views of the State of Nebraska or DHHS. Funding was also received from the Liz’s Legacy fund through Kicks for a Cure. Dr. H.T.L.’s work was partially funded through the Charles F. and Mary C. Heider Chair in Cancer Research, which he held at Creighton University. D.G.E. is supported by the all Manchester NIHR Biomedical Research Centre (IS-BRC-1215-20007).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Melanie A. Price, Henry T. Lynch.
References
- 1.Bosch N, Junyent N, Gadea N, Brunet J, Ramón y Cajal T, Torres A, et al. What factors may influence psychological well being at three months and one year post BRCA genetic result disclosure? Breast. 2012;21:755–760. doi: 10.1016/j.breast.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Graves KD, Vegella P, Poggi EA, Peshkin BN, Tong A, Isaacs C, et al. Long-term psychosocial outcomes of BRCA1/BRCA2 testing: differences across affected status and risk-reducing surgery choice. Cancer Epidemiol. Biomark. Prev. 2012;21:445–455. doi: 10.1158/1055-9965.EPI-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halbert CH, Stopfer JE, McDonald J, Weathers B, Collier A, Troxel AB, et al. Long-term reactions to genetic testing for BRCA1 and BRCA2 mutations: does time heal women’s concerns? J. Clin. Oncol. 2011;29:4302–4306. doi: 10.1200/JCO.2010.33.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AW, Dougall AL, Posluszny DM, Somers TJ, Rubinstein WS, Baum A. Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psychooncology. 2008;17:767–773. doi: 10.1002/pon.1291. [DOI] [PubMed] [Google Scholar]
- 5.Beran TM, Stanton AL, Kwan L, Seldon J, Bower JE, Vodermaier A, et al. The trajectory of psychological impact in BRCA1/2 genetic testing: does time heal? Ann. Behav. Med. 2008;36:107–116. doi: 10.1007/s12160-008-9060-9. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MD, Peshkin BN, Hughes C, Main D, Isaacs C, Lerman C. Impact of BRCA1/BRCA2 mutation testing on psychologic distress in a clinic-based sample. J. Clin. Oncol. 2002;20:514–520. doi: 10.1200/JCO.2002.20.2.514. [DOI] [PubMed] [Google Scholar]
- 7.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 8.King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091–1092. doi: 10.1001/jama.2014.12483. [DOI] [PubMed] [Google Scholar]
- 9.Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Liede A, Mansfield CA, Metcalfe KA, Price MA, Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. Snyder C, et al. Preferences for breast cancer risk reduction among BRCA1/BRCA2 mutation carriers: a discrete-choice experiment. Breast Cancer Res. Treat. 2017;165:433–444. doi: 10.1007/s10549-017-4332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom. Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Graves KD, Wenzel L, Schwartz MD, Luta G, Wileyto P, Narod S, et al. Randomized controlled trial of a psychosocial telephone counseling intervention in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol. Biomark. Prev. 2010;19:648–654. doi: 10.1158/1055-9965.EPI-09-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringwald J, Wochnowski C, Bosse K, Giel KE, Schäffeler N, Zipfel S, et al. Psychological distress, anxiety, and depression of cancer-affected BRCA1/2 mutation carriers: a systematic review. J. Genet. Couns. 2016;25:880–891. doi: 10.1007/s10897-016-9949-6. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe KA, Mian N, Enmore M, Poll A, Llacuachaqui M, Nanda S, et al. Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res. Treat. 2012;133:735–740. doi: 10.1007/s10549-011-1941-0. [DOI] [PubMed] [Google Scholar]
- 15.Thewes B, Meiser B, Hickie IB. Psychometric properties of the Impact of Event Scale amongst women at increased risk for hereditary breast cancer. Psychooncology. 2001;10:459–468. doi: 10.1002/pon.533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.